Professional Documents
Culture Documents
Dr Mirza's Guide to Myelopoiesis (10p
Uploaded by
Hamilton SebastineOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dr Mirza's Guide to Myelopoiesis (10p
Uploaded by
Hamilton SebastineCopyright:
Available Formats
Dr.
Talaat Mirza Myelopoiesis 10 pages
MYELOPOIESIS
Myelopoiesis is the process of making new WHITE blood cells (myeloid cells).
There are two major types of myeloid cells: 1- granuloid, and 2- non-granuloid. As
the names imply, granuloid cells have granules (in the cytoplasm) where as non-
granuloid are devoid of such granules.
GRANULOID CELLS
Three sub types of granuloid cells are present in BM and peripheral blood (pb):
1- The Poly-Morphonuclear-Neutrophil (pmn) and the band (the stage preceding
the pmn in maturation) compromising up to 70% of pb. These cells have very
fine (minute) granules all over the cytoplasm. The color of these granules is
identical to the color of the RBC's.
2- The basophil making up to 1% of pb. These cells are characterized by the
presence of very large dark blue-black granules all over the cell. The
granules are very basophilic hence the color is very dark blue.
3- The eosinophil making up 1-5% of pb. These cells are characterized by the
presence of very large bright red or pink granules all over the cytoplasm.
The granules are very acidophilic, hence the color is very bright red acquired
by taking up the eosin dye.
NON-GRANULOID CELLS
Two major groups of cells are non-granuloid: 1- the lymphoid cells, and 2- the
monocytoid/macrophage ones. In this section of the myelopoiesis, only
monocytes/macrophages development and differentiation is discussed. Lymphoid
Dr. Talaat Mirza Myelopoiesis Page 1 of 10
Dr. Talaat Mirza Myelopoiesis 10 pages
cells will not be discussed due to their different stem cell origin. Lymphpoiesis is
going to be discussed in detail, in the next section.
COMMITMENT TO DIFFERENTIATION
The pluripotent stem cell differentiates into a more specialized cell known as the
CFU-GEMM. The CFU-GEMM (also known as CFU-mix) is the earliest form of
stem cell committed to myeloid differentiation. As mentioned in the previous lecture,
CFU-GEMM can give rise to only 4 cell lines (granuloid, erythroid, monocytoid, and
megakaryocytoid). Specific myeloid growth factors such as G-CSF, M-CSF, and
GM-CSF act on the CFU-GEMM to enhance its differentiation to one of these
myeloid cell lines. On the other hand, CFU-GEMM's exposed to erythropoietin
(especially in the absence of G-CSF, M-CSF, or GM-CSF) would differentiate to
erythroid cells. Erythroid differentiation is going to be discussed in detail, in later
lectures.
Once the CFU-GEMM is exposed to G-CSF and other supporting GF’s, the stem cell
further commits it self to granuloid differentiation ending in neutrophils, basophils,
and/or eosinophils. Similarly, if it was exposed to M-CSF and other supporting
GF’s, the CFU-GEMM further commits it self to monocyte/macrophage
differentiation ending in monocytes and/or macrophages.
Important features in morphological evaluation
The following considerations are to be followed whenever a cell is to be evaluated or
identified, morphologically:
1- Cell size: normal red blood cells have a diameter of about 7 microns (μ). One
should use the RBC as a reference of size. For example, a small resting
lymphocyte is about 9 microns (therefore slightly bigger than a normal RBC)
Dr. Talaat Mirza Myelopoiesis Page 2 of 10
Dr. Talaat Mirza Myelopoiesis 10 pages
however a mature monocyte is about 17 microns (more than twice the size of a
normal RBC).
2- Shape: the shape is evaluated noting criteria such as, shape of nucleus (round,
oval, or irregular), color (of nucleus & cytoplasm), regularity of the cytoplasm,
multi lobulated nucleus versus single, etc.
3- Nucleus: Size and shape of the nucleus must be evaluated noting whether it is
single or multiple. Also, the appearance of chromatin (smooth or condensed).
The more condensed the chromatin, the more mature the cell (immature
cells, e.g., blasts, have very smooth chromatin). Presence or absence of
nucleoli should be observed because mature cells do not have nucleoli.
