Professional Documents
Culture Documents
Clinical Laboratory Report: Page 1 of 2
Uploaded by
Praveen kumarOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Clinical Laboratory Report: Page 1 of 2
Uploaded by
Praveen kumarCopyright:
Available Formats
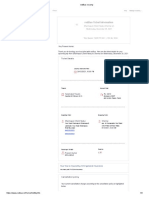
CLINICAL LABORATORY REPORT
NAME : Mr ARAVINDKUMAR OP/IP : OP/OP21-122450
UHID .NO : 12018066860 Requested On : 12/01/2022 12:41 PM
AGE / SEX : 29 Y 10 M 26 D /Male DOB : 15/02/1992 Collected On : 12/01/2022 08:29 PM
Ref. By : ER CARE GROUP Reported On : 12/01/2022 11:41 PM
INVESTIGATION TEST METHOD RESULT UNIT BIOLOGICAL REFERENCE INTERVAL
Molecular
SARS-COV-2
Swab
Test Name and Method :SARS-COV-2 REAL TIME PCR (Qualitative)
Specimen :NASAL SWAB/ORAL SWAB
Result:
SARS-COV-2 : POSITIVE
Comments:
A Negative result does not preclude SARS-CoV-2 and should not be used as the sole basis for patient management
decisions. In clinically suspected patients and those who had contact with a known positive patient, it is advisable to retest
in a fresh sample after 48 to 72 hours. (Clinical Sensitivity of the test is 60-80% )
A Positive result comes after thorough check of the controls , Each batch Validity ensured in addition to validating each Kit
prior to clinical diagnostic use . The analytical sensitivity of the assay is 95 % .Therefore , false positives are ruled out by all
means .
ICMR ID : 433829833
Note :
• ICMR Recommended kits are used for reporting . All the Positive cases will be notified to ICMR for further
Surveillance .
• Borderline positive cases (Ct Value > 30) may give variable results on repeat testing . The possible reasons could be
the variations in the kits and instruments used.
• Clinical correlation with patient history , radiology findings and co-infection with other viruses is essential for
individual case management . Ct values will not be shared in this test as it is a qualitative test and subjective to various
parameters . Ct values has no correlation with severity and has no implications on patient management .
Factors leading to false negative RT- PCR report :
• Inadequate specimen collection , poor quality of sample and non-representative sample .
• Sample collected too early or too late in the infection , improper sample handling and shipment .
• Technical reasons – PCR inhibitor , analytical sensitivity of kit used .
• Active recombination and / mutations in target genes used for detection of SARS-CoV- 2 virus .
Reference :
• Labcorp COVID-19 RT-PCR test EUA Summary / COVID -19 RT-PCR test (laboratory corporation of America).
** End of Report **
Ms Sharmila S DR. MUKUL VIJ, MD,PDCC DR. SUBHA S MD.,DNB
Sr.CONSULTANT PATHOLOGIST Consultant Microbiologist & Head
Result Entered / Verified By: Page 1 of 2 13-01-2022 12:01:27 AM
CLINICAL LABORATORY REPORT
Ms Sharmila S DR. MUKUL VIJ, MD,PDCC DR. SUBHA S MD.,DNB
Sr.CONSULTANT PATHOLOGIST Consultant Microbiologist & Head
Result Entered / Verified By: Page 2 of 2 13-01-2022 12:01:27 AM
You might also like
- Test Name Result Bio. Ref. Range Unit Method: Nasopharyngeal and Oropharyngeal SwabDocument2 pagesTest Name Result Bio. Ref. Range Unit Method: Nasopharyngeal and Oropharyngeal SwabGovind Arun KamatNo ratings yet
- Management by ObjectivesDocument30 pagesManagement by ObjectivesJasmandeep brar100% (4)
- Bar Q Salaries Part 2Document5 pagesBar Q Salaries Part 2Brigette DomingoNo ratings yet
