Professional Documents
Culture Documents
Name: Yinjia "Ivan" Wang Lab Station: 9 Reading Orientation
Name: Yinjia "Ivan" Wang Lab Station: 9 Reading Orientation
Uploaded by
Ivan Wang0 ratings0% found this document useful (0 votes)
10 views2 pages- Purify PCR products using spin columns, eluting DNA into labeled microcentrifuge tubes
- Run gel electrophoresis with 5 μL purified PCR samples and loading dye in labeled tubes alongside ladder and controls
- Use gel image to determine DNA amounts, then set up Sanger sequencing reactions in labeled tubes using appropriate primers and DNA amounts to reach a final volume of 12 μL
Original Description:
UC Berkeley Bio1AL
Original Title
Ivan - Flowchart 7
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document- Purify PCR products using spin columns, eluting DNA into labeled microcentrifuge tubes
- Run gel electrophoresis with 5 μL purified PCR samples and loading dye in labeled tubes alongside ladder and controls
- Use gel image to determine DNA amounts, then set up Sanger sequencing reactions in labeled tubes using appropriate primers and DNA amounts to reach a final volume of 12 μL
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views2 pagesName: Yinjia "Ivan" Wang Lab Station: 9 Reading Orientation
Name: Yinjia "Ivan" Wang Lab Station: 9 Reading Orientation
Uploaded by
Ivan Wang- Purify PCR products using spin columns, eluting DNA into labeled microcentrifuge tubes
- Run gel electrophoresis with 5 μL purified PCR samples and loading dye in labeled tubes alongside ladder and controls
- Use gel image to determine DNA amounts, then set up Sanger sequencing reactions in labeled tubes using appropriate primers and DNA amounts to reach a final volume of 12 μL
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
Flowchart 7 “16” or “rpo”.
Place appropriate spin
Name: Yinjia “Ivan” Wang columns into these tubes. This tube will
Lab Station: 9 be used to collect DNA.
Reading orientation: Top → Down in each - Dispense 30 uL of ELUTE buffer
column. Left column, then right column. (Purple tube) directly to the white silica
matrix in the center of spin columns.
Safety: Close the cap of spin column. The
- Safety goggles collection tube remains open. Let
- Lab coat columns stand for 1 min and then
- Gloves centrifuge for 1 min.
- Remove spin columns and sicard them
Prepare a flowchart detailing the necessary into the solid waste beaker. Do not
steps to analyze your PCR Product. discard the 1.5 mL microcentrifuge
tubes - these contain purified PCR-
Cleanup amplified DNA frags. Cap and place in
rack at workstation.
- Label the side of the capless collection
tubes, and lid of the spin columns and Gel Electrophoresis
orange-colored BIND tubes with group
number and sample name. - Take two clear 0.5 mL tubes and use a
Sharpie to label one “16” and the other
- Use P200 to transfer 50 uL of each of
“rpo.”
PCR samples into the corresponding - Use a P20 micropipette to remove 5 µL
orange-colored tubes (containing 250 uL from each of your purified PCR samples
of BIND buffer. and place it in the appropriately labeled
- Use P1000 to everything (300 uL total) 0.5 mL tube. Do not throw away the rest
to the center of the appropriately labeled of the purified PCR sample, as you will
use this to set up your sequencing
spin column.
reaction.
- Balance two samples on the centrifuge. - Use a P20 to add 15 µL of the 1.33X
Centrifuge for 60 seconds at 10k X g. loading dye to the 5 µL of sample you
(DNA will bind to the silica matrix in just pipetted into the 0.5 mL tube.
the spin column Gently pipette up and down to mix the
- Discard flow through into the waste solutions.
beaker. Replace columns back into the - The instructor will load lanes 1 and 12
with 20 µL each of negative control
empty collection tube.
reactions for 16S and rpoB. The
- Wash spin columns by adding 700 uL of instructor will also load lanes 3 and 4 of
WASH Buffer (Green colored tube) in the Bench 1 gel with positive control
each columns and centrifuge 60s. reactions for 16S and rpoB.
