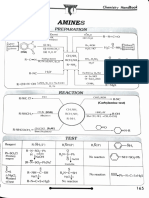
Professional Documents
Culture Documents
Conversion Chart Organic Chemistry
Uploaded by
VishakhaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Conversion Chart Organic Chemistry
Uploaded by
VishakhaCopyright:
Available Formats
Road Map (Flow Chart) of Conversions (Aliphatic compounds)
Nitrites KNO,
P0a NaNO,1ICt (HONO)
-N
R-CH-ONO Partial 1Hydrolysis Amide
RCH,CONH,H
-0,
NIfy
H,COOH - RCH,COðÑH,O RCH.CONH
4[H]LLiAlH
AmineLiAL,_Nitroalkancso AgNO
R-CH-NHa 40RCH,-NG% KCN Cyanide LiAIH 1 aminc NaNO,ICT 1 alcohol
RCH,C=N 44) RCH,CH NH, -N, -1,0 RCH,CH,OH
0HCHO R"-CH-OH 1° Alcohol
NaOR"_ (Gi) M,o
Ether. Tamine
yarolysis HCOOH
RCH0-R (wliamson's RCH,NH,]|
@RCHO
synthesis)
AgCN Isocyanidc
LRCH,NC.
LiAlH,2 amin
4[H]RCH.NHCH
Grignard reagent
RCH,MgXor R'MgX
(1) HO CHOH 2Alcohol
Alkane (where R"=RCH)* RC=0
OH
r
3° Alcohol
RCH-CH-R(Wun
reaction)
(i) H,O
NallAcetoneAlkyl iodide E ) co, COOH Carboxylic acid
()
Alkyl fluoride ction) R-CHl Ho
Si
Vic.diol dy
R-CHF (Swar's
reaction 2 Alcohol ro or PC Ketone
.
Alkyl halide
RCH OHLiAH or HyPd
C=O
Vic. dibromide CHX ACL, Br)|
aslogca
os K,Cr;0,M,S0, or KMn0,/H or OH P0A
Acid anhydride
HO
NHCOONH, EOJ,0".
ey
(Patial oxidation)
g 0FEC(dehydrogenation)Aldehyde
AIkane RedP+H1° Alcohoil E,0JH* C Amide lysisCyanideLiAIH
H,ORCONH,Partinl EydroysisR-C
0a 1° AmineNaNOHCI 1° Alcoho
Alkyne Alkene
R-CH, J 473 K R-CH,OHoUS"K [H]
or oens reagent LKCOOH N [H]R-CHNH 4 tONO)
UIO
NaBH, H/Pd or or LiAIH,
Cone. H,S0 43 K orNao (Hofmann's
fmann'sBr
KOH
Rror
Bromamide
H,OH Alcohol R-CH
OH
(Markovnikov addiion of H,0 CN
Cyanohydrin Acyl chloride
Kolbe's AIK
RCOONa Electrolysis reaction))
oa
RCOC
R-R 1°
R-NH
Amine
B,H,
(HBO-Antimarkovnikov
of l1,0)
Zn-ig/1C
Aldehydeketone (Clemmensen's
() H,0/OH
reduction
addition
Alcohol
Alkane
Ether
URCH),0
nd
R-CH
cOOH
OH
ahlydroxOH
carboxylic acid
ROIUE
Ester Ester Isocyanide
RCOOCHR 0OR,on1* CI RNC
0 NH,NH; (in) KOH/ethylene glycol
Pa
(Wolfi kasfiner reduction)
R OH
CH,CH,
OcoCH,
CH, (Clemmensen
Zn-lHg/HCI
s reduction)
Ethylbenzene 0 CO COOH
CH,COCi (Acetylation)
Pyridine,-HC coo
CHcOCUAICly (1) H
OH alicylic acid
(CHCO)Oi' Acery salicylie
(Acctylation)-CH,COOH
acid (Aspirtn
ACctophenone CH-C-CH,
ag
9 CHyMgl i) H,O X(Wiliamson's synthesis)
(ongnard Te3gcni) 3° Alcohol H
-C,Hs CHO
Alkylaryl
cJLcOCVAIC. S0,H cther
NaOl/fusion Salicylaldechyuc
--
Benzophenone
oleum
S03)
LC, (Sod.Phenoxide) aorNaOH
(F1,SO4* HCI
Sulphonation
CH-CH
IH2S0, CH-0-0-H
ur
Fuming
HPO/AICI H,OH
-ClcocH, (Acetone)
(propcne) RCOOHI C- -R"
CHyctH-Cf, Cumene Cumene hydroperoxide
Zndust/A (RCO)
onc. HPO,+ H,0/A -ZnO
or
HNO,+ -N,-I -
Hr
RCOCUPyridine Ester
Nitr: conc. N,BF4
tration) .-HX
--N
HSO HB4(Fluoroboric
An
NO NH acid)
d) NX
Sn/HCl or NaNO,/HX (X= CI, Br)
FeHCl or 273-278
H/Pd. C>H,OH
*KOHa
reaction)
reictio Benzoquinone
hylam ne
(Gattermmann
neyer's CuCNKCN
CHNH
NC (Carbyla ande
CuHX (Sandmeyer's
CI,Br