4- Cytoplasm: color (light blue or gray, dark blue, reddish, etc.), presence or
absence of granules, presence of vacuoles, shape of cytoplasm (regular versus
irregular), presence of appendages (projections) from the cytoplasm, presence
of strange looking bodies (inclusions), etc.
5- Nucleus to Cytoplasm (N/C, also N:C) ratio: early immature cells are
characterized by extremely large nuclei, that is, their nucleus could occupy
up to 95% off cell size with very little cytoplasm evident. As cells mature,
nucleus size decreases and cytoplasm increases to reach about 50% or even
less in certain cells, e.g., plasma cell (a type of B-lymphocyte, to be discussed
in detail later).
Dr. Talaat Mirza Myelopoiesis Page 3 of 10
Dr. Talaat Mirza Myelopoiesis 10 pages
MATURATION SEQUENCE
The first step in myeloid differentiation is the commitment of the pluripotent stem to
become the multipotent stem cell (CFU-GEMM, also known as CFU-mix). The
CFU-GEMM then differentiates furthermore to give rise to the first morphologically
identified myeloid cell, the Myeloblast. From the myeloblast, five morphologically
different maturation forms are produced (not including the blast itself). Therefore, the
stages in the myeloid differentiation are: 1- myeloblast, 2- promyelocyte, 3-
myelocyte, 4- metamyelocyte, 5- band, and 6- neutrophil (pmn). These sequences
also exist for the basophil and the eosinophil, however, in writing them, the words
basophilic or eosinophilic precede the maturation stage, e.g., basophilic promyelocyte,
or eosinophilic metamyelocyte, etc. Morphologically, blasts are only identified as
BLASTS without any specification of the type whether, granuloid, monocytoid,
lymphoid, or else (see below further explanation).
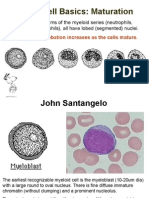
1- The myeloblast: This is the earliest morphologically identified myeloid cell.
The blast is impossible to distinguish from a CFU-GEMM or even a CFU-
S (the pluripotent) stem cell on the basis of morphology only. Only CD
markers, molecular, and biological assays can differentiate among these cells.
It is a very large cell of up to 20 μ in size (about 3 times the size of a normal
RBC). The nucleus occupy more than 85% of the cell (i.e., very high N:C
ratio) with very smooth chromatin, and 1 to 3 nucleoli could be seen. The
very scant cytoplasm present is usually light to slightly dark blue in color, with
no granules or vacuoles of any type. The blasts compromise about 1-2% of
the total nucleated cells in the BM, and not seen in pb.
Dr. Talaat Mirza Myelopoiesis Page 4 of 10
Dr. Talaat Mirza Myelopoiesis 10 pages
2- The promyelocyte (pro's): these cells are very similar to the blast in
everything except the cytoplasm, where the primary pinkish-red granules
could be prominently seen. These non-specific primary granules, known as
the Azurophilic granules are synthesized in this stage and contain
Myeloperoxidase (MPO) enzyme (the hallmark mark of neutrophilic series).
This enzyme remains active until the mature neutrophil stage. It is the
principal enzyme involved in neutrophil phagocytosis (killing of foreign
bodies, e.g., bacteria). Primary granules are made and lost in this stage
(however the enzyme remains until pmn stage). Other molecules found in
the granules include: proteases, lysozymes, muramidase, etc. Pro’s make up to
5% of total nucleated cells in the BM, and not seen in pb.
3- The myelocyte (myelo's): the cell has a wide range of size (16-24 μ) with
many different criteria than the previous 2 stages. The nucleus is usually
round but could be oval with slightly condensed chromatin, and no nucleoli.
The N/C ratio is smaller than previous 2 (less than 70%). The cytoplasm is
pale gray to pinkish due to the presence of secondary granules (also known as
specific granules), which are made in this stage but unseen due to their very
small size. Secondary granules contain Alkaline Phosphatase, acid
hydrolases, lysozymes, and other proteins, but no MPO. The myelocyte is the
last stage capable of mitosis, i.e., cells in the following stages cannot
proliferate (make more of them selves) but only mature (differentiate) to gain
more specific functional abilities. Myelo’s make up to 20% of BM and not
seen in pb.