- Indian Ordnance FactoryDocument2 pagesIndian Ordnance FactoryAniket ChakiNo ratings yet
- Summary Studying Public Policy Michael Howlett CompleteDocument28 pagesSummary Studying Public Policy Michael Howlett CompletefadwaNo ratings yet
- ABMOM q2 mod5OrgAndMngmnt Motivation - Leadership and Communication in Organizations-V2Document18 pagesABMOM q2 mod5OrgAndMngmnt Motivation - Leadership and Communication in Organizations-V2Zoren Jovillanos EmbatNo ratings yet
- Too Much Time in Social Media and Its Effects On The 2nd Year BSIT Students of USTPDocument48 pagesToo Much Time in Social Media and Its Effects On The 2nd Year BSIT Students of USTPLiam FabelaNo ratings yet
- Attachment 05 - BFD, ELD and P&I Diagrams-PearlDocument77 pagesAttachment 05 - BFD, ELD and P&I Diagrams-Pearlum er100% (1)
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodShravan RajavathNo ratings yet
- Organisational Structure of NetflixDocument2 pagesOrganisational Structure of NetflixAnkita Das57% (7)
- Test Report: Investigation Observed Value Unit Biological Ref. Interval Specimen Covid-19 Virus Qualitative PCRDocument2 pagesTest Report: Investigation Observed Value Unit Biological Ref. Interval Specimen Covid-19 Virus Qualitative PCRRahul DesardaNo ratings yet
- Department of Molecular Biology:: Mr. B.Uday Kumar ReddyDocument1 pageDepartment of Molecular Biology:: Mr. B.Uday Kumar ReddychenchuNo ratings yet
- Jitendra RTPCRDocument2 pagesJitendra RTPCRMohan KumarNo ratings yet
- Molecular Biology: Lab ID Reference No Name MRN ID Sample NoDocument1 pageMolecular Biology: Lab ID Reference No Name MRN ID Sample NoMuhammadnasidiNo ratings yet
- MR - AJINKYA KASAR LabReportNew-4Document2 pagesMR - AJINKYA KASAR LabReportNew-4Ajinkya kasarNo ratings yet
- Muhammad Zahid - F - 09022022002550Document1 pageMuhammad Zahid - F - 09022022002550Alyaan ChNo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Not Detected Target (S) N Gene, Orf1Ab GeneDocument2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Not Detected Target (S) N Gene, Orf1Ab Genemanwanimuki12No ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument4 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range Methodsourabhshrivastava80No ratings yet
- Covid Test Result Deepti PadteDocument2 pagesCovid Test Result Deepti PadteDeepti PadteNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesMohammed Shafi CpNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodRutuja ShindeNo ratings yet
- Sargam SoodDocument1 pageSargam SoodMayank JunejaNo ratings yet
- 04 Feb 2022Document1 page04 Feb 2022Ramanan RajaNo ratings yet
- Madhan - 642161200148401 2Document2 pagesMadhan - 642161200148401 2madhanNo ratings yet
- Rashidmalayankandi 20220218072328774Document1 pageRashidmalayankandi 20220218072328774ramsheed ramsheedNo ratings yet
- Covid-19 (Sars-Cov-2) Qualitative Real Time PCR: Department of Molecular BiologyDocument1 pageCovid-19 (Sars-Cov-2) Qualitative Real Time PCR: Department of Molecular Biologyಅ ಪರಿಚಿತNo ratings yet
- Covid-19 (Sars-Cov-2) Qualitative Real Time PCR: Department of Molecular BiologyDocument1 pageCovid-19 (Sars-Cov-2) Qualitative Real Time PCR: Department of Molecular Biologyಅ ಪರಿಚಿತNo ratings yet
- Rajat GoswamiDocument2 pagesRajat GoswamiNM KPTNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesMohammed Shafi CpNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesMohammed Shafi CpNo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)Document2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)manwanimuki12No ratings yet