CuX/HX (S
IX=
reaction) LiAl
or Na /Ni
Dchzylamin nine
NaOH 623 K, 300 atm HA
CONH2
-Na
(Fitigreaction)
. Soda_
COONa
BryNaOH
(Hofmann-Bromamide
mann Lime/a
/
.
Dipneny Benzamide degradation)
attcm
Etards reaction) HCTT zene NaOH
() Cro, in (CH,CO),0 (1) H,0"
O nhyd
*CGCo,CI,
CoONa
CHO COOH
CH,CI CH,OH
K,Cr0,/H or
Cylhv KMnO Road Map (Flow Chart) of Conversions
or SOCI, Cus75
PCI,
OH COCI (Aromatic compounds)
HCI,
AqKOH
CO duction
und
-H,O
Acyl choride
Zn-HgHCI, 4q)_ (CIemmensenS rcauction)
(i) alk KMnO/KOH (ii) H,O"
You might also like
- G R Reduction AlkaneDocument43 pagesG R Reduction AlkaneManthan HaritashNo ratings yet
- Aldol Reaction - ChemistryDocument7 pagesAldol Reaction - ChemistryGamer HelperNo ratings yet
- Chem 212 Alkyl Halide Problems 2Document1 pageChem 212 Alkyl Halide Problems 2kevinamy100% (1)
- Chem 212 Alkyl Halide Problems 3Document1 pageChem 212 Alkyl Halide Problems 3kevinamyNo ratings yet
- Roadmap Problem - 9Document1 pageRoadmap Problem - 9abhyudaipathwayNo ratings yet
- Chem 212 Alkyl Halide Problems 4Document1 pageChem 212 Alkyl Halide Problems 4kevinamyNo ratings yet
- CH CH CHCH CH H CH CH CH CH CH CH H CH: Byvineet Khatri SirDocument13 pagesCH CH CHCH CH H CH CH CH CH CH CH H CH: Byvineet Khatri Sirsarvesh goyalNo ratings yet
- AIEEE Chemistry Quick ReviewDocument1 pageAIEEE Chemistry Quick ReviewYashwanth KalyanNo ratings yet
- Solution - Colligative Properties Solutions PDFDocument25 pagesSolution - Colligative Properties Solutions PDFGOURISH AGRAWALNo ratings yet
- Organic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideDocument9 pagesOrganic Reagents: 1. Alcoholic KOH 2. Aluminium EthoxideAarya Nandal100% (1)
- 01 D and F Block Elements Theory Final EDocument17 pages01 D and F Block Elements Theory Final Etech 2 life100% (1)
- H.D.A. 2021Document54 pagesH.D.A. 2021Every Time Chemistry [ ETC]No ratings yet
- Redox RxnsDocument30 pagesRedox RxnsJolaine ValloNo ratings yet
- Alkenes Alkynes Oxidation PDFDocument32 pagesAlkenes Alkynes Oxidation PDFRamuNo ratings yet
- Organic 6 CDocument26 pagesOrganic 6 CDr.Rajarshi PatelNo ratings yet
- Navneet Jethwani Geometrical Optics: Organic ChemistryDocument40 pagesNavneet Jethwani Geometrical Optics: Organic ChemistrySubhrota Pradhan100% (1)
- Telegram Channel for JEE ToppersDocument267 pagesTelegram Channel for JEE ToppersGyanesh DwivediNo ratings yet
- 12th Chem Exemplar PDFDocument288 pages12th Chem Exemplar PDFRalston King Stulla ChambersNo ratings yet
- Solved Example: Chemistry For Neet & AiimsDocument24 pagesSolved Example: Chemistry For Neet & AiimsAnup KNo ratings yet
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDocument2 pagesOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- GRiGNARD REAGENT!!Document22 pagesGRiGNARD REAGENT!!GazalNo ratings yet
- Mole Concept-2: Oxidation, Reduction, and Balancing Redox EquationsDocument38 pagesMole Concept-2: Oxidation, Reduction, and Balancing Redox EquationsR S.NagiNo ratings yet