Dr. Talaat Mirza Myelopoiesis Page 5 of 10
Dr. Talaat Mirza Myelopoiesis 10 pages
4- The metamyelocyte (meta's): the meta is similar to myelo in a lot of things
except that its nucleus is not round or oval anymore, rather the nucleus takes
a kidney, bean, or peanut shape. The cytoplasm is more pinkish than the
previous stage due to presence of the very tiny secondary granules. Meta’s
make up to 30% of BM and rarely seen in pb (only in stress situation where a
large number of granuloid cells are released in to pb to fight a severe infection,
for instance).
5- The band: the last stage before the fully mature and differentiated neutrophil
(pmn). The nucleus has started to lobulate forming a specific shape of a
“horse shoe” and the filaments which, attach (connect) the different lobes have
not yet, developed. Bands spend about 24 hours in pb to become fully mature
lobulated pmn’s. Bands can make up to 20% of pb differential count.
6- The neutrophil (pmn's): the cell is unique in its possession of a multi-lobed
nucleus. Usually 2-3 lobes could be seen, attached to each other by very fine
filaments (thread like inclusions). The color of cytoplasm is identical to that
of RBC’s with very fine tiny granules all over. Neutrophils make up to 65%
of pb differential count.
Dr. Talaat Mirza Myelopoiesis Page 6 of 10
Dr. Talaat Mirza Myelopoiesis 10 pages
OTHER GRANULOID CELLS
Two granuloid cells exist in normal pb and BM known as: 1- the basophil, and 2- the
eosinophil. These cells rise from the same CFU-GEMM as the neutrophil. The
earliest form to be recognized in the bone marrow is at the PROMYELOCYTE
stage. In other words, the blast cell of all myeloid cells is exactly the same, and
could not be distinguished, from one to another. Basophilic (or eosinophilic)
promyelocytes, (as well as their following stages), are recognized by their
characteristic granules. That is, the presence of cytoplasmic dark blue to black
granules for basophils, and bright red ones for the eosinophilic lineage.
The basophil (baso's)
Similar to mature neutrophil but could be slightly bigger than pmn’s. The very
obvious "hallmark" criterion for its identification is the presence of very large dark
blue-blackish granules all over the cell. Even though, the granules are cytoplasmic,
they might appear as if they are nuclear, as well. Basophils and their tissue
equivalent, the mast cells, are very important mediators of allergic reactions,
especially in the “Immediate Hypersensitivity Reaction”. These cells release
HISTAMINE (a powerful chemotactic factor), Platelet Activating Factor (PAF), and
Arachadonic acid. Basophils are rarely seen in pb or BM having a differential pb
count of about 1%.
The eosinophil (eos)
Eosinophils have large refractive bright pinkish-red granules that are restricted to the
cytoplasm and do not overlap the nucleus (as was the case with baso’s). Eosinophils
are increased in parasitic infections (as an inflammatory response), allergy, and
certain skin diseases. Considerably rare eos are found at about 5% of the normal pb
differential count.
Dr. Talaat Mirza Myelopoiesis Page 7 of 10
Dr. Talaat Mirza Myelopoiesis 10 pages
NEUTROPHIL PHAGOCYTOSIS
The major function of neutrophils is in the defense and killing of foreign invading
organisms (mainly, bacteria). The process is known as phagocytosis and involves
specialized premeditated plan:
1- Motility: also known, as “diapedesis” is the process that allows pmn’s free
migration among tissues and pb. Usually, pmn’s move (migrate) freely in pb,
but in response to chemotactic factors, pmn’s can direct themselves toward the
stimulus. Chemotactic factors are certain compounds such as bacterial
endotoxins, C5a (a complement factor), or Arachadonic acid. Tissue that have
been exposed to bacterial insult, releases such compounds leading to pmn’s
migration to the site of infection.
2- Recognition: antibodies or complement fragments attached to the invading
organism help in identifying the invader (a process known as “opsonization”).
Some opsonins include compounds such as C3b (a complement fragment), Ig
G antibody, or fibronectin.
3- Ingestion: the cytoplasm of pmn’s extends outward (in appendages) known
as “psuedopods” until it fuses together around the foreign body (surrounds it)
forming a “phagosome”.