- Medical Laboratory Report: Specimen Nasopharyngeal / Oropharyngeal Swab Covid-19 Qualitative PCRDocument1 pageMedical Laboratory Report: Specimen Nasopharyngeal / Oropharyngeal Swab Covid-19 Qualitative PCRSuhas KandNo ratings yet
- Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRDocument2 pagesSars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRRajat RanjanNo ratings yet
- HIMESH DUG - 8e6bDocument1 pageHIMESH DUG - 8e6bAshwani SehgalNo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Not Detected Target (S) N Gene, Orf1Ab GeneDocument2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Not Detected Target (S) N Gene, Orf1Ab Genemanwanimuki12No ratings yet
- Molecular Microbiology: Test Result Reference RangeDocument1 pageMolecular Microbiology: Test Result Reference RangeSalman AbdelkhalekNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Miss. Dodla GaganamokshaDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Miss. Dodla GaganamokshaDv ScNo ratings yet
- Department of Molecular Biology and Cytogenetics:: Master Dodla Venkata SanjeethDocument1 pageDepartment of Molecular Biology and Cytogenetics:: Master Dodla Venkata SanjeethDv ScNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodAshwini PrinceNo ratings yet
- Medical Laboratory Report: Specimen Nasopharyngeal / Oropharyngeal Swab Covid-19 Qualitative PCRDocument1 pageMedical Laboratory Report: Specimen Nasopharyngeal / Oropharyngeal Swab Covid-19 Qualitative PCRVivek SahNo ratings yet
- Specimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)Document2 pagesSpecimen Nasopharyngeal Swab & Throat Swab Sars-Cov-2 Rna Target (S)manwanimuki12No ratings yet
- Sajal AgarwalDocument1 pageSajal AgarwalMayank JunejaNo ratings yet
- Covid-19 RT PCRDocument1 pageCovid-19 RT PCRSanu DandotiyaNo ratings yet
- MR Praveen Borkar 002Document1 pageMR Praveen Borkar 002vansh taraNo ratings yet
- Covid-19 RT-PCRDocument2 pagesCovid-19 RT-PCRAmit ShindeNo ratings yet
- SMSHLD tPEkEzDocument1 pageSMSHLD tPEkEzPranjal JindalNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodPritam JanaNo ratings yet
- Raghavendra RTPCR 15 01 2022Document2 pagesRaghavendra RTPCR 15 01 2022Rithvik ShettyNo ratings yet
- Sars-Cov-2: Empowers To Live WellDocument2 pagesSars-Cov-2: Empowers To Live WellAkhil KNo ratings yet
- TestReport 2200200991Document1 pageTestReport 2200200991Harshvardhan KhatodNo ratings yet
- Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRDocument2 pagesSars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRBATARNo ratings yet
- TestReport - 22 06 2021 - Apollo 2471624375836407Document2 pagesTestReport - 22 06 2021 - Apollo 2471624375836407thakuryaNo ratings yet
- Test Result Report: Interpretation GuidelinesDocument2 pagesTest Result Report: Interpretation GuidelinesVivek VinuNo ratings yet
- Lab-Result - Ronal Saisayado - 2871970 - 21209876Document1 pageLab-Result - Ronal Saisayado - 2871970 - 21209876Kalam ManaluNo ratings yet
- 12/11/2022 2:19:00PM: 12/11/2022 2:19:52PM:12/11/2022 9:19:52PM: FinalDocument2 pages12/11/2022 2:19:00PM: 12/11/2022 2:19:52PM:12/11/2022 9:19:52PM: FinalDheeraj KumarNo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodGirija Prasad SwainNo ratings yet