- Discard flow-through into liquid waste. - Lanes 2 and 11 will be loaded with 20
Replace the columns in to the collection µL of a 1Kb ladder DNA solution
tubes and centrifuge for an additional (marker DNA of known size and
concentration). The gels should be
minute(remove residual wash buffer).
loaded according to Figure 5. Maps for
- Label 2 empty 1.5 mL microcentrifuge loading the lanes are also next to each
tubes with section number, group gel.
number, and
- Use a P20 to load 20 µL from each of - Set up the two sequencing reaction for
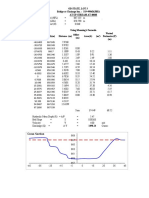
your reactions into the appropriate well. rpoB by solving for the
Use a new tip for each reaction. Use two - ng Estimated DNA Amount
hands to steady the tip over the well. Be - ng/uL Estimated DNA
careful not to punch the pipette tip Concentration (= i / 5 uL)
through the gel. - uL needed for 30 ng of DNA
- Once the entire gel is loaded, press and (30 ng / ii ng /uL)
release the 30 minute button to start a 30 - Label two sterile 0.5 mL centrifuge
minute run. The light will change to a tubes with your group number and
steady green. After 30 minutes, the gel “rpoF” (for forward) or “rpoR” (for
automatically shuts off. You will hear a reverse). Set up the reactions using the
rapid beeping and the light will flash table below (Table 2).
red. Press and release the 30 minute - Place your sequencing reactions in the
button to stop the beeping. GREEN rack on instructor bench into
- Your instructor will collect your gel, appropriate labeled wells. Your
place it in the LED illuminator, and instructor will add 1 uL of appropriate
photograph it. You will use this primer to each tube and transfer the
photograph to analyze your results. The sample to the BLUE rack. Results come
SYBR Safe binds to the DNA and next week.
fluoresces when exposed to the blue
light. You will compare the position and
fluorescence intensity of your bands to
the position and fluorescence of various
DNA bands in the ladder DNA. The
expected sizes of your DNA products
are 1,484 bp for 16S rRNA and 1,112 bp
for rpoB.
- Make sure that you get a picture or print
out of your gel image, as you will need
it for your post-lab.
Prepare a Flowchart on how to set up your
sequencing reactions once you have the data
from the gel.
16S
- Set up the two sequencing reactions for
16s rRNA sample by solving the
- ng Estimated DNA Amount
- ng/uL Estimated DNA
Concentration (= i / 5 uL)
- uL needed for 30 ng of DNA
(30 ng / ii ng /uL)
- Label two sterile 0.5 ml centrifuge tubes
with your group number and “16F”
forward and “16R” reverse.
- Determine the amount of water required
to reach a final volume of 12 uL for
each reaction.
rpoB
You might also like
- 1D Native Barcoding Genomic DNA (With EXP-NBD104, EXP-NBD114, and SQK-LSK109)Document9 pages1D Native Barcoding Genomic DNA (With EXP-NBD104, EXP-NBD114, and SQK-LSK109)Lucas Sousa Neves Andrade100% (1)
- Komatsu 980E-4 SuspensionsDocument28 pagesKomatsu 980E-4 Suspensionsveeresh100% (1)
- Mini Preparation PROTOCOLDocument3 pagesMini Preparation PROTOCOLKeith Arora-WilliamsNo ratings yet
- Agilent 2100 Bioanalyzer ProtocolDocument2 pagesAgilent 2100 Bioanalyzer Protocol박동민No ratings yet
- Lab 5 Photosynthesis FlowcharttDocument3 pagesLab 5 Photosynthesis FlowcharttIvan WangNo ratings yet
- Micro Array Practical MethodDocument12 pagesMicro Array Practical MethodpriyaaNo ratings yet
