Professional Documents
Culture Documents
Renal Pathology I. Clinical Manifestations of Renal Diseases
Uploaded by
Krisha Marie Badillo0 ratings0% found this document useful (0 votes)
53 views18 pagesThis document discusses renal pathology and glomerular diseases. It describes the clinical manifestations of renal diseases including azotemia, uremia, nephritic syndrome, and nephrotic syndrome. Pathologic responses in the glomerulus include hypercellularity, crescent formation, basement membrane thickening, hyalinosis, and sclerosis. Glomerular diseases are mainly caused by immune mechanisms such as the in situ formation of immune complexes, antibodies against glomerular basement membrane components, and the deposition of circulating immune complexes. This can lead to inflammation, proliferation, and fibrosis in the glomerulus and tubulointerstitial injury. Specific conditions discussed include nephritic syndrome, proliferative glomerulone
Original Description:
Original Title
RENAL PATHOLOGY
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document discusses renal pathology and glomerular diseases. It describes the clinical manifestations of renal diseases including azotemia, uremia, nephritic syndrome, and nephrotic syndrome. Pathologic responses in the glomerulus include hypercellularity, crescent formation, basement membrane thickening, hyalinosis, and sclerosis. Glomerular diseases are mainly caused by immune mechanisms such as the in situ formation of immune complexes, antibodies against glomerular basement membrane components, and the deposition of circulating immune complexes. This can lead to inflammation, proliferation, and fibrosis in the glomerulus and tubulointerstitial injury. Specific conditions discussed include nephritic syndrome, proliferative glomerulone
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
53 views18 pagesRenal Pathology I. Clinical Manifestations of Renal Diseases
Uploaded by
Krisha Marie BadilloThis document discusses renal pathology and glomerular diseases. It describes the clinical manifestations of renal diseases including azotemia, uremia, nephritic syndrome, and nephrotic syndrome. Pathologic responses in the glomerulus include hypercellularity, crescent formation, basement membrane thickening, hyalinosis, and sclerosis. Glomerular diseases are mainly caused by immune mechanisms such as the in situ formation of immune complexes, antibodies against glomerular basement membrane components, and the deposition of circulating immune complexes. This can lead to inflammation, proliferation, and fibrosis in the glomerulus and tubulointerstitial injury. Specific conditions discussed include nephritic syndrome, proliferative glomerulone
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 18
RENAL PATHOLOGY leukocytes; trigger – activation of
I. Clinical Manifestations of Renal Diseases coagulation factors
Azotemia o Basement membrane thickening – seen in EM
o Increased BUN and creatinine d/t decreased GFR Deposition of immune complexes
o Both in AKI and CKI Increased synthesis of protein components
Uremia (eg diabetic glomerulosclerosis)
o Symptomatic azotemia Formation of additional matrix layers (eg
o Failure of renal excretory function MPGN)
o Metabolic and endocrine changes o Hyalinosis
o Secondary involvement of the GIT, peripheral Accumulation of homogenous and
nerves, and heart eosinophilic material
Nephritic syndrome Hyalin – extracellular amorphous,
o Inflammatory glomerular disease composed of plasma proteins
o Acute onset hematuria, diminished GFR, mild- o Sclerosis
moderate proteinuria, hypertension Deposition of extracellular collagenous
o Classic presentation of APSGN matrix
nephrotic syndrome Mesangial in diabetic glomerulosclerosis
o Heavy proteinuria (> 3.5g/d), hypoalbuminemia, Pathogenesis - mainly immune mechanisms (most
severe edema, hyperlipidemia, lipiduria commonly autoantibody-mediated)
1. In Situ Formation of Immune Complexes
AKI
o Antigens – intrinsic or extrinsic
o Rapid decline in GFR (hours to days) with
o Classic example: membranous nephropathy
dysregulation of fluid and electrolyte balance,
retention of metabolic wastes (local formation of immune complexes by
o Most severe form: oliguria / anuria antibodies reactive with endogenous antigens)
o IF: Granular pattern of immune deposition –
CKD
localized Ag-Ab interaction
o Persistent GFR < 60 mL/min/1.73m 2 for > 3 mo
o EM: discrete subepithelial electron-dense
and/or persistent albuminuria
deposits (with resultant host responses, cause
o End result of all chronic renal parenchymal
thickened BM appearance)
diseases (MC DM and HTN)
2. Antibodies Directed Against Normal Components of
ESRD
the GBM
o GFR < 5% of normal
o Intrinsic antigens homogenously distributed
o Terminal stage of uremia
along entire length of GBM
Renal tubular defects
IF: Diffuse linear pattern
o Polyuria, nocturia, electrolyte disorders
o < 5% cases but causes severe necrotizing and
crescentic glomerular damage
II. Glomerular Diseases
o Clinical syndrome of RPGN
3. Deposition of Circulating Immune Complexes
o Localization because of physicochemical
properties of complexes and hemodynamic
factors peculiar to the glomerulus
o Antigens
Endogenous – SLE, IgA nephropathy