- 13 DPP 03g Goc Excel Acid+BaseDocument5 pages13 DPP 03g Goc Excel Acid+BasekljNo ratings yet
- 7 Coordination CompoundsDocument329 pages7 Coordination CompoundsArka100% (1)
- Isomerism - Handwritten NotesDocument7 pagesIsomerism - Handwritten Notesgovind_galamNo ratings yet
- Alkyl HalidesDocument20 pagesAlkyl HalidesShivam Gupta0% (1)
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDocument2 pagesHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- Chapter 8 Reactions of AlcoholsDocument12 pagesChapter 8 Reactions of AlcoholsRoberto SIlvaNo ratings yet
- TEST 2 GOC & POC Tough by S.K.sinha See Chemistry Animations atDocument3 pagesTEST 2 GOC & POC Tough by S.K.sinha See Chemistry Animations atmyiitchemistryNo ratings yet
- Alkyl Halides and Aryl Halides SkillsDocument23 pagesAlkyl Halides and Aryl Halides SkillsNETHAKANI SUJATHA100% (1)
- Alkyl Aryl Halides PDFDocument21 pagesAlkyl Aryl Halides PDFLakshit SanghrajkaNo ratings yet
- Chemistry Concepts on Aromaticity and ReactivityDocument4 pagesChemistry Concepts on Aromaticity and ReactivitySubhadeepNo ratings yet
- Reducing Agents ListDocument1 pageReducing Agents ListSourabh DhavalaNo ratings yet
- Organic Chemistry - General Organic ChemistryDocument79 pagesOrganic Chemistry - General Organic ChemistryTetakali SandeepNo ratings yet
- Prince Singh: Physical & Inorganic ChemistryDocument5 pagesPrince Singh: Physical & Inorganic ChemistryJatin SinglaNo ratings yet
- Aldehydes & Ketones (Booklet-2Document15 pagesAldehydes & Ketones (Booklet-2kraken monsterNo ratings yet
- Alkyl Halides & Aryl Halides-02 - Solved ProblemsDocument13 pagesAlkyl Halides & Aryl Halides-02 - Solved ProblemsRaju SinghNo ratings yet
- Reactions of Alkyl Halides-GDocument37 pagesReactions of Alkyl Halides-Gnicole_oropezaNo ratings yet
- IIT JEE Chemistry Revision on Liquid Solutions and Colligative PropertiesDocument5 pagesIIT JEE Chemistry Revision on Liquid Solutions and Colligative PropertiesJatin Singla100% (1)
- Reductions, oxidations, substitutions and rearrangementsDocument9 pagesReductions, oxidations, substitutions and rearrangementsArka MukhopadhyayNo ratings yet
- Ape Assignment 3Document7 pagesApe Assignment 3Atharva KulkarniNo ratings yet
- Haloalkanes and Haloarenes1Document15 pagesHaloalkanes and Haloarenes1Poorni RenuNo ratings yet
- Organic Chemistry Revision for JEE AdvancedDocument35 pagesOrganic Chemistry Revision for JEE AdvancedSubhrota PradhanNo ratings yet
- IsomerismDocument62 pagesIsomerismsubesinghNo ratings yet
- Carbonyl Compound WorksheetDocument25 pagesCarbonyl Compound WorksheetOmendra SinghNo ratings yet
- Part - I: Objective Questions: Section A: Geometrical IsomerismDocument10 pagesPart - I: Objective Questions: Section A: Geometrical IsomerismTejas pawarNo ratings yet
- Review Sheet On Determining Term SymbolsDocument8 pagesReview Sheet On Determining Term SymbolsMaria AnwarNo ratings yet
