Professional Documents
Culture Documents
Activity C32: Acid-Base Titration (PH Sensor)
Activity C32: Acid-Base Titration (PH Sensor)
Uploaded by
Ngọc Phương Anh NguyễnOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Activity C32: Acid-Base Titration (PH Sensor)
Activity C32: Acid-Base Titration (PH Sensor)
Uploaded by
Ngọc Phương Anh NguyễnCopyright:
Available Formats
Name _____________________ Class ______________ Date _________
Activity C32: Acid-Base Titration
(pH Sensor)
Concept DataStudio ScienceWorkshop (Mac) ScienceWorkshop (Win)
Acids, bases & salts C32 Titration.DS C32 Acid-Base Titration C32_ACID.SWS
Equipment Needed Qty Equipment Needed Qty
pH Sensor (CI-6507A) 1 Wash bottle 1
Base and support rod (ME-9355) 1 Protective gear PS
Beaker, 250 mL 6 Chemicals and Consumables Qty
Buret, 50 mL 1 Buffer solution: high pH 100 mL
Clamp, buret (SE-9446) 2 Buffer solution: low pH 100 mL
Graduated cylinder 1 Hydrochloric acid, unknown 10 mL
Magnetic stirrer and stir bar 1 Sodium hydroxide, 0.10 molar 100 mL
Pipette, 10 mL 1 Water, distilled 500 mL
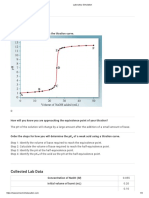
What Do You Think?
In this activity you can use a computer to measure pH as you add 0.10 molar sodium hydroxide
to hydrochloric acid of unknown concentration. Can you use a graph of pH versus volume to
determine the equivalence point? Can you use the volume of the sodium hydroxide titrant used
at the equivalence point to determine the molarity of the hydrochloric acid?
Take time to answer the ‘What Do You Think?’ question(s) in the Lab Report section.
Background
A titration is a process used to determine the volume of a solution needed to
react with a given amount of another substance. For example, when a
hydrochloric acid solution is titrated with a sodium hydroxide solution, the
pH of the acidic solution is initially low. As the base is added, the change in
pH is quite gradual until close to the equivalence point, where equimolar
amounts of acid and base have been mixed. Near the equivalence point, the
pH increases very rapidly. The change in pH then becomes more gradual again before leveling
off with the addition of excess base.
Hydrogen ions from the hydrochloric acid react with hydroxide ions from the sodium hydroxide
in a one-to-one ratio to produce water in the overall reaction:
H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) ---> H2O + Cl-(aq) + Na+(aq)
Because the overall reaction is one-to-one, the number of moles of hydrochloric acid equals the
number of moles of sodium hydroxide. The molarity of the hydrochloric acid is as follows:
C32 © 1999 PASCO scientific p. 307
Chemistry Labs with Computers Student Workbook
C32: Acid-Base Titration 012-07005A
p. 308 © 1999 PASCO scientific C32
Name _____________________ Class ______________ Date _________
SAFETY REMINDERS
Wear protective gear.
Follow directions for using the equipment.
Handle and dispose of all chemicals and solutions properly.
CAUTION: Never pipette by mouth. Always use a pipette bulb or a pipette pump. Be careful when
handling any acid or base solutions.
For You To Do
Titrate hydrochloric acid solution, HCl, with a basic sodium hydroxide solution, NaOH, of
known molarity. Use the pH Sensor to measure the change in pH of the acid solution. Use
DataStudio or ScienceWorkshop to record the change in pH of the acid and the volume of basic
solution added. Use the software to display your data and use your
data to determine the concentration (molarity) of the acid solution.
PART I: Computer Setup
1. Connect the ScienceWorkshop interface to the computer, turn
on the interface, and turn on the computer.
2. Connect the DIN plug of the pH Sensor to Analog Channel A
on the interface.
3. Open the file titled as shown;
DataStudio ScienceWorkshop (Mac) ScienceWorkshop (Win)
C32 Titration.DS C32 Acid-Base Titration C32_ACID.SWS
• The DataStudio file has a Digits display, a Table display, and a Graph display of pH versus
volume. Read the Workbook display for more information.
• The ScienceWorkshop document has a Digits display, a Table display and a Graph display
of the pH versus volume.
• Data recording is set at one measurement per second (1 Hz). Data recording is also set so
that you can manually enter the volume of the sodium hydroxide titrant.