4- De-granulation: this is the process where pmn’s granules empty their load
(compounds) into the phagosome.
5- Killing: this achieved by the release of cytotoxic compounds such as
superoxide and hydrogen peroxide (aided by the Myeloperoxidase enzyme).
Other lysozymes also, act on degeneration of the bacterial cell by lysing its
cell wall. Finally, the invading cell is gradually lysed and the whole complex
(pmn including the invading bacteria) is disposed.
Dr. Talaat Mirza Myelopoiesis Page 8 of 10
Dr. Talaat Mirza Myelopoiesis 10 pages
MONOCYTIC MATURATION
Monocytes and macrophages (activated form of monocytes found mostly in tissues)
are derived from the same stem cell that granuloid cells (pmn’s, baso’s, and eos) are
derived from, which is the CFU-GEMM (CFU-mix). Similar to neutrophil
maturation, the first identifiable cell morphologically is the blast. However, unlike,
neutrophils, there are only 3 maturational stages (cells) in the monocytic series.
These stages are: 1- the monoblast, 2- the promonocyte, and finally, the mature
monocyte.
1- The monoblast:
Morphologically, the monoblast is undistiguishable from the myeloblast. That is, it is
a very large cell of up to 20 μ in size (about 3 times the size of a normal RBC). The
nucleus occupy more than 85% of the cell (i.e., very high N:C ratio) with very smooth
chromatin, and 1 to 3 nucleoli could be seen. The very scant cytoplasm present is
usually light to slightly dark blue in color, with no granules or vacuoles of any type.
Only CD markers, biological assays, and advanced molecular techniques can
differentiate between monoblast and myeloblast.
2- The promonocyte:
This is similar to the promyelocyte in everything, except they don’t have the typical
primary (reddish pink) granules of the promyelocytes. That is, their shape, size,
chromatin quality, and cytoplasm are very much similar to that of promyelocytes.
They also resemble myeloblasts but they might not have nucleoli.
3- The monocyte:
The monocyte ranges in size from 12 to 22 μ but appear large on stained blood films.
They look larger than neutrophils because they strongly adhere to the slide surface.
Dr. Talaat Mirza Myelopoiesis Page 9 of 10
Dr. Talaat Mirza Myelopoiesis 10 pages
The nucleus is very variable in shape where it could be round, horse-shoe, or
lobulated, with possible convolutions and/or folding of the chromatin. The cytoplasm
is irregular in shape with dull, faded, grayish-blue color and very fine granules. The
cytoplasm could have some projections (psuedopods) and may contain vacuoles.
Monocytes constitute 2-10% of pb differential count.
The main function of monocytes is phagocytosis of invading organisms, even though
they are a little slower and less efficient than neutrophils. Furthermore, monocytes
are involved in T cell mediated immunity. Monocytes can live up to 3 days but tissue
macrophages (i.e., the monocytes that got localized inside the tissues) can live for
several months in the tissues.