- Report-882110000039522 Ms - SADHANAPAL-Z4445870-0767002515097 25mar2022 132833Document2 pagesReport-882110000039522 Ms - SADHANAPAL-Z4445870-0767002515097 25mar2022 132833SamiliciousfreshNo ratings yet
- Laboratory Investigation Report: Kindly Correlate With Clinical FindingsDocument1 pageLaboratory Investigation Report: Kindly Correlate With Clinical FindingsProxyNo ratings yet
- NIYASrtpctDocument1 pageNIYASrtpctniyasNo ratings yet
- 09 Feb 2022Document1 page09 Feb 2022Anonymous dH3DIEtzNo ratings yet
- 23/5/2021 1:25:00PM:24/5/2021 9:16:56PM: 291038543 Received Self Male Age:53 Years:24/5/2021 1:43:29PMDocument2 pages23/5/2021 1:25:00PM:24/5/2021 9:16:56PM: 291038543 Received Self Male Age:53 Years:24/5/2021 1:43:29PMNikhil JainNo ratings yet
- MR - Saurabh Vinaykumar Shukla-1Document1 pageMR - Saurabh Vinaykumar Shukla-1KAUSHAL KUMAR SHUKLANo ratings yet
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range Methodmaneesh babuNo ratings yet
- Dds DDocument1 pageDds DHarish KumarNo ratings yet
- DownloadDocument1 pageDownloadSAI SHARANNo ratings yet
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodRahul SankaranNo ratings yet
- Diagnostics: Sars - Cov - 2 Real Time PCRDocument2 pagesDiagnostics: Sars - Cov - 2 Real Time PCRJas Karan SinghNo ratings yet
- Obi Pcos DN - S.mukilkumarDocument13 pagesObi Pcos DN - S.mukilkumarPraveen kumarNo ratings yet
- Statement 1687884970915Document20 pagesStatement 1687884970915Praveen kumarNo ratings yet
- Joining ChecklistDocument1 pageJoining ChecklistPraveen kumarNo ratings yet
- Dharmapuri Chennai: 11:25 PM 4:40 AMDocument2 pagesDharmapuri Chennai: 11:25 PM 4:40 AMPraveen kumarNo ratings yet
- M0114 - Mmaskinds - PPM - January-2022Document3 pagesM0114 - Mmaskinds - PPM - January-2022Praveen kumarNo ratings yet
- N0078 - Nu Tech - PPM - January-2022Document3 pagesN0078 - Nu Tech - PPM - January-2022Praveen kumarNo ratings yet
- m0907 - M Tom - PPM - January-2022Document3 pagesm0907 - M Tom - PPM - January-2022Praveen kumarNo ratings yet
- FRM Exam Results View - JSPDocument1 pageFRM Exam Results View - JSPPraveen kumarNo ratings yet
- S. Raman: Mobile: +91 9600883617Document3 pagesS. Raman: Mobile: +91 9600883617Praveen kumarNo ratings yet
- Kyb Motorcycle Suspension India Pvt. LTD.: Pay Slip Cum Time Card Form-25B For The Month of May 2021Document1 pageKyb Motorcycle Suspension India Pvt. LTD.: Pay Slip Cum Time Card Form-25B For The Month of May 2021Praveen kumarNo ratings yet
- Cbs LM 00474160000017783Document2 pagesCbs LM 00474160000017783Praveen kumarNo ratings yet
- Redbus Ticket Information: Bus Tickets Ryde Redrail RpoolDocument3 pagesRedbus Ticket Information: Bus Tickets Ryde Redrail RpoolPraveen kumarNo ratings yet
- Gmail - Your Amazon - in Order of Samsung Galaxy M20...Document2 pagesGmail - Your Amazon - in Order of Samsung Galaxy M20...Praveen kumarNo ratings yet
- Praveen Kumar S: Mobile: +91 9791368654, +91-8248734157 Skype Number: +91 9791368654Document4 pagesPraveen Kumar S: Mobile: +91 9791368654, +91-8248734157 Skype Number: +91 9791368654Praveen kumarNo ratings yet
- Upplier Valuation Odel Work Sheet: Godrej Material Handling Supplier Evaluation ModelDocument42 pagesUpplier Valuation Odel Work Sheet: Godrej Material Handling Supplier Evaluation ModelPraveen kumarNo ratings yet
- Cbs LM 12938980000010640Document1 pageCbs LM 12938980000010640Praveen kumarNo ratings yet