PART II: Sensor Calibration and Equipment Setup
Calibrate the Sensor
• To calibrate the pH Sensor you will need the following: wash bottle, distilled water, three
beakers, buffer solutions of high pH (e.g. pH 10) and low pH (e.g. pH 4), pH Sensor.
• Put distilled water into the wash bottle and into one of the beakers. Put about 100 mL of
the high pH buffer solution in one of the other two beakers and about 100 mL of the low
pH buffer solution into the third beaker.
C32 © 1999 PASCO scientific p. 309
Chemistry Labs with Computers Student Workbook
C32: Acid-Base Titration 012-07005A
1. Remove the pH electrode from its bottle of buffer solution. Connect the electrode to the pH
Sensor amplifier. To connect the electrode, push the BNC plug onto the receptacle on the
Sensor amplifier and turn the BNC plug clockwise until it ‘clicks’ into place.
Remove the
bottle of Connect to
buffer the sensor.
solution.
2. Use the wash bottle to rinse the end of the electrode. Soak the pH electrode in the beaker of
distilled water for 10 minutes.
3. In the Experiment Setup window, double-click the pH Sensor icon.
• In DataStudio, the Sensor Properties window will open. Click the ‘Calibration’ tab. In
ScienceWorkshop, the Sensor Setup window will open.
4. Calibrate with the high pH buffer solution.
Put the end of the pH electrode into the high pH buffer solution.
Check the voltage under ‘Current Reading’ in DataStudio or next to ‘Cur Value:’ in
ScienceWorkshop.
p. 310 © 1999 PASCO scientific C32
Name _____________________ Class ______________ Date _________
When the voltage stabilizes, click the ‘Take Reading’ button under ‘High Point’ in
DataStudio or the ‘Read’ button in the row for ‘High Value:’ in ScienceWorkshop.
Enter the pH value of the buffer solution.
5. Thoroughly rinse the pH electrode with distilled water and dry it with a tissue.
6. Calibrate with the low pH buffer solution.
Put the end of the pH electrode in the low pH buffer solution.
Check the voltage under ‘Current Reading’ in DataStudio or next to ‘Cur Value:’ in
ScienceWorkshop.
When the voltage stabilizes, click the ‘Take Reading’ button under ‘Low Point’ in
DataStudio or the ‘Read’ button in the row for ‘Low Value:’ in ScienceWorkshop.
Enter the pH value of the buffer solution. Click OK to return to the Experiment Setup
window.
7. Thoroughly rinse the pH electrode with distilled water and dry gently.
Equipment Setup
• For this part you will need the following: hydrochloric acid
solution, distilled water, 250-mL beakers, 50-mL buret, pH
Sensor, buret clamps, base and support rod, magnetic stirrer
and stir bar, 0.10 molar sodium hydroxide solution.
1. Put 50 mL of distilled water into a clean dry 250-mL
beaker.
B
u
ret
2. Use a pipette to add 10.00 mL of the hydrochloric acid
solution into the beaker with the distilled water.
3. Carefully add a spin bar to the beaker. Place the beaker on
the magnetic stirrer.
4. Use a clamp and base and support rod to position the pH
electrode so the end of the electrode is in the acid solution,
but will not interfere with the spin bar.
5. Rinse the 50-mL buret with a few milliliters of the 0.100
molar sodium hydroxide solution. Dispose of the rinse
solution as directed.
6. Use another clamp to support the 50-mL buret so the end of
the buret is above the acid solution. (Be sure the buret valve
is closed!)
7. Fill the buret with 0.100 molar sodium hydroxide solution.
Be sure to start the titration with the buret filled exactly to
M
ta
irg
n
e
rtic
the 0.00 mL mark.
S
8. Turn on the magnetic stirrer. (Note: If a magnetic stirrer is
not available, carefully stir the solution with a stirring rod.)
C32 © 1999 PASCO scientific p. 311
Chemistry Labs with Computers Student Workbook
C32: Acid-Base Titration 012-07005A
9. Record the precise concentration of the sodium hydroxide solution in the Lab Report
section.
PART III: Data Recording – Acid-Base Titration
• Note: Data recording goes faster if one person controls the buret value and reads the
volume of titrant added to the acid while a second person operates the computer and enters
the volumes.
• In DataStudio, arrange the displays so you can see the Table. In ScienceWorkshop, arrange
the displays so you can see the Digits display.
