Professional Documents
Culture Documents
POGIL Activity - Collision Theory
Uploaded by
Mika VaughnCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
POGIL Activity - Collision Theory
Uploaded by
Mika VaughnCopyright:
Available Formats
Collision Theory - Impact for a Chemical Reaction
Why?
The collision theory states that a chemical reaction can only occur between
particles when they collide (hit each other). The collision between reactant particles
is necessary but not sufficient for a reaction to take place. The collisions also have
to be effective. It is important to understand the exact nature of an effective
collision since this determines whether or not particles actually react with each
other and form new products.
Learning Objectives
• Identify the requirements needed for a successful reaction to occur between
reactant particles.
Success Criteria
• Explain the meaning of an effective collision.
• Explain the requirements needed for a reaction to occur between reactant
particles.
Resources
• Judith Gould, Three Strikes Equals a Hit, STANYS Science Teacher Bulletin
(2000).
• Zumdahl, Zumdahl and DeCoste. 2002. World of Chemistry. Houghton Mifflin,
pp. 537 - 541
Prerequisites
• Chemical reaction nomenclature
• Balancing chemical reactions
• Lewis structures (electron-dot-diagrams)
New Concepts
• Collision theory
• Effective collision
• Activation energy
©POGIL 2005, 2006 1/4
Authored by: Bryan Horan; Revised by: Kelly Levy and Kenneth Levy
Edited by Linda Padwa and David Hanson, Stony Brook University
Collision Theory
Model: Collision Theory
In the picture below, the baseball bat represents Reactant A and the baseball
represents Reactant B. A reaction will only be successful if the batter hits a homerun. If
the batter does not hit a homerun, the reaction will be considered a failure. Now, read
the four scenarios below and answer the key questions that follow.
Scenario 1: The pitcher throws a fastball down the middle of the plate. The batter takes
a mighty swing and totally misses the ball. The umpire yells, "Strike one!"
Scenario 2: The pitcher throws an off-speed pitch and the batter checks his swing. The
batter just barely makes contact with the ball and it dribbles down in front of the batter’s
feet into foul territory. The umpire yells, "Foul ball; strike two!"
Scenario 3: The pitcher throws a curve ball that looks like it might catch the outside
corner of the plate. The batter swings with all his strength, but the bat grazes the
underside of the ball and the ball skews off to the right, flying into the crowd. The umpire
yells, "Foul ball, still two strikes!"
Scenario 4: The pitcher throws another fastball down the middle of the plate. The batter
swings and wallops the ball high into the air and the ball clears the center field wall that
reads 410 feet. The ump yells, "Homerun!"
©POGIL 2005, 2006 2/4
Authored by: Bryan Horan; Revised by: Kelly Levy and Kenneth Levy
Edited by Linda Padwa and David Hanson, Stony Brook University
Collision Theory
Key Questions
1. Did a reaction take place between Reactant A and Reactant B in Scenario 1?
Why or why not? Explain your reasoning in terms of the nature of the collision.
2. Did a reaction take place between Reactant A and Reactant B in Scenario 2?
Why or why not? Explain your reasoning in terms of the nature of the collision.
3. Did a reaction take place between Reactant A and Reactant B in Scenario 3?
Why or why not? Explain your reasoning in terms of the nature of the collision.
4. Did a reaction take place between Reactant A and Reactant B in Scenario 4?
Why or why not? Explain your reasoning in terms of the nature of the collision.
5. Based on your responses to Key Questions 1-4 and your reasoning, what insight
has your team gained about the term effective collision?
6. Based on your answer to Key Question 5, complete the following statement:
Collision theory states that a reaction is most likely to occur if…
©POGIL 2005, 2006 3/4
Authored by: Bryan Horan; Revised by: Kelly Levy and Kenneth Levy
Edited by Linda Padwa and David Hanson, Stony Brook University
Collision Theory
7. With your group, develop a different analogy/model to explain the collision
theory to someone who is not in your group.
Exercise
1. Hydrogen gas and iodine vapor combine to form hydrogen iodide gas, as shown
in the equation H2 + I2 → 2 HI. Using the representations shown below, draw a
diagram to show an orientation for the reactant molecules that could produce an
effective collision capable of producing two hydrogen iodide molecules.
H2 I2 HI
2. Using the representations shown in question 1, draw a diagram to show an
orientation for the reactant molecules that would NOT produce an effective
collision.