Exogenous – infections (streptococcal,
hepatitis B and C, Treponema pallidum,
Plasmodium falciparum)
Mechanisms of Injury after Immune Complex
Formation
o Elicitation of a local inflammatory response
o Antibodies may activate complement and
engage Fc receptors on leukocytes and
mesangial cells
o Leukocytic infiltration and proliferation of
Pathologic Responses of the Glomerulus to Injury mesangial and endothelial cells
o Hypercellularity o EM: electron dense deposits (ICs)
Endocapillary proliferation: Proliferation of o IF: granular deposits
mesangial or endothelial cells + Infiltration o Once deposited, ICs may be degraded by
of leukocytes infiltrating neutrophils and
Formation of crescents: proliferating monocytes/macrophages, mesangial cells, and
glomerular epithelial cells and infiltrating endogenous proteases
If repeatedly deposited for prolonged 2. Tubular Injury and Interstitial Fibrosis
periods however, it leads to membranous / o Ischemia of tubular segments downstream from
membranoproliferative GN sclerotic glomeruli, inflammation, od loss of
o Localization of ICs peritubular capillary blood supply
Antigen Cross GBM Resultant IC o Proteinuria can also cause direct injury to and
Cationic + Subepithelial activation of tubular cells
Anionic - Subendothelial Activated tubular cells express adhesion
Neutral Mesangial molecules and elaborate proinflammatory
Large ICs usually not nephritogenic – cytokines, chemokines, and GFs,
cleared by phagocytes contributing to interstitial fibrosis
Influenced by glomerular dynamics, A. Nephritic Syndrome
mesangial function, and integrity of charge- Glomerular inflammation – injury of capillary walls
selective barrier of the glomerulus – permits blood passage
variable pattern in various forms of GN Hematuria, proteinuria (subnephrotic, +/- edema),
Subendothelial and mesangial – accessible azotemia, and hypertension
to circulation and > likely to be involved in Typical presentation of most proliferative GN
inflammatory processes requiring Reduced GFR – oliguria, fluid retention, azotemia
interaction with WBCs Hypertension – fluid retention and renin release from
Subepithelial – often non-inflammatory ischemic kidneys
Other Possible Mechanisms of Injury 1. Acute Proliferative (Post-infectious and Infection-
o T-cell mediated immune reactions associated) GN
o Activation of alternative complement pathway o Proliferation of glomerular cells + leukocytic
Occurs in dense deposit disease (MPGN infiltrate
type II) and C3 glomerulopathies o Mainly caused by ICs
o Podocyte injury Antigen exogenous (post-infectious) or
Mediators of Glomerular Injury endogenous (SLE)
o Cells o Poststreptococcal GN – usually appears 1-4
Neutrophils and monocytes – result of weeks after streptococcal infection of the
activation of complement (C5a) and Fc pharynx or skin (impetigo); most common in
receptors; neutrophil release of proteases, children 6-10 years old
oxygen-derived free radicals, and Group A beta-hemolytic streptococci (90%
arachidonic acid metabolites types 12, 4, and 1) – identified by typing of
Macrophages and T cells M protein of bacterial cell walls
Platelets Latent period = time required for antibody
Resident glomerular cells (mesangial cells) – production and immune complex formation
produce inflammatory mediators; may (+) serum antistreptococcal antibody titer
initiate inflammation even in the absence of Low serum complement (C3) because of
leukocyte infiltration consumption of complement components
o Soluble mediators Granular immune deposits in glomeruli
Complement activation – chemotactic Principal antigenic determinant:
products, membrane attack complex (C5b- streptococcal pyogenic exotoxin B (Spec B)
C9); some diseases have defective – humplike deposits
regulation (eg C3 glomerulopathies) Outset: inciting antigens are exogenously
Eicosanoids, nitric oxide, angiotensin, planted from the circulation in the
endothelin subendothelium → in situ IC formation →
Cytokines (IL-1 and TNF) dissociate, migrate across the GBM, and
Chemokines (eg monocyte chemoattractant reform on the subepithelial side of the GBM
protein 1, growth factors) o Other infections with the same
Coagulation system – fibrin often (+) in the pathomechanism:
glomeruli and Bowman space in GN Bacterial – staphylococcal endocarditis,
Mechanisms of Progression pneumococcal pneumonia,
o Once any renal disease destroys functioning meningococcemia
nephrons and reduces the GFR to 30-50% of Viral – hepatitis B and C, mumps, HIV,
normal, progression to end-stage renal failure varicella, mononucleosis
proceeds at a steady rate, independent of the Parasitic – malaria, toxoplasmosis
original insult o Morphology
1. Glomerulosclerosis Enlarged hypercellular glomeruli
o Can lead to proteinuria and increasing functional - Infiltration by neutrophils and
impairment monocytes
- proliferation of endothelial and Produced predominantly by the
mesangial cells proliferation of epithelial cells lining the
- crescent formation (severe cases) Bowman capsule and by the infiltration of
Proliferation and leukocyte infiltration – monocytes and macrophages (+/-
global and diffuse (all glomerular lobules neutrophils and lymphocytes)
involved) o Pathogenesis
Swelling of endothelial cells Anti-GBM Ab-mediated (1/5)