1. When you are ready, start recording data. (Hint: Click ‘Start’ in DataStudio or click ‘REC’
in ScienceWorkshop. )
• In DataStudio, the ‘Start’ button changes to a ‘Keep’ button (
). In ScienceWorkshop, the ‘Keyboard
Sampling’ window opens.
p. 312 © 1999 PASCO scientific C32
Name _____________________ Class ______________ Date _________
2. In DataStudio, the first row of the Table shows the beginning value of pH.
In ScienceWorkshop, the Digits display shows the pH reading.
NOTE: Do not add any NaOH titrant for the first reading.
When the pH value stabilizes, click the ‘Keep’ button in DataStudio and then click the
Tab key to move to the next row in the Table. In ScienceWorkshop, type in “0.00” in the
Keyboard Sampling window for Entry #1, and then click the ‘Enter’ button ( ).
• In DataStudio, the Table shows the first ‘pH, volume’ pair in
the first row. In ScienceWorkshop, the ‘Keyboard Sampling’
dialog box changes to show the number you entered.
3. Open the buret's valve and add the first amount of NaOH. Add
enough titrant to raise the pH by 0.15 units and then close the
valve.
4. After the titrant has been added and the pH reading stabilizes,
type the total volume of NaOH added into the next row in the
Table in DataStudio and then click the ‘Keep’ button. In ScienceWorkshop, type the
volume into the ‘Keyboard Sampling’ window and click the ‘Enter’ button.
5. Add the next increment of NaOH (enough to raise the pH by 0.15 units). When the pH
stabilizes, type in the total amount of NaOH added and then click the ‘Keep’ button and
then the Tab key (in DataStudio) or click the ‘Enter’ button (in ScienceWorkshop).
6. Continue adding NaOH solution in amounts that raise the pH by about 0.15 units and type
in the total volume of NaOH added each time.
7. When pH 3.5 is reached, change to 2-drop increments. Continue to type in the total volume
of NaOH added after each increment.
8. After pH 10 is reached, again add larger increments of NaOH (enough to raise the pH by
about 0.15 units) and type in the total volume of NaOH added.
9. Add NaOH until the pH remains constant. Stop data recording. (Hint: In DataStudio, click
the ‘Stop’ button ( ). In ScienceWorkshop, click the ‘Stop Sampling’ button (
). The Keyboard Sampling window will automatically close.)
10. When data recording stops, turn off the magnetic stirrer. Remove the pH sensor from the
solution. Rinse the pH sensor in distilled water and dry the sensor gently.
11. Dispose of the solution in the beaker as instructed.
• If time permits, repeat the procedure.
C32 © 1999 PASCO scientific p. 313
Chemistry Labs with Computers Student Workbook
C32: Acid-Base Titration 012-07005A
Analyzing the Data
1. Use the ‘Smart Tool’ in DataStudio or the ‘Smart Cursor’ in ScienceWorkshop to find the
value of the pH in the plot of Run #1 just before the largest pH increase. Record the X-
coordinate as the “NaOH volume added before largest pH increase”.
2. Move the ‘Smart Tool’ or ‘Smart Cursor’ to the place in the plot of Run #1 just after the
largest pH increase. Record the X-coordinate as the “NaOH volume added after largest pH
increase”.
3. Calculate and record the average of the NaOH volumes just before and just after the largest
pH increase.
4. Move the ‘Smart Tool’ or ‘Smart Cursor’ to the point on the plot of Run #1 where the pH
is 7.0 (or as close as possible). Record the X-coordinate as the “NaOH volume added at the
equivalence point.
• The average of the volume of NaOH just before and just after the largest pH increase
should equal the volume of NaOH added at pH = 7.
5. Calculate the number of moles of NaOH used.
6. Use the equation for the neutralization reaction to calculate the number of moles of HCl
used.
• Recall that you pipetted out 10.00 mL of the unknown HCl solution for the titration.
7. Calculate the HCl concentration in mole/liter (mol/L).
Optional
• If you did two titrations, determine the average concentration of HCl.
Record your results in the Lab Report section.
p. 314 © 1999 PASCO scientific C32
Name _____________________ Class ______________ Date _________
Lab Report - Activity C32: Acid-Base Titration
What Do You Think?
In this activity you can use a computer to measure pH as you add 0.10 molar sodium hydroxide
to hydrochloric acid of unknown concentration. Can you use a graph of pH versus volume to
determine the equivalence point? Can you use the volume of the sodium hydroxide titrant used
at the equivalence point to determine the molarity of the hydrochloric acid?