- GRIGNARD REAGENTS, REDUCTION & ALKANESDocument52 pagesGRIGNARD REAGENTS, REDUCTION & ALKANESPRIYANSHU KUMARNo ratings yet
- Reduction, Oxidation - Hydrolysis Exercise PDFDocument24 pagesReduction, Oxidation - Hydrolysis Exercise PDFGOURISH AGRAWAL100% (3)
- Organic Chemistry Fiitjee Flowcharts PDFDocument12 pagesOrganic Chemistry Fiitjee Flowcharts PDFTanishq VermaNo ratings yet
- Ionic EquilibriumDocument55 pagesIonic Equilibriumharshul jainNo ratings yet
- Reaction IntermediatesDocument20 pagesReaction IntermediatesSacchitDShethNo ratings yet
- Organic Chemistry Final Exam - Questions OnlyDocument9 pagesOrganic Chemistry Final Exam - Questions OnlybrookNo ratings yet
- ORGANIC CHEMISTRY DPPDocument7 pagesORGANIC CHEMISTRY DPPAshish RanjanNo ratings yet
- 12 Chemistry Keypoints Revision Questions Chapter 12Document20 pages12 Chemistry Keypoints Revision Questions Chapter 12sangam patraNo ratings yet
- Amines HandbookDocument2 pagesAmines HandbookSuhani SubratNo ratings yet
- CHEM-353-LECTURE 2Document10 pagesCHEM-353-LECTURE 2Caleb AsharleyNo ratings yet
- Seatwork Preparation and Chemical Reactions of Organic CompoundsDocument6 pagesSeatwork Preparation and Chemical Reactions of Organic CompoundsSo FiaNo ratings yet
- REDUCTIONS FinalDocument11 pagesREDUCTIONS Finalgamer boomerNo ratings yet
- Heterocyclic ReactionsDocument2 pagesHeterocyclic Reactionsthat's niceNo ratings yet
- ANNUAL Time Table Class 10 & 12Document1 pageANNUAL Time Table Class 10 & 12VishakhaNo ratings yet
- CHEMISTRY (043) SYLLABUS FOR CLASS XIDocument11 pagesCHEMISTRY (043) SYLLABUS FOR CLASS XIshivam namdevNo ratings yet
- Estimating Vitamin C in Food & DrinksDocument30 pagesEstimating Vitamin C in Food & Drinksdan jose skNo ratings yet
- Estimating Vitamin C in Food & DrinksDocument30 pagesEstimating Vitamin C in Food & Drinksdan jose skNo ratings yet
- Guidelines Practical Term II 24022022Document16 pagesGuidelines Practical Term II 24022022Chandu NirmalkarNo ratings yet
- Applications of Weak Acid Cation Resin in Waste TreatmentDocument9 pagesApplications of Weak Acid Cation Resin in Waste TreatmentSandeep MishraNo ratings yet
- SPM Chemistry Form 5 NotesDocument16 pagesSPM Chemistry Form 5 NotesHongYu Hui100% (4)
- Activity 1 Nomenclature of Organic Compounds (PB)Document10 pagesActivity 1 Nomenclature of Organic Compounds (PB)Sittie Neharah S. MapandiNo ratings yet
- 5 6194909700736155970Document6 pages5 6194909700736155970Ayur GreenNo ratings yet
- Assignment 25 Carboxylic AcidsDocument8 pagesAssignment 25 Carboxylic Acidsbob jizzleNo ratings yet
- Synthesis and Characterization of Acrylated EpoxidizedDocument10 pagesSynthesis and Characterization of Acrylated EpoxidizedCalin MihaelaNo ratings yet
- Carboxylic Acid and DerivativesDocument8 pagesCarboxylic Acid and Derivativesmark angel andayaNo ratings yet