Resultant obliteration of capillary lumen - Linear deposits of IgG and C3 in the
+/- interstitial edema and inflammation, GBM
tubular RBC casts - May cross react with pulmonary
IF: granular deposits of IgG and C3 alveolar BMs and cause pulmonary
(sometimes IgM) in the mesangium and hemorrhage (Goodpasture syndrome)
along the GBM – common antigen: non-collagenous
- ICs universally present but often focal portion of alpha-3 chain of collagen
and sparse type IV
EM: discrete amorphous electron-dense - Tx: plasmapheresis, corticosteroids,
deposits on the epithelial side of the cytotoxic agents (can reverse
membrane (appear as humps) – ICs at the pulmonary hemorrhage and renal
subepithelial surface failure in Goodpasture syndrome)
- Subendothelial deposits common Immune complex deposition (1/4)
especially in early disease course - Granular deposits of antibodies and
o Clinical Features complement
Young child abruptly develops malaise, - May be idiopathic
fever, nausea, oliguria, and hematuria - IC nephritides: PSGN, lupus nephritis,
(smoky or cola urine) 1-2 weeks after sore IgA nephropathy, HSP
throat recovery - Tx: address underlying cause
- Dysmorphic RBCs [casts] in urine, mild Pauci-immune crescentic GN
proteinuria (<1 g/d), periorbital - (-) anti-GBM Ab or IC
edema, and mild to moderate - Circulating antineutrophil cytoplasmic
hypertension antibodies (ANCAs) – cytoplasmic or
Adults – onset atypical (sudden appearance perinuclear stain
of hypertension or edema with increased - May be a component of systemic
BUN) vasculitis but often limited to the
- may be subclinical (seen only on kidneys (idiopathic)
screening for microscopic hematuria in Formerly Now
epidemics) c-ANCA PR3-ANCA
> 95% affected children recover – tx aimed p-ANCA MPO-ANCA
at maintaining sodium and water balance o Morphology
- < 1% develop RPGN Kidneys enlarged and pale with petechial
- Remainder undergo slow progression hemorrhages on cortical surfaces
to chronic GN Focal and segmental necrosis
- Poor prognostic factors: prolonged and Variable proliferation
persistent heavy proteinuria and Pauci-immune: segmental glomerular
abnormal GFR necrosis and crescents adjacent to
Disease less benign in adults glomerular segments uninvolved by
- Only 60% of sporadic cases recover inflammatory or proliferative changes
promptly Fibrin strands prominent between cellular
- Remainder experience persistence layers in the crescents
(may recover, develop chronic GN, or IF
develop RPGN) - IC-mediated: granular immune
2. Crescentic (Rapidly Progressive) Glomerulonephritis deposits
(RPGN) - Goodpasture syndrome: linear GBM
o Severe glomerular injury without a specific fluorescence for Ig and complement
etiology denoted - Pauci-immune: little to no deposition
o Rapid and progressive loss of renal function of immune reactants
associated with severe oliguria and signs of EM: GBM rupture
nephritic syndrome - Allows leukocytes, plasma proteins,
o If untreated, death from renal failure within and inflammatory mediators to reach
weeks to months the urinary space and trigger crescent
o (+) crescents in most of the glomeruli formation
Crescents undergo organization with time High Low (albumin, transferrin)
and foci of segmental necrosis resolve as Low Low and high (globulin)
segmental scars (normalization only Vulnerable to infection
possible with early aggressive tx) o Especially staphylococcal and pneumococcal
o Clinical Features o Caused by loss of immunoglobulins in urine
Hematuria with RBC casts (+) thrombotic complications
Moderate proteinuria (occasionally o Caused by loss of anticoagulants in urine
nephrotic) o Consequence: renal vein thrombosis (particularly
Variable hypertension and edema with membranous nephropathy)
Renal involvement often progressive and Pathogenesis
culminates in severe oliguria o Children – often due to a primary kidney disease
Tx: steroids, cytotoxic agents; however, o Adults – often associated with a systemic disease
many patients later on require RRT (MC diabetes, amyloidosis, and SLE)
especially if disease is discovered at a late o Most important primary glomerular lesions:
stage minimal change disease, membranous
B. Nephrotic Syndrome nephropathy, and FSGS
1. Membranous Nephropathy
o Diffuse thickening of the glomerular capillary
wall due to the accumulation of deposits
containing Ig along the subepithelial side of the
BM
o 75% primary; remainder secondary with the
following associations:
Drugs: penicillamine, captopril, gold, NSAIDs
Malignancy: carcinoma of the lung and
colon; melanoma
SLE
Infection: chronic hepatitis B and C, syphilis,
schistosomiasis, malaria
Other autoimmune disorder: thyroiditis
o Pathogenesis
Chronic immune-complex mediated disease
Caused by a derangement in glomerular capillary Primary – antibodies to a renal autoantigen
walls resulting in increased permeability to plasma - PLA2R (membrane protein at the basal
proteins surface of the glomerular epithelial
o Massive proteinuria (> 3.5 g / d) cell) antigen in 60-70%
o Hypoalbuminemia (< 3 g / dL) – urine loss > liver - ICs deposited at subepithelial BM
production, increased renal catabolism of Paucity of neutrophils, monocytes, or
filtered albumin platelets in the glomeruli
o Generalized edema – soft and pitting; most MAC – activates glomerular epithelial and