Data Table
Use volume in liters for the calculations.
# moles NaOH = volume x molarity.
# moles HCl = # moles NaOH
C32 © 1999 PASCO scientific p. 315
Chemistry Labs with Computers Student Workbook
C32: Acid-Base Titration 012-07005A
p. 316 © 1999 PASCO scientific C32
Name _____________________ Class ______________ Date _________
Trial 1 Trial 2
Volume HCl mL mL
Concentration of NaOH M M
NaOH volume added before largest pH increase mL mL
NaOH volume added after largest pH increase mL mL
Average of NaOH before and after pH increase mL mL
NaOH volume added at equivalence point mL mL
Moles NaOH mol mol
Moles HCl mol mol
Concentration of HCl mol/L mol/L
Average (HCl) M
Question
1. Based on your data, what is the concentration of hydrochloric acid?
C32 © 1999 PASCO scientific p. 317
Chemistry Labs with Computers Student Workbook
C32: Acid-Base Titration 012-07005A
p. 318 © 1999 PASCO scientific C32
You might also like
- Chemistry LabsDocument25 pagesChemistry LabsManushka Thomas100% (2)
- Weak Acid Strong Base Titration LabDocument8 pagesWeak Acid Strong Base Titration Labapi-265089380100% (1)
- Advanced Pharmaceutical analysisFrom EverandAdvanced Pharmaceutical analysisRating: 4.5 out of 5 stars4.5/5 (2)
- UTech, Ja. - Summary of Undergraduate Courses of StudyDocument28 pagesUTech, Ja. - Summary of Undergraduate Courses of StudyManushka ThomasNo ratings yet
- Experiment 1&2Document8 pagesExperiment 1&2Fatima AhmedNo ratings yet
- Determination of Acetic Acid in VinegarDocument6 pagesDetermination of Acetic Acid in VinegarTishko0% (1)
- CWV 24 COMP Acid - Base - Titration PDFDocument8 pagesCWV 24 COMP Acid - Base - Titration PDFTha KantanaNo ratings yet
- 25 Titration Diprotic AcidDocument8 pages25 Titration Diprotic AcidAngel DediosNo ratings yet
- PH Meter It's Applications: Practical Advance Pharmaceutical AnalysisDocument19 pagesPH Meter It's Applications: Practical Advance Pharmaceutical AnalysisarjunkumbharchemNo ratings yet
- S Determination of Phosphoric Acid Content in SoftdrinksDocument5 pagesS Determination of Phosphoric Acid Content in SoftdrinksMike Anderson0% (1)
- Lab R.2 - Concentration of An Acid - Three WaysDocument6 pagesLab R.2 - Concentration of An Acid - Three WaysAdarsh Raj TiwariNo ratings yet
- Potentiometric TitrationDocument9 pagesPotentiometric Titrationiah_guevarraNo ratings yet
- Public Health EngineeringDocument48 pagesPublic Health EngineeringrajendrakumarNo ratings yet
- CHEM A 24 COMP Half TitrationDocument4 pagesCHEM A 24 COMP Half TitrationSung Hoon ParkNo ratings yet
- PH Determination: B. General TestsDocument3 pagesPH Determination: B. General TestshemajsuryaNo ratings yet
- EPA Method 3101Document3 pagesEPA Method 3101skrim240No ratings yet
- Acid - Base Lab 2016 BegleyDocument7 pagesAcid - Base Lab 2016 BegleyIsaac SnitkoffNo ratings yet
- Lab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, KaDocument10 pagesLab 3: Introduction To Acids Base Chemistry Part A Experimental Determination of Acid Dissociation Constant, Kaenock yegonNo ratings yet
- Ascorbic Acid Titration Summer 2019 One PeriodDocument9 pagesAscorbic Acid Titration Summer 2019 One PeriodTaiga KagamiNo ratings yet
- Potentiometric Titration CurvesDocument5 pagesPotentiometric Titration CurvesDavid GrahamNo ratings yet
- Soft DrinksDocument3 pagesSoft DrinkskiiieeeshieeeNo ratings yet
- Volatile Acids: Sodium Hydroxide Method Method 8218 100-2400 MG/L CH Cooh Digital TitratorDocument4 pagesVolatile Acids: Sodium Hydroxide Method Method 8218 100-2400 MG/L CH Cooh Digital Titratoralexis villalobosNo ratings yet