- Hill Reaction of Photosynthesis-Effects of Selected HerbicidesDocument4 pagesHill Reaction of Photosynthesis-Effects of Selected HerbicidesBryan AbarcaNo ratings yet
- Ligation Sequencing GDNA - Native Barcoding (SQK-LSK109 With EXP-NBD104 and EXP-NBD114) - PromethionDocument6 pagesLigation Sequencing GDNA - Native Barcoding (SQK-LSK109 With EXP-NBD104 and EXP-NBD114) - PromethionAna Cecília PaulaNo ratings yet
- PTC PCR Reaction Week 3 February 2018Document2 pagesPTC PCR Reaction Week 3 February 2018Isini sehansa amarathungaNo ratings yet
- dATA SHEET Plant Rna Purification1Document2 pagesdATA SHEET Plant Rna Purification1Luis Arístides Torres SánchezNo ratings yet
- MwPIXBqzx Brky3 CgsrroIG58NGOd Rna 20 Isolation 20 and 20 Reverse 20 Transcription 20 Protocol 20 Cells 20 inDocument4 pagesMwPIXBqzx Brky3 CgsrroIG58NGOd Rna 20 Isolation 20 and 20 Reverse 20 Transcription 20 Protocol 20 Cells 20 inLuis Ángel Rod FloNo ratings yet
- RNA Isolation ProtocolDocument4 pagesRNA Isolation ProtocolSannan TareenNo ratings yet
- Fósforo Total Faixa Alta 2 To 20 PPM (Método 10209 TNT845)Document8 pagesFósforo Total Faixa Alta 2 To 20 PPM (Método 10209 TNT845)Jair BenNo ratings yet
- Sewage Testing Protocols For Sars-Cov-2Document5 pagesSewage Testing Protocols For Sars-Cov-2Prem KumarNo ratings yet
- Polymerase Chain Reaction and Agarose Gel ElectrophoresisDocument4 pagesPolymerase Chain Reaction and Agarose Gel ElectrophoresisEdward HuNo ratings yet
- Serum Preparation: Step ActionDocument9 pagesSerum Preparation: Step Actionlee tohNo ratings yet
- Fosforo Reactivo Metodo CeldaDocument6 pagesFosforo Reactivo Metodo CeldaSoporte EcolabNo ratings yet
- Agilent 2100 Bioanalyzer AbstractDocument5 pagesAgilent 2100 Bioanalyzer Abstract박동민No ratings yet
- Practical Biotech MSCDocument6 pagesPractical Biotech MSCEr Parshant SaharanNo ratings yet
- Fósforo Total Faixa Baixa - 0.05 To 1.50 PPM de P (Método 10209 - 10210 - TNT843)Document8 pagesFósforo Total Faixa Baixa - 0.05 To 1.50 PPM de P (Método 10209 - 10210 - TNT843)Jair BenNo ratings yet
- Lab Protocol 5Document6 pagesLab Protocol 51701emgNo ratings yet
- Week 6 Generating RNA W20 RRG T7Document3 pagesWeek 6 Generating RNA W20 RRG T7Empowering YouthNo ratings yet
- Efficient Adaptor Ligation For The Preparation of DsDNA Libraries Using Blunt-TA Ligase Master MixDocument10 pagesEfficient Adaptor Ligation For The Preparation of DsDNA Libraries Using Blunt-TA Ligase Master MixMoni Becerra WongNo ratings yet
- Nextera XT DNA Library Prep KitDocument6 pagesNextera XT DNA Library Prep KitAhmadNo ratings yet
- Practical 3 Ion Exchange ChromatographyDocument2 pagesPractical 3 Ion Exchange ChromatographyWavhudi MudauNo ratings yet
- Preparation For WDYTYA 2Document2 pagesPreparation For WDYTYA 2Louis AmorNo ratings yet
- Rapid Sequencing gDNA - Field Sequencing Kit (SQK-LRK001) - MinionDocument3 pagesRapid Sequencing gDNA - Field Sequencing Kit (SQK-LRK001) - MinionMarNo ratings yet
- Kit de ExtraccionDocument4 pagesKit de ExtraccionBenavidez RicardoNo ratings yet
- Nab Activity Procedure ModifiedDocument2 pagesNab Activity Procedure ModifiedRubén MartínezNo ratings yet
- Total Soil DNA and RNA Extraction and PurificationREVISEDDocument6 pagesTotal Soil DNA and RNA Extraction and PurificationREVISEDnanabiogenomNo ratings yet