©POGIL 2005, 2006 4/4
Authored by: Bryan Horan; Revised by: Kelly Levy and Kenneth Levy
Edited by Linda Padwa and David Hanson, Stony Brook University
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Factors Affecting The Rate of Chemical Reactions: Before You ReadDocument9 pagesFactors Affecting The Rate of Chemical Reactions: Before You ReadMika VaughnNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Introduction To The Gas LawsDocument5 pagesIntroduction To The Gas LawsMika VaughnNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- POGIL Activity - Collision TheoryDocument4 pagesPOGIL Activity - Collision TheoryMika VaughnNo ratings yet
- NonClinical Labs Inspections List (10-1-2000 Through 9-27-2020)Document78 pagesNonClinical Labs Inspections List (10-1-2000 Through 9-27-2020)Mika VaughnNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Balancing Redox Reactions by The Ion-Electron Method AcidDocument3 pagesBalancing Redox Reactions by The Ion-Electron Method AcidMika VaughnNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Cathodic and Anodic ProtectionDocument2 pagesCathodic and Anodic ProtectionMika VaughnNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Expt #1 - Molecular Modeling of Alkanes Assigned Reading/Viewing - Lab Manual, #1, Expt 18a. Hyperchem InstallationDocument9 pagesExpt #1 - Molecular Modeling of Alkanes Assigned Reading/Viewing - Lab Manual, #1, Expt 18a. Hyperchem InstallationMika VaughnNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
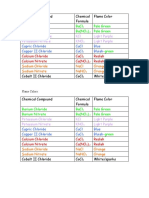
- Flame Colors:: Barium Chloride Bacl Pale Green Barium Nitrate Ba (No) Pale GreenDocument1 pageFlame Colors:: Barium Chloride Bacl Pale Green Barium Nitrate Ba (No) Pale GreenMika VaughnNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Chapter10-Physical Significance of IntegralDocument66 pagesChapter10-Physical Significance of IntegralMika VaughnNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Corrosion Behavior Between Dental Implant AbutmentDocument3 pagesCorrosion Behavior Between Dental Implant AbutmentMika VaughnNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Electrical QuantitiesDocument34 pagesElectrical QuantitiesMika VaughnNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Carib Studies Module 2 NotesDocument96 pagesCarib Studies Module 2 NotesMika Vaughn100% (1)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- BIOL 1263 Living Organisms II Tutorial Questions-Mycology SectionDocument2 pagesBIOL 1263 Living Organisms II Tutorial Questions-Mycology SectionMika VaughnNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Collision Theory Is The Key To Understanding Why Some Reactions Are Faster Than OthersDocument16 pagesCollision Theory Is The Key To Understanding Why Some Reactions Are Faster Than OthersRicki HanNo ratings yet
- Chemical Kinetics CH 290Document263 pagesChemical Kinetics CH 290jastin michaelNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Collision Theory: Effective Collision Occurs WhenDocument3 pagesCollision Theory: Effective Collision Occurs WhenLi Ching HoeNo ratings yet
- Ib Chemistry: Topic 6 Chemical KineticsDocument18 pagesIb Chemistry: Topic 6 Chemical KineticsThe Entangled Story Of Our WorldNo ratings yet
- The Collision Theory and Factors Affecting The Rate of Chemical ReactionDocument28 pagesThe Collision Theory and Factors Affecting The Rate of Chemical ReactionRolan Picones BaltazarNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Lab Report Batch ReactorDocument21 pagesLab Report Batch Reactornaneesa_190% (30)
- Chemistry PDF PDFDocument311 pagesChemistry PDF PDFÇhí Igwe - Kanu100% (1)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Kuliah 2 - Reaksi MolekulerDocument38 pagesKuliah 2 - Reaksi MolekulerHerald MatiusNo ratings yet
- ATOPCV1 3 4 Collision Theory of Reaction Rates and Its LimitationsDocument15 pagesATOPCV1 3 4 Collision Theory of Reaction Rates and Its LimitationsmevadatinkalNo ratings yet
- Chemical Reactions: Physical Vs Chemical ChangesDocument7 pagesChemical Reactions: Physical Vs Chemical ChangesIfra HassanNo ratings yet
- Chemical KineticsDocument7 pagesChemical KineticsdineshnpNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Energy and Chemical Change Grade 11Document14 pagesEnergy and Chemical Change Grade 11Reitumetse MolefeNo ratings yet
- KINETICSDocument47 pagesKINETICSMarilia BonorinoNo ratings yet
- Module 4Document24 pagesModule 4MARIE ANN DIAMANo ratings yet
- Chapter 2-Measurements and CalculationsDocument39 pagesChapter 2-Measurements and CalculationsAref DahabrahNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Please Note:: Submitting Multiple Images or Naming Your PDF Incorrectly Will Slow Down Your Application ProcessDocument18 pagesPlease Note:: Submitting Multiple Images or Naming Your PDF Incorrectly Will Slow Down Your Application Processjerzie cheethamNo ratings yet
- Boardworks IBO Chemistry Diploma A-Level Mapping GridDocument28 pagesBoardworks IBO Chemistry Diploma A-Level Mapping GridMary MannuNo ratings yet
- 4th QUARTER-Module-6-CHEMICAL REACTIONSDocument12 pages4th QUARTER-Module-6-CHEMICAL REACTIONSStray DogsNo ratings yet
- Collision TheoryDocument66 pagesCollision TheoryPAUL BENEDICT MORENO MENDOZANo ratings yet
- A270134180 - 23715 - 17 - 2019 - Chemical Kinetics - 1Document50 pagesA270134180 - 23715 - 17 - 2019 - Chemical Kinetics - 1omer faruqeNo ratings yet
- Matriculation Chemistry (Reaction Kinetics) Part 4Document13 pagesMatriculation Chemistry (Reaction Kinetics) Part 4ridwan100% (1)
- 2 RemovedDocument1 page2 RemovedNo NameNo ratings yet
- Experiment 1Document3 pagesExperiment 1Myzhel InumerableNo ratings yet
- AP Chemistry, Kinetics Lab ReportDocument15 pagesAP Chemistry, Kinetics Lab ReportSebatHian Santiago60% (5)
- Handout Powerpoint Chem 301 PharChm1Document101 pagesHandout Powerpoint Chem 301 PharChm1Mikee MeladNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Collision Theory PDFDocument48 pagesCollision Theory PDFOliric FabiolasNo ratings yet
- Rates of ReactionDocument6 pagesRates of ReactionRednaxela OnalaNo ratings yet
- CHM 432Document16 pagesCHM 432Amirah NajihahNo ratings yet
- Statistical ThermodynamicsDocument40 pagesStatistical ThermodynamicsAli Amiri0% (1)
- Rate LawsDocument20 pagesRate LawsReginal MoralesNo ratings yet