- Naming AlcoholsDocument9 pagesNaming AlcoholsMary Margaret "MM" A. Avena0% (1)
- PDF Carboxylic AcidsDocument45 pagesPDF Carboxylic AcidsRegina Maree BermudezNo ratings yet
- WBJEE 2019 Chemistry Question Answerkey SolutionsDocument21 pagesWBJEE 2019 Chemistry Question Answerkey SolutionsANIKET ROYNo ratings yet
- 2019MTEQtr1G12-STEM - Chem2Document7 pages2019MTEQtr1G12-STEM - Chem2Elcid BocacaoNo ratings yet
- 2015 YJC H2 Chem 2015 Prelim Suggested AnswersDocument23 pages2015 YJC H2 Chem 2015 Prelim Suggested AnswerswaimoeNo ratings yet
- Alcohols, Phenols and EpoxidesDocument134 pagesAlcohols, Phenols and EpoxidesStudent 365100% (1)
- Polymers - Library ContentDocument141 pagesPolymers - Library ContentCH M RehanNo ratings yet
- Jumbo Home Test-1 - FinalDocument59 pagesJumbo Home Test-1 - FinalSayali SachinNo ratings yet
- Biology Notes of First Year - Notes of 1st YearDocument190 pagesBiology Notes of First Year - Notes of 1st YearAli Ayan100% (2)
- Cheat Code For Organic Conversion RXN (10.8.2020) (1) - Removed - Removed - RemovedDocument53 pagesCheat Code For Organic Conversion RXN (10.8.2020) (1) - Removed - Removed - Removedpiyushnag40No ratings yet
- EDEXCEL A2 CHEMISTRY UNIT 5 January 2011Document24 pagesEDEXCEL A2 CHEMISTRY UNIT 5 January 2011Ghaleb W. MihyarNo ratings yet
- Module 5 Review of Basic Organic CompoundsDocument18 pagesModule 5 Review of Basic Organic CompoundsBig BrotherNo ratings yet
- Assignment-1-Cbse Question Bank Chapter-12-Aldehydes, Ketones & Carboxylic AcidsDocument9 pagesAssignment-1-Cbse Question Bank Chapter-12-Aldehydes, Ketones & Carboxylic AcidsSHUBHAMNo ratings yet
- Anisole SynthesisDocument6 pagesAnisole SynthesisManoj Tiwari0% (1)
- Time: 1 Hrs Max. Marks: 75 Single Correct: OH H OH H CH Oh CH Oh ODocument5 pagesTime: 1 Hrs Max. Marks: 75 Single Correct: OH H OH H CH Oh CH Oh Olakshmi.vedanarayanan7785No ratings yet
- Functional Groups: Gonzales - Gregorio - Madriñan - Maglaya 1nur5 - Group 5Document27 pagesFunctional Groups: Gonzales - Gregorio - Madriñan - Maglaya 1nur5 - Group 5Ella Cabales GonzalesNo ratings yet
- Imidazoline-Théorie Ferm1954Document21 pagesImidazoline-Théorie Ferm1954Belkhadem FatimaNo ratings yet
- Learning chart for aliphatic organic chemistry reactionsDocument3 pagesLearning chart for aliphatic organic chemistry reactionsHet GalaNo ratings yet
- Carboxylic Acid Lab ReportDocument11 pagesCarboxylic Acid Lab ReportTHASVIN OFFICIAL NETWORKNo ratings yet
- Cytec Cymel ResinsDocument9 pagesCytec Cymel ResinsHarshad PorwalNo ratings yet
- Organic Chemistry - Chapter 17 - Introduction To Carbonyl Chemistry Oxidation-ReductionDocument10 pagesOrganic Chemistry - Chapter 17 - Introduction To Carbonyl Chemistry Oxidation-ReductionSairille ManejaNo ratings yet
- Gen Chem 1 Q2 Module 3Document24 pagesGen Chem 1 Q2 Module 3Joshua RaninNo ratings yet
- Structure of A Carboxylic AcidDocument7 pagesStructure of A Carboxylic AcidKajal SinghNo ratings yet