- PH, TA& % AcidityDocument41 pagesPH, TA& % Acidityasutoshpanda618No ratings yet
- Lab 1 AcidityDocument8 pagesLab 1 AcidityEngr Arafat QubatiNo ratings yet
- ProjectDocument2 pagesProjectCindy Mae A. PogoyNo ratings yet
- Analysis of Soda Ash: ExperimentDocument6 pagesAnalysis of Soda Ash: ExperimentyzzacamilleaNo ratings yet
- STEM 6 Determining The Concentration of Acetic Acid in Vinegar Via Acid Base TitrationDocument11 pagesSTEM 6 Determining The Concentration of Acetic Acid in Vinegar Via Acid Base TitrationNEIL MAXI LATOGNo ratings yet
- Experiment 4Document7 pagesExperiment 4Lakshmankumar TjpsNo ratings yet
- Experiment 4 PDFDocument7 pagesExperiment 4 PDFsaiNo ratings yet
- PH and Acidity of CoffeeDocument4 pagesPH and Acidity of Coffeeoshlaura0% (1)
- Ppotentiometric Titration of Benzoic Acid With 0.1M Sodium HydroxideDocument23 pagesPpotentiometric Titration of Benzoic Acid With 0.1M Sodium HydroxideCristine ConcepcionNo ratings yet
- Fluoride Electrode ManualDocument26 pagesFluoride Electrode ManualNilesh SomtiyaNo ratings yet
- K00326 - 20181121132815 - Lab Manual SKU1023Document26 pagesK00326 - 20181121132815 - Lab Manual SKU1023Kamilia AfiqahNo ratings yet
- PH Electrode: (Order Code PH-BNC)Document4 pagesPH Electrode: (Order Code PH-BNC)Jack Eito NeoNo ratings yet
- B04 Catalase EnzymeDocument11 pagesB04 Catalase EnzymeAlysa PoralNo ratings yet
- CWV 35 COMP Phosphoric - Acid PDFDocument4 pagesCWV 35 COMP Phosphoric - Acid PDFNaveen KumarNo ratings yet
- 12 - Clear Soda Titration Lab 2023Document4 pages12 - Clear Soda Titration Lab 2023Mathar BashirNo ratings yet
- PH Meters Purdue University Instrument Van Project Acid-Base Titration Using A PH MeterDocument5 pagesPH Meters Purdue University Instrument Van Project Acid-Base Titration Using A PH MeterNatsu PatnaikNo ratings yet
- Chemistry Lab Manual Final PDFDocument38 pagesChemistry Lab Manual Final PDFpvnchemNo ratings yet
- No 3Document12 pagesNo 3Punit Ratna ShakyaNo ratings yet
- Lab Activity 3 - Acid-Base Titration (Revised) PDFDocument7 pagesLab Activity 3 - Acid-Base Titration (Revised) PDFFranzei CandelariaNo ratings yet
- DOC3165301165Document6 pagesDOC3165301165Carlos berrios CanalNo ratings yet
- Quality Control For The Athenium Baking Soda CompanyDocument21 pagesQuality Control For The Athenium Baking Soda CompanyJack DupeeNo ratings yet
- Reinhard 1.0Document11 pagesReinhard 1.0Felix YeboahNo ratings yet
- PH, EC and TDSDocument7 pagesPH, EC and TDSBrijesh Kumar MishraNo ratings yet
- 668 锌20度 1.8 30秒 tds PDFDocument4 pages668 锌20度 1.8 30秒 tds PDFjkljNo ratings yet
- Titration VDocument24 pagesTitration VwaefsNo ratings yet
- Ice Melt Fast by Which Substance AddingDocument20 pagesIce Melt Fast by Which Substance AddingthakdeerNo ratings yet
- Chemistry ProjectDocument20 pagesChemistry ProjectManash BorahNo ratings yet
- Titration of Citric Acid CHEM 103 LabDocument4 pagesTitration of Citric Acid CHEM 103 LabTANMAY ANANDNo ratings yet
- Assessment 2 - Titration Practical - 2022 This Is RealDocument8 pagesAssessment 2 - Titration Practical - 2022 This Is RealUntitled N/ANo ratings yet
- Determining The Amount of Acetic Acid in VinegarDocument2 pagesDetermining The Amount of Acetic Acid in VinegarAadhi JNo ratings yet
- Titration Notes: MethodDocument3 pagesTitration Notes: MethodArSlanRahatNo ratings yet