marked in the periorbital region and dependent mesangial cells – induced to liberate
portions of the body; if severe, may lead to proteases and oxidants that cause capillary
pleural effusion and ascites wall injury
Decreased intravascular colloid osmotic IgG4 – principal Ig deposited in most cases
pressure of membranous nephropathy
Sodium and water retention o Morphology
- hypovolemia-enhanced renin secretion LM: glomeruli appear normal in early
increases aldosterone production disease or exhibit uniform diffuse thickening
- SNS activation of the glomerular capillary wall
- Reduction in the secretion of EM: thickening d/t electron-dense deposits
natriuretic factors (ICs) between the BM and epithelial cells
o Hyperlipidemia and lipiduria with effacement of podocyte foot processes
Increased lipoprotein synthesis in the liver, - BM material laid down in between
abnormal transport of circulating lipid deposits – appear as irregular spikes
particles, and decreased lipid catabolism protruding from the GBM (best seen by
Lipids appear in urine as free fat or oval fat silver stains which color the BM but
bodies (lipoprotein resorbed by tubular not the deposits black)
epithelial cells then shed with injured cells - Spikes thicken with time to produce
detached from BM) domelike protrusions and eventually
Proteinuria selectivity Protein MW close over immune deposits – buried
within a markedly thickened irregular - Changes completely reversible after
membrane corticosteroid tx (proteinuria
IF: granular deposits of Ig and complement remission)
- Immunostain for PLA2R or THSD7a PCT cells laden with lipid and protein d/t
Segmental sclerosis with disease tubular reabsorption of lipoproteins that
advancement have passed through the diseased glomeruli
Epithelial cells of PCT contain protein - “lipoid nephrosis”
reabsorption droplets IF: (-) Ig or complement deposits
Considerable interstitial mononuclear cell o Clinical Features
inflammation Despite massive proteinuria, renal function
o Clinical Features remains good
Insidious onset nephrotic syndrome (-) hypertension or hematuria
15% have non-nephrotic proteinuria Proteinuria highly selective (MC albumin)
Hematuria and mild hypertension in 15-35% Dramatic response to corticosteroids (>90%
cases patients respond)
Course generally indolent - Proteinuria may recur in some patients
Non-selective proteinuria – non-responsive – may become steroid-dependent or
to corticosteroids resistant (but still resolves usually by
Remission in 40% patients puberty)
- > in women and non-nephrotic range Excellent long-term prognosis (even in
proteinuria adults who respond slower to steroids)
Progression associated with increasing May also follow NSAID tx usually in
glomeruli sclerosis, renal insufficiency, and association with acute interstitial nephritis
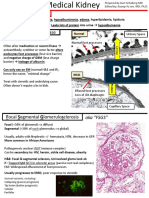
hypertension development 3. Focal Segmental Glomerulosclerosis (FSGS)
Clinical Progression % o Primary FSGS – MC cause of nephrotic syndrome
Persistent proteinuria 60 in the US
Renal failure, death 10 o MC manifests as acute or subacute onset
CKD, ESRD 40 nephrotic syndrome or non-nephrotic
Recurs in 40% patients who underwent proteinuria
transplant Hypertension, microscopic hematuria, and
2. Minimal Change Disease some degree of azotemia
o Benign disorder characterized by diffuse o Classification
effacement of foot processes (detected only on Primary (idiopathic)
EM) Associated with other known conditions
o MC cause of nephrotic syndrome in children (HIV, heroin, sickle cell disease, morbid
(peaks at 2-6 y/o) obesity)
o Pathogenesis – immunologic despite absence of Secondary event (scarring of a previously
immune deposits active necrotizing lesion – eg focal GN (IgA
Association with respiratory infection or nephropathy))
routine immunization Component of adaptive response to renal
Response to immunosuppressives tissue loss (renal ablation)
(corticosteroids) - Congenital (renal agenesis/dysplasia)
Association with atopy (eczema, rhinitis) - Acquired (reflux nephropathy)
- Increased prevalence of certain HLA - Advanced disorders (hypertensive
haplotypes nephropathy)
Increased incidence in Hodgkin lymphoma Inherited – mutation in genes encoding slit
Primary podocytopathy diaphragm proteins (podocin, alpha-actinin
o Morphology 4, TRPC6)
LM: normal glomeruli o Clinical Features
EM: GBM normal; principal lesion at visceral > incidence of hematuria, reduced GFR, and
epithelial cells hypertension
- Uniform and diffuse effacement of foot Non-selective proteinuria
processes – reduced to a rim of Poor response to corticosteroids
cytoplasm with loss of recognizable Progression to CKD (50% develop ESRD
intervening slit diaphragms within 10 years)
- D/dx (foot process effacement but o Pathogenesis
glomeruli appear abnormal): Ultrastructural hallmark: epithelial damage
membranous glomerulopathy, diabetic Degeneration and focal disruption of
nephropathy visceral epithelial cells with effacement of
foot processes (D/dx: MCD)
- Caused by circulating factors and segmental deposition of plasma
genetic defects affecting the slit proteins along the capillary wall
diaphragm (hyalinosis)
Hyalinosis and sclerosis – d/t entrapment of - (+) lipid droplets and foam cells