- To Estimate The Serum Alkaline PhosphateDocument3 pagesTo Estimate The Serum Alkaline Phosphatedr ahsanNo ratings yet
- CH1801 C5 Plasmid IsolationDocument7 pagesCH1801 C5 Plasmid IsolationTanisha ChowdharyNo ratings yet
- Lab3 EnzymesDocument10 pagesLab3 EnzymesAhtziri MartinezNo ratings yet
- Hela Cell Preparation For Total Rna Isolation (Rneasy Mini Kit QIAGEN)Document4 pagesHela Cell Preparation For Total Rna Isolation (Rneasy Mini Kit QIAGEN)Masudul AlamNo ratings yet
- Fosforo TotalDocument8 pagesFosforo TotalLuis Angel HdezNo ratings yet
- MCB 251 Lab PCRDocument8 pagesMCB 251 Lab PCRDan BorsNo ratings yet
- 3bsmt1 Bobier, Ashwelldonne Molbio PCRDocument9 pages3bsmt1 Bobier, Ashwelldonne Molbio PCRAshwell Donne BobierNo ratings yet
- 2019-NCoV Quick Start Protocol (CY5-IC)Document7 pages2019-NCoV Quick Start Protocol (CY5-IC)Rmd ChanelNo ratings yet
- Activity 1. PCR Amplification of 16S RDNA For Bacterial IdentificationDocument8 pagesActivity 1. PCR Amplification of 16S RDNA For Bacterial Identificationgarcia.zaniabNo ratings yet
- 4R6Lab3 Dead Time 2017Document2 pages4R6Lab3 Dead Time 2017tarek moahmoud khalifaNo ratings yet
- RNAcovDocument7 pagesRNAcovDavid Custodio ZegarraNo ratings yet
- NemaidDocument23 pagesNemaidmohammadi2No ratings yet
- IMHM 311: Week 4Document1 pageIMHM 311: Week 4Janine Alicia VargasNo ratings yet
- PDH Mak183bulDocument4 pagesPDH Mak183bulArantxa SanchezNo ratings yet
- Igem Training 2Document34 pagesIgem Training 2api-343562611No ratings yet
- Measure AmyDocument2 pagesMeasure Amytuan vănNo ratings yet
- Transfusion Microbiology LaboratoryDocument36 pagesTransfusion Microbiology LaboratorykhadijahabibabdullahiNo ratings yet
- Expression and Purification of His-Tagged Proteins Expressed in E. Coli (Price Lab)Document3 pagesExpression and Purification of His-Tagged Proteins Expressed in E. Coli (Price Lab)Raja GunalanNo ratings yet
- Quick: Dual-Luciferase Reporter Assay and Dual-Luciferase Reporter 1000 Assay SystemsDocument2 pagesQuick: Dual-Luciferase Reporter Assay and Dual-Luciferase Reporter 1000 Assay SystemsVijay Kumar ReddyNo ratings yet
- Alp PDFDocument1 pageAlp PDFDhita Ariefta PNo ratings yet
- PMT 324 Molecular Biology of The GeneDocument6 pagesPMT 324 Molecular Biology of The GeneBlameless ArikoNo ratings yet
- SCR 066Document4 pagesSCR 066Linh ĐỗNo ratings yet
- QPCR SopDocument7 pagesQPCR SopAna Marina SantosNo ratings yet
- Hach 8317 Cu Ver 2Document8 pagesHach 8317 Cu Ver 2edi_munawarNo ratings yet
- SOP009 DNA& RNA Extraction From Fecal SamplesDocument6 pagesSOP009 DNA& RNA Extraction From Fecal SamplesHayleyNo ratings yet
- Rnasimple Total Rna KitDocument7 pagesRnasimple Total Rna KitHicha LidyaNo ratings yet
- Edge Lab - Agilent Microarray Labeling Protocol - 6/18/12: 6.5-7 Hours (Including 1 Hour To Allow Samples To Thaw)Document7 pagesEdge Lab - Agilent Microarray Labeling Protocol - 6/18/12: 6.5-7 Hours (Including 1 Hour To Allow Samples To Thaw)bakafish007No ratings yet
- Phosphorus, Reactive (Orthophosphate) : Molybdovanadate Method Method 8114 1.0 To 100.0 MG/L PO (HR) Test N Tube VialsDocument6 pagesPhosphorus, Reactive (Orthophosphate) : Molybdovanadate Method Method 8114 1.0 To 100.0 MG/L PO (HR) Test N Tube VialsOudah AliNo ratings yet
- Experimental approaches to Biopharmaceutics and PharmacokineticsFrom EverandExperimental approaches to Biopharmaceutics and PharmacokineticsNo ratings yet