- Clay and Cement ContaminationDocument5 pagesClay and Cement ContaminationGNo ratings yet
- 06 and 07 Standardization of NaOH and Acid Base TitrationDocument16 pages06 and 07 Standardization of NaOH and Acid Base TitrationTyler Hardy80% (5)
- Titration Diprotic AcidDocument9 pagesTitration Diprotic AcidjaNo ratings yet
- Draft Oecd Guideline For The Testing of ChemicalsDocument7 pagesDraft Oecd Guideline For The Testing of ChemicalsMauricio SilvestriNo ratings yet
- Lab 27Document3 pagesLab 27api-239505062No ratings yet
- 1Document8 pages1Isma WantiNo ratings yet
- Chemistry ProjectoDocument20 pagesChemistry ProjectoPragya SahooNo ratings yet
- Practical Manual of Analytical ChemistryFrom EverandPractical Manual of Analytical ChemistryRating: 4.5 out of 5 stars4.5/5 (3)
- Revision Notes BiologyDocument8 pagesRevision Notes BiologyManushka ThomasNo ratings yet
- Bio Test On Asexual Reproduction in PlantsDocument2 pagesBio Test On Asexual Reproduction in PlantsManushka ThomasNo ratings yet
- The Emerging Professional Assignment 2: Individual: (Reflective E-Journal: 30%) Due: Dec. 13, 2021Document2 pagesThe Emerging Professional Assignment 2: Individual: (Reflective E-Journal: 30%) Due: Dec. 13, 2021Manushka ThomasNo ratings yet
- Course Outline EMERGING - PROFESSIONAL - Courseoutline - SJTC - 2020 - CorrectedDocument7 pagesCourse Outline EMERGING - PROFESSIONAL - Courseoutline - SJTC - 2020 - CorrectedManushka ThomasNo ratings yet
- Biology Lab Work. 2022Document5 pagesBiology Lab Work. 2022Manushka ThomasNo ratings yet
- EP Assignment 1 2021Document6 pagesEP Assignment 1 2021Manushka ThomasNo ratings yet
- Orientation Programme 2021 7a5b6Document40 pagesOrientation Programme 2021 7a5b6Manushka ThomasNo ratings yet
- Managing Diversity in Jamaican ClassroomDocument2 pagesManaging Diversity in Jamaican ClassroomManushka Thomas100% (1)
- Revision Notes BiologyDocument8 pagesRevision Notes BiologyManushka ThomasNo ratings yet
- Chemistr Y Notes: - Solencia HamiltonDocument56 pagesChemistr Y Notes: - Solencia HamiltonManushka ThomasNo ratings yet
- CSEC Chem P1 Solutions 12 18Document1 pageCSEC Chem P1 Solutions 12 18Manushka ThomasNo ratings yet
- Bio 2020 p1Document15 pagesBio 2020 p1Manushka ThomasNo ratings yet
- 5 Grade - Lesson 1.3 Dissolving and Back Again: ObjectiveDocument4 pages5 Grade - Lesson 1.3 Dissolving and Back Again: ObjectiveManushka ThomasNo ratings yet
- Function and Structure of The CellDocument5 pagesFunction and Structure of The CellManushka ThomasNo ratings yet
- Jan 2011 p3Document11 pagesJan 2011 p3Manushka Thomas0% (1)
- Additivity of Heats of Reaction: Hess's LawDocument4 pagesAdditivity of Heats of Reaction: Hess's LawManushka ThomasNo ratings yet
- 5 Grade - Lesson 1.3 Dissolving and Back Again: ObjectiveDocument4 pages5 Grade - Lesson 1.3 Dissolving and Back Again: ObjectiveManushka ThomasNo ratings yet
- Engineering Physics-II: Unit-I Crystal Structures and X-Ray DiffractionDocument24 pagesEngineering Physics-II: Unit-I Crystal Structures and X-Ray DiffractionJag Ram100% (1)
- Chem 321 Lecture 7 - Gravimetric Analysis: Student Learning ObjectivesDocument6 pagesChem 321 Lecture 7 - Gravimetric Analysis: Student Learning ObjectivesrtyiookNo ratings yet
- Experiment 1-3 Identify Weak Acid Using Titration CurveDocument4 pagesExperiment 1-3 Identify Weak Acid Using Titration CurveGianne OngNo ratings yet
- Uv Visible SpectrosDocument50 pagesUv Visible SpectrosVacker Guzel50% (2)