plasma proteins in hyperpermeable foci and EM: diffuse effacement of foot processes
increased ECM deposition - Also focal detachment of epithelial
Recurrence of proteinuria after transplant cells and denudation of underlying
(sometimes within 24 h) with subsequent GBM
progression to overt lesions in some IF: IgM and C3 in sclerotic areas and/or
patients – may be d/t an unknown mesangium
circulating factor Disease progression – increasing sclerosis
o Inherited forms with pronounced tubular atrophy and
NPHS1 interstitial fibrosis
- Chromosome 19q13 Morphologic variant: collapsing
- Encodes nephrin (key component of glomerulopathy
the slit diaphragm) - Retraction and/or collapse of the
- Mutation - congenital nephrotic entire glomerular tuft +/- FSGS lesions
syndrome (Finnish type) - Proliferation and hypertrophy of
NPHS2 podocytes
- AR; chromosome 1q25-q31 - Idiopathic, drug-associated
- Encodes podocin (also part of the slit (pamidronate)
diaphragm) - Most characteristic lesion of HIV-
- Mutation – steroid-resistant nephrotic associated nephropathy
syndrome of childhood onset - Associated with prominent tubular
Gene encoder of podocyte actin-binding injury with formation of microcysts and
protein alpha-actinin 4 interstitial inflammation
- AD; insidious onset but high rate of - Poor prognosis
renal insufficiency progression o Clinical Features
Gene encoder of TRPC6 Little tendency for spontaneous remission
- Increased calcium flux in podocytes Better prognosis in children
- Some adult-onset FSGS Variable steroid response and progression
o Renal ablation FSGS to renal failure
A secondary form – complication of 25-50% recurrence in transplant patients
[non]glomerular diseases (reflux Prognostic factors: degree of proteinuria,
nephropathy, unilateral agenesis) renal insufficiency at diagnosis, histology
- May lead to progressive - Collapsing variant – poor prognosis
glomerulosclerosis and renal failure - Tip variant – good prognosis
- Glomerulosclerosis initiated by the 4. HIV-Associated Nephropathy
adaptive change occurring in the o HIV – direct or indirect causation of renal
relatively unaffected glomeruli complications (eg with drugs or infection)
(undergo hypertrophy – hypertension o 5-10% of patients with HIV
both systemic and intraglomerular) o Morphology
Endothelial and visceral epithelial cell injury Collapsing variant of FSGS
- Denuding of foot processes → protein Focal cystic dilation of tubule segments –
permeability and accumulation in the filled with proteinaceous material, inflamed
mesangial matrix and fibrotic
- Proliferation of mesangial cells, Tubuloreticular inclusions within
infiltration by macrophages, increased endothelial cells on EM (also seen in SLE) –
ECM accumulation modifications of endoplasmic reticulum
- Segmental and eventual global induced by circulating interferon-alpha
sclerosis o HIV-associated FSGD primarily due to G1/G2 risk
Prominent role: TGF-beta alleles for APOL1
Tx: RAAS inhibitors 5. Membranoproliferative Glomerulonephritis (MPGN)
o Morphology o A pattern of immune-mediated injury (not a
LM: focal and segmental lesions may specific disease)
involve only a minority of the glomeruli o Types
(may be missed if the biopsy specimen I – deposition of ICs with IgG and complement
contains insufficient glomeruli) II – dense deposit disease – complement
- Sclerotic segments – collapse of activation; a form of C3 glomerulopathy (C3
capillary loops, increased matrix, and present in GBM but not in dense deposits)
o Histology (C3NeF) that binds C3 convertase and
Alterations in GBM prevents its inactivation → persistent C3
Accumulation of mesangial matrix activation and hypocomplementemia
Proliferation of glomerular cells Decreased C3 synthesis by the liver
Leukocyte infiltration +/- associated Factor H mutation
Deposits in mesangial regions and Morphology
glomerular capillary walls - Mesangial proliferative pattern – MC
o 10% of nephrotic syndrome cases in children in - Defining feature in EM: permeation of
young adults; mostly associated other systemic the lamina densa of the GBM by a
disorders ribbonlike homogenous extremely
o Pathogenesis electron-dense material of unknown
Type I – ICs in glomerulus and activation of composition
both classical and alternative complement - IF: C3 (+) in irregular granular or linear
pathways foci in BMs on either side but not
Unknown antigens; believed to be from within dense deposits; also in
hepatitis B and C mesangium as circular aggregates
o Morphology (mesangial rings)
Large and hypercellular glomeruli - (-) IgG, C1q, C4
Hypercellularity from mesangial and Clinical Features
endocapillary proliferation - Primarily affects children and young
Accentuated lobular appearance of adults
glomeruli due to the proliferating mesangial - Nephritic or nephrotic syndrome
cells and increased mesangial matrix - Poor prognosis (50% progress to ESRD)
GBM thickened – “double contour” or “tram - 90% recur in transplant patients
track” appearance especially in silver or PAS 6. Fibrillary GN
stains o Fibrillary deposits in the mesangium and
- Duplication / splitting of BM d/t new glomerular capillary walls that resemble amyloid
BM synthesis in response to fibrils superficially