Dr. Talaat Mirza Myelopoiesis Page 10 of 10
You might also like
- Dr Mirza's Guide to Myelopoiesis (10pDocument10 pagesDr Mirza's Guide to Myelopoiesis (10pHamilton SebastineNo ratings yet
- HEMATOPOIESISDocument9 pagesHEMATOPOIESISKIPRUTO DENNISNo ratings yet
- HEMATOLOGY 1 Hematopoiesis Notes 1Document10 pagesHEMATOLOGY 1 Hematopoiesis Notes 1sansastarkNo ratings yet
- Heamtopoises ReviewerDocument17 pagesHeamtopoises ReviewerClyde BaltazarNo ratings yet
- Here, Only 1 Few Stages Undergo Subdivision (Sabi Ni Maam)Document6 pagesHere, Only 1 Few Stages Undergo Subdivision (Sabi Ni Maam)Jaenie Grace AliganNo ratings yet
- Describe The Principles of Blood Cell MaturationDocument11 pagesDescribe The Principles of Blood Cell MaturationnotsoninjaninjaNo ratings yet
- MT0831 Complete Hematology Report CompilationDocument234 pagesMT0831 Complete Hematology Report CompilationsazunaxNo ratings yet
- White Blood Cell (Leucocyte)Document77 pagesWhite Blood Cell (Leucocyte)Dr.Gomathi sivakumarNo ratings yet
- CHAPTER 12: Leukocyte Development Kinetics, and FunctionsDocument7 pagesCHAPTER 12: Leukocyte Development Kinetics, and FunctionsPrimo GeorgeNo ratings yet
- Hematology 425, LeukopoiesisDocument56 pagesHematology 425, LeukopoiesishanzukikNo ratings yet
- Basophilia and RBC Maturation StagesDocument6 pagesBasophilia and RBC Maturation StagesDANIELLE JOYCE BACSALNo ratings yet
- Hema 2Document35 pagesHema 2Angela ReyesNo ratings yet
- Leukopoeisis 210325144405Document41 pagesLeukopoeisis 210325144405Gon FreecssNo ratings yet
- Topic 1Document7 pagesTopic 1Jacqueline Tungcul DonatoNo ratings yet
- CML Philadelphia ChromosomeDocument7 pagesCML Philadelphia ChromosomeSaima IramNo ratings yet
- HEMOSTASISDocument6 pagesHEMOSTASIScatuiraneljhayyyNo ratings yet
- White Cell and Platelet MorphologyDocument7 pagesWhite Cell and Platelet Morphologylamjo5750% (1)
- Basic Tissues Lectures 31,32 - Blood Clotting I & II - Dr. SitaraDocument10 pagesBasic Tissues Lectures 31,32 - Blood Clotting I & II - Dr. SitaraNYUCD17No ratings yet
- Leukocyte's Classification, Structure, Function, and AssessmentDocument32 pagesLeukocyte's Classification, Structure, Function, and Assessmenthayamitib11No ratings yet
- Most Common Type of Wbcs in Adults: Nucleus: Usually Trilobulated Cytoplasm: Pink To Tan With Violet or LilacDocument21 pagesMost Common Type of Wbcs in Adults: Nucleus: Usually Trilobulated Cytoplasm: Pink To Tan With Violet or LilacRod PasionNo ratings yet
- 5.0 LeukopoiesisDocument37 pages5.0 LeukopoiesisJunior SataNo ratings yet
- Week 1 - Hematology 2 LectureDocument8 pagesWeek 1 - Hematology 2 LectureRubenne Miles ElagasNo ratings yet
- Praktikum MDTDocument16 pagesPraktikum MDTnanda andhyka100% (1)
- Haem 2 Revision Sept 2010Document170 pagesHaem 2 Revision Sept 2010saint5470100% (1)
- GRANULOPOIESIS (Autosaved) .PPTX OKLADocument93 pagesGRANULOPOIESIS (Autosaved) .PPTX OKLAPrincewill SeiyefaNo ratings yet
- ERYTHROPOIESISDocument17 pagesERYTHROPOIESISsureshNo ratings yet
- MegakaryopoiesisDocument41 pagesMegakaryopoiesisHazel FlorentinoNo ratings yet
- Hematopoietic DevelopmentDocument6 pagesHematopoietic DevelopmentSea ClestNo ratings yet
- PBL-3: Effects of Smoking and Gutka on HealthDocument8 pagesPBL-3: Effects of Smoking and Gutka on HealthRomi RajaNo ratings yet
- Hematology Section OverviewDocument76 pagesHematology Section OverviewDr.A SHAHID SiddiquiNo ratings yet
- Plant Cell Biology #2Document34 pagesPlant Cell Biology #2Anirudh Moza 16009No ratings yet
- MegakaryopoiesisDocument13 pagesMegakaryopoiesisAezel CruzNo ratings yet