- Sponification of Edible OilDocument2 pagesSponification of Edible OilUsman GhaniNo ratings yet
- Outline: Week 1: Introduction To Analytical ChemistryDocument6 pagesOutline: Week 1: Introduction To Analytical ChemistryJuren LasagaNo ratings yet
- Chapter 1: Introduction: Overview of The Fundamental of Analytical Chemistry & Its ApplicationDocument12 pagesChapter 1: Introduction: Overview of The Fundamental of Analytical Chemistry & Its ApplicationMuhammad FawwazNo ratings yet
- Ahmmed (2011) Mathematical Model For Neutralization SystemDocument8 pagesAhmmed (2011) Mathematical Model For Neutralization SystemJuan Jose SossaNo ratings yet
- HPLC Method DevelopmentDocument55 pagesHPLC Method DevelopmentSanthi KumarNo ratings yet
- 07 Chapter 10 (Compiled)Document86 pages07 Chapter 10 (Compiled)Haziq KhairiNo ratings yet
- 7 Repl - Quant Blue Dye in Comm Drinks - ModFall2012Document7 pages7 Repl - Quant Blue Dye in Comm Drinks - ModFall2012Zohaib AliNo ratings yet
- 04 Column Distillation - Internal BalancesDocument152 pages04 Column Distillation - Internal BalancesAsad Saeed100% (1)
- Solvolysis of Salt of A Weak Acid and Weak BaseDocument11 pagesSolvolysis of Salt of A Weak Acid and Weak BaseNitty MeYa100% (1)
- NOTES Acid Bases and SaltsDocument18 pagesNOTES Acid Bases and SaltsRaghav JiNo ratings yet
- Praktikum BiokimiaDocument148 pagesPraktikum BiokimiaZuniva AndanNo ratings yet
- Report Thin Layer Chromatography On Lipid DetectionDocument14 pagesReport Thin Layer Chromatography On Lipid Detectionatiqah0% (1)
- Mass Relationship in Chemical Reaction - Atomic MassDocument5 pagesMass Relationship in Chemical Reaction - Atomic MassLovely RamNo ratings yet
- Acid-Base Titration Using Method of Double IndicatorsDocument13 pagesAcid-Base Titration Using Method of Double IndicatorsShaker MahmoodNo ratings yet
- Unit4 ADocument2 pagesUnit4 AsaraNo ratings yet
- EDTA Titration of Calcium and MagnesiumDocument3 pagesEDTA Titration of Calcium and MagnesiumAnonymous NxpnI6jC67% (3)
- Identification of Essential Oil Components by Gas ChromatographyDocument811 pagesIdentification of Essential Oil Components by Gas ChromatographyKre LyNo ratings yet
- Application of Sublimation As General Method of PurificationDocument1 pageApplication of Sublimation As General Method of PurificationWnz NaiveNo ratings yet
- 1 ThesisDocument236 pages1 ThesisLong ManNo ratings yet
- Spectrophotometric Determination of Loratadine in Bulk and Pharmaceutical FormulationsDocument3 pagesSpectrophotometric Determination of Loratadine in Bulk and Pharmaceutical FormulationsRicha NurselvianaNo ratings yet
- A Presentationn On FtirDocument7 pagesA Presentationn On FtirmtariqjanNo ratings yet
- Instant Notes in Analytical Chemistry - D. KealeyDocument353 pagesInstant Notes in Analytical Chemistry - D. KealeySteveManningNo ratings yet
- Chem 26.1 Lab ManualExpts3-5Document18 pagesChem 26.1 Lab ManualExpts3-5Dam Yeo WoolNo ratings yet
- Determination of Psilocybin in Hallucinogenic Mushrooms by Reversed-Phase Liquid Chromatography With Fluorescence DetectionDocument7 pagesDetermination of Psilocybin in Hallucinogenic Mushrooms by Reversed-Phase Liquid Chromatography With Fluorescence DetectionBen MartinezNo ratings yet
- Chapt 03 Sect 7 To 11Document15 pagesChapt 03 Sect 7 To 11Jesse McClure100% (1)
- Chemistry Atoms First 2nd Edition Burdge Solutions ManualDocument49 pagesChemistry Atoms First 2nd Edition Burdge Solutions Manualkaitlynmosleyewigyrapof100% (29)