subendothelial IC deposits (-) staining however for Congo red
- Between duplicated BMs, (+) cellular o LM: mesangioproliferative or
elements membranoproliferative
- (+) crescents o IF: IgG4, C3, Ig-K and L light chains
Type I – discrete subendothelial electron- o Nephrotic syndrome, hematuria, progressive
dense deposits renal insufficiency
- Granular IgG and C3 deposition (also o Recurs in transplants
C1q and C4) o Marker: DNAJB9
o Clinical Features C. Other Glomerular Diseases
MC present in adolescence with nephrotic 1. IgA Nephropathy (Berger Disease)
syndrome and a nephritic component o Prominent IgA deposits in the mesangial regions
Few spontaneous remissions and hematuria
Slowly progressive but unremitting course o MC type of GN worldwide
o Secondary MPGN o Mild proteinuria; rarely may develop nephrotic
Often type 1; arises from: syndrome and present with crescentic GN
- Chronic IC deposition (SLE), hepatitis B, o Typically an isolated renal disease but IgA may
hepatitis C with cryoglobulinemia, also be in other systemic disorders such as HSP
endocarditis, infected ventriculoatrial Secondary IgA nephropathy may occur in
shunts, chronic visceral abscess, HIV, liver and intestinal disease
schistosomiasis o Pathogenesis: multi-hit etiology
- Alpha 1 antitrypsin deficiency Increased plasma polymeric IgA
- Malignancy – chronic lymphocytic - Aberrant glycosylation
leukemia - Defect in normal formation or
o Dense Deposit Disease attachment of O-linked glycans to the
Excessive activation of alternative hinge region of IgA1
complement pathway Aberrantly glycosylated IgA either deposited
Decreased serum C3, normal C1 and C4 by itself in glomeruli or it forms ICs in the
Decreased serum Factor B and properdin circulation with IgG autoantibodies – later
Glomerular deposits: C3 and properdin (not on deposited in the mesangium
IgG) Mesangial deposits activate mesangial cells
> 70% patients have a circulating to proliferate, produce ECM, and secrete
autoantibody termed C3 nephritic factor cytokines and GFs
Inflammatory cell recruitment - Sx appear at 5-20 y/o
Activation of alternate pathway – (+) C3 and - Overt renal failure in 20-50 y/o males
(-) C1q and C4 in glomeruli b. Thin Basement Membrane Nephropathy
HLA genotypes may be linked (Benign Familial Hematuria)
Increased synthesis of abnormal IgA may Familial asymptomatic hematuria – MC
occur in response to RT/GIT exposure to uncovered on routine urinalysis
environmental agents Morphologic diffuse thinning of the GBM to
- Gluten enteropathy (celiac disease) 150-225nm (normal: 300-400 nm in healthy
- Liver disease adults)
o Morphology - varies +/- mild-moderate proteinuria
Normal or mesangial widening and Renal function normal – excellent prognosis
endocapillary proliferation Affects 1% of the general population; MC
Focal proliferative GN autosomal
Overt crescentic GN Mutation in genes encoding alpha 3 or 4
IF: characteristic mesangial deposition of chains of collagen
IgA D. Glomerular Lesions Associated with Systemic Diseases
- Often with C3 and properdin, and less 1. Lupus Nephritis
IgG or IgM o Hematuria, nephritic syndrome, RPGN<
- (-) early complement components nephrotic syndrome, ARF/CRF, hypertension
EM: electron dense deposits in the 2. Henoch-Schonlein Purpura
mesangium (sparse at capillary wall) o Purpura + abdominal pain and intestinal bleed +
o Clinical Features arthralgia + renal abnormalities (not all needed
MC in older children and young adults for diagnosis)
Mostly present with gross hematuria after o Skin: arm and leg extensors, buttocks
infection of RT > GIT or UT o Abdomen: pain, vomiting, intestinal bleed
- Lasts for several days, subsides, then o Renal (1/3): hematuria, nephritic syndrome,
returns every few months nephrotic syndrome
Variable subsequent course Few adults develop RPGN with many
Increased progression risk: old age of onset, crescents
heavy proteinuria, hypertension, and o MC in children 3-8 y/o – excellent prognosis
glomerulosclerosis o 1/3 patients have atopy (onset after URTI)
Frequent recurrence in transplant o IgA deposition in mesangium (also C3)
2. Hereditary Nephritis o Morphology
o Mutations in collagen genes that manifest Mild to diffuse mesangial and endocapillary
primarily with glomerular injury proliferation to crescentic GN (vary)
a. Alport Syndrome Pathognomonic on IF: IgA deposition
Mutations affecting type IV collagen (GBM, (sometimes with IgG and C3) in the
eye lens, cochlea) resulting in hematuria mesangium (sometimes to capillary loops)
with progression to CRF Skin lesions – subepidermal hemorrhages
- Large deletions in alpha-5 chain and a necrotizing vasculitis of dermal
(COL4A5) associated with early stage vessels (also with the same deposition)
ESRD - Vasculitis rare in the kidney
With nerve deafness (may require sensitive 3. Diabetic Nephropathy
testing) and eye disorders (lens dislocation, 4. Other
posterior cataracts, and corneal dystrophy) a. Goodpasture syndrome, microscopic
X-linked (males – full syndrome, female polyangiitis, granulomatosis with
heterozygotes – hematuria) > AR and AD polyangiitis – foci of glomerular necrosis
90% affected males progress to ESRD before and crescent formation
40 y/o b. Essential mixed cryoglobulinemia –
Morphology cryoglobulins principally of IgG-IgM