- Soft Tissue Special Issue: Selected Topics in The Pathology of Adipocytic TumorsDocument11 pagesSoft Tissue Special Issue: Selected Topics in The Pathology of Adipocytic TumorsCynthia BarrientosNo ratings yet
- Haematopoiesis: Production of Blood CellsDocument46 pagesHaematopoiesis: Production of Blood Cellslow_sernNo ratings yet
- Red Blood Cells..: Erythropoiesis.Document10 pagesRed Blood Cells..: Erythropoiesis.lubna aloshibiNo ratings yet
- Anatomic Sciences PDFDocument656 pagesAnatomic Sciences PDFFatima Nurjanna AwaliNo ratings yet
- Lab 9Document40 pagesLab 9bob0% (1)
- Hem 07Document13 pagesHem 07api-3706483No ratings yet
- Cell Cycle and DivisionDocument47 pagesCell Cycle and DivisionMadhura ShekatkarNo ratings yet
- Haematopoiesis: Jump To Navigation Jump To SearchDocument16 pagesHaematopoiesis: Jump To Navigation Jump To SearchHashim uddinNo ratings yet
- 5.1 Replication and Division of Nuclei and Cells-MinDocument3 pages5.1 Replication and Division of Nuclei and Cells-MinShean LimNo ratings yet
- Granulocytic Series: General CommentsDocument2 pagesGranulocytic Series: General Commentselishaswaih15No ratings yet
- Cell DivisionDocument20 pagesCell DivisionABHINABA GUPTANo ratings yet
- White and Red Cells DevelopementDocument18 pagesWhite and Red Cells Developementsaint5470No ratings yet
- Biology 3rd Edition Brooker Solutions Manual DownloadDocument11 pagesBiology 3rd Edition Brooker Solutions Manual DownloadPorfirio Dambrosio100% (21)
- L 11 MeiosisDocument39 pagesL 11 MeiosissNo ratings yet
- The Fundamental Unit of LifeDocument5 pagesThe Fundamental Unit of Lifevivek_theeasywaysNo ratings yet
- Cell Cycle (Rubina)Document35 pagesCell Cycle (Rubina)RubinaNo ratings yet
- Biology ExamDocument17 pagesBiology ExamSai Adrienne RealubitNo ratings yet
- HistoDocument13 pagesHistoJAWAD, JAINA A.No ratings yet
- Leukocyte Development HEMADocument1 pageLeukocyte Development HEMANikoh Anthony EwayanNo ratings yet
- Cell Cycle and Cell Division Class 11 Notes. " (Cbse)Document8 pagesCell Cycle and Cell Division Class 11 Notes. " (Cbse)i uploadNo ratings yet
- Erythropoiesis Formation Red Blood CellsDocument8 pagesErythropoiesis Formation Red Blood CellsMohammed R.HusseinNo ratings yet
- BSC Lecture Notes Semester 1Document7 pagesBSC Lecture Notes Semester 1satasha28No ratings yet
- Cytology 2Document19 pagesCytology 2Dennis AgyemfraNo ratings yet
- Cell DivisionDocument20 pagesCell DivisionSoumodip ChakrabortyNo ratings yet
- Cell DivisionDocument16 pagesCell Divisionh6wcr2wgdfNo ratings yet
- A Simple Guide to the Blood Cells, Related Diseases And Use in Disease DiagnosisFrom EverandA Simple Guide to the Blood Cells, Related Diseases And Use in Disease DiagnosisNo ratings yet
- Embryo Development StagesDocument29 pagesEmbryo Development StagesHamilton SebastineNo ratings yet
- 1.health Education For 200LDocument32 pages1.health Education For 200LHamilton SebastineNo ratings yet
- Biostatistics Lecture NotesDocument86 pagesBiostatistics Lecture NotesHamilton SebastineNo ratings yet
- 1.health Education For 200LDocument32 pages1.health Education For 200LHamilton SebastineNo ratings yet
- Pathology REP FinalDocument15 pagesPathology REP Finalswerelloxsthe3rdNo ratings yet
- Bard Magnum Manual de UsuarioDocument144 pagesBard Magnum Manual de UsuarioNelson LebronNo ratings yet
- UM-DP12-25R UM-DP20-25R: The World's First Ultrasonic Probes With Dual-Plane Reconstruction Scanning CapabilityDocument3 pagesUM-DP12-25R UM-DP20-25R: The World's First Ultrasonic Probes With Dual-Plane Reconstruction Scanning CapabilityRicardo PaterninaNo ratings yet