- Transmitters in the Visual ProcessFrom EverandTransmitters in the Visual ProcessS.L. BontingNo ratings yet
- 6.DC Motor InterfaceDocument51 pages6.DC Motor InterfaceGowtham PalanirajanNo ratings yet
- Thailand ReportDocument18 pagesThailand ReportE32 ESTERLINENo ratings yet
- Theodor Reuss Oto Rituals: Lecture For The Ivth Degree (Fragment Only)Document2 pagesTheodor Reuss Oto Rituals: Lecture For The Ivth Degree (Fragment Only)Eduardo CostaNo ratings yet
- Literary Criticism Module 9 12Document8 pagesLiterary Criticism Module 9 12Maranding vinersNo ratings yet
- Mendeley Teaching PresentationDocument36 pagesMendeley Teaching PresentationpatrickNX9420No ratings yet
- April 29 Homework Solutions: Mechanical Engineering 390 Fluid MechanicsDocument6 pagesApril 29 Homework Solutions: Mechanical Engineering 390 Fluid MechanicsLaliethaNo ratings yet
- SerializabilityDocument10 pagesSerializabilityKunj PatelNo ratings yet
- BISE Peshawar Board Inter Part 1, 2 Supplementary Exams Date Sheet 2013Document2 pagesBISE Peshawar Board Inter Part 1, 2 Supplementary Exams Date Sheet 2013Shawn ParkerNo ratings yet
- Unit 2.2 MmworldDocument41 pagesUnit 2.2 MmworldAveryNo ratings yet
- Electrostatics Bes 117Document6 pagesElectrostatics Bes 117Joyjoy C LbanezNo ratings yet
- Inspiration To Reality PDFDocument11 pagesInspiration To Reality PDFmelanieeeeNo ratings yet
- Ametek XG-1500Document6 pagesAmetek XG-1500onurNo ratings yet
- Modulos CNC Siemens Fresadora MVK 2500Document5 pagesModulos CNC Siemens Fresadora MVK 2500Arnoud NegraoNo ratings yet
- ELMACH Packages India Pvt. LTD - EDocument3 pagesELMACH Packages India Pvt. LTD - EYosses Sang NahkodaNo ratings yet
- Virtue EthicsDocument10 pagesVirtue Ethicsjayanema100% (2)
- Problems in Heat ConductionDocument32 pagesProblems in Heat ConductionistopiNo ratings yet
- Hydraulic - 319+990 PDFDocument8 pagesHydraulic - 319+990 PDFArun ChauhanNo ratings yet
- MAX16910 200ma, Automotive, Ultra-Low Quiescent Current, Linear RegulatorDocument15 pagesMAX16910 200ma, Automotive, Ultra-Low Quiescent Current, Linear RegulatorcarlosNo ratings yet
- Composite Propeller ConstructionDocument8 pagesComposite Propeller Construction이재국No ratings yet
- Carescape v100 BrochureDocument2 pagesCarescape v100 BrochureNirmayati YatiNo ratings yet
- MIT3 40JF09 Lec02-Basic CrystallographyDocument7 pagesMIT3 40JF09 Lec02-Basic CrystallographychandrasmgNo ratings yet
- Drama 7 Week 14 Lesson PlanDocument9 pagesDrama 7 Week 14 Lesson Planapi-533472774No ratings yet
- Introduction To Risk: Learning OutcomesDocument22 pagesIntroduction To Risk: Learning OutcomesChristen CastilloNo ratings yet
- ECE 4316: Digital Signal Processing: Dr. Hany M. ZamelDocument23 pagesECE 4316: Digital Signal Processing: Dr. Hany M. ZamellovelearnNo ratings yet
- Updated 2017 2022 PIP As Input To FY 2020 Budget Preparation Chapter 7 As of 12april19Document9 pagesUpdated 2017 2022 PIP As Input To FY 2020 Budget Preparation Chapter 7 As of 12april19detailsNo ratings yet
- The Teach For India FellowshipDocument31 pagesThe Teach For India FellowshipSynonymousNo ratings yet
- Interest Rate ModelsDocument188 pagesInterest Rate Modelsdoc_oz3298No ratings yet
- STEM Maker Education - Space ToolsDocument4 pagesSTEM Maker Education - Space ToolsRokenbokNo ratings yet
- Thesis On Employee Performance ManagementDocument5 pagesThesis On Employee Performance Managementbkx8wryp100% (2)