- GBM: irregular foci of thickening complexes – induce cutaneous vasculitis,
alternating with thinning synovitis, and MPGN type 1; often
- Pronounced splitting and lamination of associated with hepatitis C infection
lamina densa → basket-weave c. Plasma cell neoplasms – secrete Ig that may
appearance also induce glomerular lesions
- Glomerulosclerosis with progression
Clinical Features III. Tubular and Interstitial Diseases
- MC presenting sign: gross or A. Acute Tubular Injury / Necrosis (ATI / ATN)
microscopic hematuria, with RBC casts Characterized by acute renal failure +/- tubular
- May develop proteinuria later epithelial cell necrosis
Most common cause of AKI (50% in hospitalized Sublethal endothelial injury → increased
patients); reversible release of endothelin and decreased
Causes production of NO and PGI2
o Ischemia (Ischemic) o Patchiness of necrosis and BM integrity allows
Intrarenal blood vessel disease repair if the precipitating cause is removed
(microangiopathies) Re-epithelialization mediated by growth
Decreased effective circulating blood factors and cytokines from tubular and
volume (hypovolemic shock) inflammatory cells
o Direct toxic injury to tubules (Nephrotoxic) Morphology
Endogenous: myoglobin, hemoglobin, o Tubular epithelial injury at multiple points along
monoclonal light chains, bile/bilirubin the nephron with large skip areas in between
Exogenous: drugs (gentamicin), often with:
radiocontrast dye, heavy metals (mercury), BM rupture (tubulorrhexis)
organic solvents (carbon tetrachloride), Occlusion of tubular lumen by casts
poison o Severity of morphologic findings doesn’t
o Combined ischemic and nephrotoxic: hemolytic correlate with severity of clinical manifestations
crises, skeletal muscle injuries o Most vulnerable: straight portion of proximal
Intratubular hemoglobin or myoglobin casts tubule and ascending thick limb in the renal
Toxic iron content medulla
Pathogenesis Distal tubule may also show focal lesions
o Tubular cell injury o Necrosis patchier in ischemic type and more
Tubular epithelial cells (especially those in extensive in toxic type
the PCT) are sensitive to ischemia and o Other findings in ischemic ATI
vulnerable to toxins Interstitial edema
Predisposing factors Accumulation of leukocytes within dilated
- Increased surface area for vasa recta
reabsorption Epithelial regeneration – flattened epithelial
- Active transport system for ions and cells with hyperchromatic nuclei and mitotic
organic acids figures
- High rate of metabolism and oxygen o Toxic ATI
consumption Most obvious findings at PCT
- Capability to resorb and concentrate Agent Morphology
toxins Mercuric Large acidophilic inclusions; later
Effects of Ischemia chloride necrotize, desquamate, and calcify
- Loss of cell polarity due to Ethylene Marked ballooning and
redistribution of membrane proteins glycol hydropic/vacuolar degeneration;
from the basolateral to the luminal calcium oxalate crystals in the lumen
surface of the tubular cells → Clinical Course (Phases)
abnormal ion transport → increased 1. Initiation
sodium delivery to the distal tubules → o 36 hours; dominated by the inciting event
inciting vasoconstriction via o Slight decline in urine output with increased BUN
tubuloglomerular feedback (lowers the o If with oliguria – d/t transient decrease in BF and
GFR to maintain distal BF) declining GFR
- Expression of cytokines and adhesion 2. Maintenance
molecules → leukocyte recruitment o Sustained decrease in UO 40-400mL/d (oliguria),
- Injured cells detach from BM → salt and water overload, increasing BUN,
luminal obstruction, increased hyperkalemia, metabolic acidosis, and other
intratubular pressure, and further manifestations of uremia
decreased GFR o Can be overcome with appropriate tx
Glomerular filtrate in the lumen of damaged 3. Recovery
tubules leak back into the interstitium → o Steady increase in urine volume that may reach
interstitial edema up to 3 L/d
o Hemodynamic alterations that cause reduced o Tubules still damaged – large amounts of water,
GFR Na, K lost in urine
Intrarenal vasoconstriction → reduced o Development of hypokalemia
glomerular BF and reduced oxygen delivery o Increased infection vulnerability
to outer medulla (thick ascending limb and o Eventually, renal tubular function is restored and
straight segment of the proximal tubule) concentrating ability improves (BUN and Cr
RAAS activation – stimulated by decreased normalize)
sodium in the tubules d/t decreased BP
o
Subtle impairments may persist but most o Morphology
patients eventually recover completely Hallmark: patchy interstitial suppurative
Prognosis inflammation intratubular aggregates of
o Depends on magnitude and duration of injury neutrophils, neutrophilic tubulitis, and
o Recovery expected if no other organs are tubular injury
seriously damaged Suppuration may occur as discrete focal
o 95% recover with supportive care abscesses or large wedge-like areas
o Mortality rate > 50% however if with multiorgan Early: neutrophilic infiltration limited to
failure tubules; later on extend to interstitium and
B. Tubulointerstitial Nephritis produce abscesses
Insidious onset; principally manifest by azotemia Glomeruli are relatively resistant to