- Explanations On How To Use This Rts Excel File: Review of Technical Specifications (RTS)Document11 pagesExplanations On How To Use This Rts Excel File: Review of Technical Specifications (RTS)Praveen kumarNo ratings yet
- Praveen Kumar S: Mobile: +91 - 8248734157, +91-97913686564Document4 pagesPraveen Kumar S: Mobile: +91 - 8248734157, +91-97913686564Praveen kumarNo ratings yet
- Part No. & Name: Gauge Name:/ Micrometer Date 20.1.18 Characteristics: Outside Diameter Specification: SKSDocument11 pagesPart No. & Name: Gauge Name:/ Micrometer Date 20.1.18 Characteristics: Outside Diameter Specification: SKSPraveen kumarNo ratings yet
- Execution Lac 415a of 2006Document9 pagesExecution Lac 415a of 2006Robin SinghNo ratings yet
- Job Description Examples - British GasDocument2 pagesJob Description Examples - British GasIonela IftimeNo ratings yet
- Sap On Cloud PlatformDocument2 pagesSap On Cloud PlatformQueen ValleNo ratings yet
- Modeling Cover Letter No ExperienceDocument7 pagesModeling Cover Letter No Experienceimpalayhf100% (1)
- Emancipation Fact SheetDocument2 pagesEmancipation Fact SheetKeelie SmithNo ratings yet
- Columbia County Property Transfers March 29-April 4Document3 pagesColumbia County Property Transfers March 29-April 4augustapressNo ratings yet
- 2B. Glicerina - USP-NF-FCC Glycerin Nutritional Statement USP GlycerinDocument1 page2B. Glicerina - USP-NF-FCC Glycerin Nutritional Statement USP Glycerinchristian muñozNo ratings yet
- 1 48 Volt Parallel Battery System PSS-SOC - Step-By-Step VolvoDocument11 pages1 48 Volt Parallel Battery System PSS-SOC - Step-By-Step VolvoEyosyas NathanNo ratings yet
- FGD MetallurgyDocument5 pagesFGD MetallurgyrajivashishNo ratings yet
- Pepsico IncDocument26 pagesPepsico IncYKJ VLOGSNo ratings yet
- Magicolor2400 2430 2450FieldSvcDocument262 pagesMagicolor2400 2430 2450FieldSvcKlema HanisNo ratings yet
- P40Agile P541 - 2 - 3 - 4 - 5 6 Guideform SpecificationDocument15 pagesP40Agile P541 - 2 - 3 - 4 - 5 6 Guideform SpecificationprinceNo ratings yet
- Unilever Financial PerformanceDocument9 pagesUnilever Financial PerformanceAbdul QayumNo ratings yet
- Chapter 3: Classical Production Models: News Vendor ModelDocument85 pagesChapter 3: Classical Production Models: News Vendor ModelmauriciovendraminNo ratings yet
- Financial Analysis of OGDCLDocument16 pagesFinancial Analysis of OGDCLsehrish_sadaqat7873100% (1)
- Mef Cecp TrainingDocument5 pagesMef Cecp TrainingShambhu KhanalNo ratings yet
- Managerial Economics - 1Document36 pagesManagerial Economics - 1Deepi SinghNo ratings yet
- MTBE - Module - 3Document83 pagesMTBE - Module - 3ABHIJITH V SNo ratings yet
- Applicant Details : Government of Tamilnadu Application Form For Vehicle E-Pass For Essential ServicesDocument1 pageApplicant Details : Government of Tamilnadu Application Form For Vehicle E-Pass For Essential ServicesŠářoĵ PrinceNo ratings yet
- Baling Press: Model: LB150S Article No: L17003 Power SupplyDocument2 pagesBaling Press: Model: LB150S Article No: L17003 Power SupplyNavaneeth PurushothamanNo ratings yet
- KINDRED HEALTHCARE, INC 10-K (Annual Reports) 2009-02-25Document329 pagesKINDRED HEALTHCARE, INC 10-K (Annual Reports) 2009-02-25http://secwatch.comNo ratings yet
- Taller Sobre Preposiciones y Vocabulario - Exhibición Comercial SergioDocument5 pagesTaller Sobre Preposiciones y Vocabulario - Exhibición Comercial SergioYovanny Peña Pinzon100% (2)