- Mzuzu UniversityDocument4 pagesMzuzu UniversityBamusi ChimwemweNo ratings yet
- Veterinary Laboratory ManualDocument44 pagesVeterinary Laboratory Manualmmohammed seidNo ratings yet
- Saran prasarana kepatuhan pada anemia aplastikDocument1 pageSaran prasarana kepatuhan pada anemia aplastikAlika Nigandiva41No ratings yet
- 10 - Growth & Reproduction Exercise 4-1Document2 pages10 - Growth & Reproduction Exercise 4-1rhdoiuaNo ratings yet
- Seerat Kaur - FEMALE - 22 Yrs +918968148007 APJ1.0020209332 5801854Document2 pagesSeerat Kaur - FEMALE - 22 Yrs +918968148007 APJ1.0020209332 5801854Seerat KaurNo ratings yet
- Anticoagulant ReversalDocument4 pagesAnticoagulant Reversalapi-647779956No ratings yet
- Poison Management HandbookDocument238 pagesPoison Management Handbookanon_32392391No ratings yet
- Mental Health Assessment of Elderly People Attending Geriatric Clinic in Medical CityDocument9 pagesMental Health Assessment of Elderly People Attending Geriatric Clinic in Medical CityenggarNo ratings yet
- Drugs in Orthodontics: G.Shekar Subramanian Ist Year PGDocument69 pagesDrugs in Orthodontics: G.Shekar Subramanian Ist Year PGShekar SubramanianNo ratings yet
- Meda QantasDocument12 pagesMeda QantasAviasiNo ratings yet
- Intro To HematologyDocument9 pagesIntro To HematologyDayledaniel SorvetoNo ratings yet
- Amsa232 PDFDocument4 pagesAmsa232 PDFSunny KumarNo ratings yet
- Home remedies for common ailmentsDocument34 pagesHome remedies for common ailmentsBrigitte Nolan100% (3)
- Pathology & Clinical Laboratory (M) SDN - BHD: Scan QR For VerificationDocument1 pagePathology & Clinical Laboratory (M) SDN - BHD: Scan QR For VerificationAzhar SabriNo ratings yet
- تجميعات سعيد لشهر نوفمبرDocument125 pagesتجميعات سعيد لشهر نوفمبرHana kNo ratings yet
- Handbook of Patient Care in Cardiac Surgery by John H. Lemmer JR., Gus J. VlahakesDocument379 pagesHandbook of Patient Care in Cardiac Surgery by John H. Lemmer JR., Gus J. Vlahakesyoshizzle129909No ratings yet
- Intracranial Brain TumorDocument24 pagesIntracranial Brain TumorheruNo ratings yet
- No Proof of COVID19 Causality - Is This The Crime of The Century - Principia Scientific IntlDocument13 pagesNo Proof of COVID19 Causality - Is This The Crime of The Century - Principia Scientific IntlDMDONo ratings yet
- 182 P - Leech Therapy - An Introduction - Matt IsaacDocument182 pages182 P - Leech Therapy - An Introduction - Matt IsaacKatyi Gyula100% (1)
- Drugs and Substance AbuseDocument4 pagesDrugs and Substance Abusevaibhavch2904No ratings yet
- Kumpulan Jurnal DengueDocument122 pagesKumpulan Jurnal DengueMas MantriNo ratings yet
- The Future of Gene EditingDocument23 pagesThe Future of Gene EditingAnurag KunwarNo ratings yet
- Illegal Drugs FlyersDocument2 pagesIllegal Drugs FlyersPCR OPERATIONNo ratings yet
- Reprocessing (Emdr) Terhadap Penurunan Skor Post Traumatic Stress Disorder (PTSD) Akut Pada Pasien PascaDocument13 pagesReprocessing (Emdr) Terhadap Penurunan Skor Post Traumatic Stress Disorder (PTSD) Akut Pada Pasien PascaRismilah MazidaNo ratings yet
- CDC - AV Fistula Graft Can Decannulation Observations ATDocument2 pagesCDC - AV Fistula Graft Can Decannulation Observations ATDewi Ratna SariNo ratings yet
- Essential guide to implementing newborn screening nationwideDocument300 pagesEssential guide to implementing newborn screening nationwideJosanne Wadwadan De CastroNo ratings yet
- Ashhad's Step 2 CK UW Notes PDFDocument166 pagesAshhad's Step 2 CK UW Notes PDFabNo ratings yet