Acute or Chronic infection (unless extensive)
o Acute Fungal – granulomatous
Interstitial edema with leukocytic Complications
infiltration of the interstitium and tubules a. Papillary necrosis
Tubular injury - Diabetics, sickle cell disease, UT
o Chronic obstruction
Infiltration with predominant mononuclear - MC bilateral
leukocytes - Cut section: tips or distal 2/3 of the
Prominent interstitial fibrosis pyramids have areas of gray-white to
Widespread tubular atrophy yellow necrosis
Differences from glomerular diseases - Microscopy: necrotic tissue shows
o Absence of nephritic / nephrotic syndrome characteristic ischemic coagulative
necrosis with preservation of tubule
o Defects in tubular function
outlines
Polyuria / nocturia (impaired ability to
b. Pyonephrosis
concentrate urine)
- Seen with total obstruction, especially
Salt wasting
if high in the UT
Diminished ability to excrete acids
- Suppurative exudate unable to drain
(metabolic acidosis)
and fills the renal pelvis, calyces, and
ureter with pus
c. Perinephric abscess
- Extension of suppurative inflammation
through the renal capsule into the
perinephric tissue
Healing occurs after the acute phase
- Neutrophilic infiltrate replaced by
macrophages, plasma cells, and
lymphocytes
- Inflammatory foci eventually replaced
by irregular scars seen on the cortical
surface as fibrous depressions
- Scars – tubular atrophy, interstitial
fibrosis, and lymphocytic infiltrate in a
characteristic patchy jigsaw pattern
with intervening preserved
parenchyma
- Pyelonephritic scar associated with
inflammation, fibrosis, and
deformation of underlying calyx and
pelvis
o Predisposing factors
Urinary tract obstruction
Instrumentation of UT
VUR
Pregnancy
Gender and age
1. Pyelonephritis
Acute 1 y/o Males (evident congenital
anomalies)
o Suppurative inflammation because of bacterial >
> 1 to 40 y/o Females
viral infection
> 40 y/o Males (prostatic Xanthogranulomatous pyelonephritis
hypertrophy, - Rare form
instrumentation) - Accumulation of foamy macrophages
Pre-existing renal lesions (scarring and intermingled with plasma cells,
obstruction) lymphocytes, polymorphonuclear
Diabetes (infection, neurogenic bladder, leukocytes, and occasional giant cells
frequent instrumentation) - Often associated with Proteus infection
Immunosuppression and immunodeficiency and obstruction
o Clinical Features - Sometimes produce large yellow
Presents as sudden onset pain at CVA and orange nodules (D/Dx – RCC)
systemic evidence of infection (malaise, o Clinical Features
fever) Silent onset or acute recurrent PN s/sx
Bladder and urethral irritation: dysuria, (back pain, fever, pyuria, bacteriuria)
frequency, urgency Late tx d/t gradual onset of renal
Pyuria – either upper or lower UT insufficiency and hypertension
involvement Loss of tubular function → polyuria and
Leukocyte casts (rich in neutrophils – pus nocturia
casts) – renal involvement Radiograph: asymmetrically contracted
Benign course if uncomplicated (resolved in kidneys with coarse scars and blunting /
a few days after antibiotic tx) Deformity of the calyceal system
If complicated, may lead to repeated Proteinuria often mild but some develop
septicemic episodes secondary FSGS
If with papillary necrosis, may lead to ARF - Proteinuria onset – poor prognostic
o Polyomavirus Infection sign – increases likelihood of ESRD
Latent infection widespread in the general progression
population C. TIN Induced by Drugs and Toxins
Reactivates with immunosuppression of the 2nd MC cause of AKI (after PN)
allograft recipient – causing transplant Mechanism
failure in 5% patients o Trigger an interstitial immunologic reaction
“Polyoma nephropathy” (acute hypersensitivity nephritis induced by
Infection of tubular epithelial cell nuclei → methicillin)
nuclear enlargement and intranuclear o Cause ATI
inclusions – seen in LM (viral cytopathic o Cause subclinical but cumulative tubular injury,
effect) taking years to result to CKD
Inclusions composed of virions arrayed in 1. Acute Drug-induced Interstitial Nephritis
distinctive crystalline-like lattices on EM o Most commonly occur with synthetic penicillins
Interstitial inflammatory response (methicillin, ampicillin), other synthetic
Tx: reduce immunosuppression antibiotics (rifampin), diuretics (thiazide),
Chronic NSAIDs, and other drugs (allopurinol, cimetidine,
o Chronic tubulointerstitial inflammation and checkpoint inhibitors)
scarring of the calyces and pelvis o Analgesic nephropathy
Only chronic PN and analgesic nephropathy Chronic TIN caused by phenacetin-
affect the calyces (clue to r/o other containing analgesics
differentials) Begins 2-40 days after drug exposure
o Morphology Fever, rash, and renal abnormalities
Gross: kidneys irregularly scarred (hematuria, mild proteinuria, and
(asymmetric if bilateral) leukocyturia (often with eos))
- D/dx: bilateral in chronic GN but - 50% have increased SCr or AKI with
symmetric oliguria
Hallmark: coarse discrete corticomedullary o Pathogenesis
scars overlying dilated blunted or deformed Idiosyncratic immune mechanism
calyces, and flattening of papillae Hypersensitivity
Scars MC at upper and lower poles (reflux - Latent period
MC in these sites) - Eosinophilia and rash
Tubules – atrophied in some areas, - Nephropathy onset not dose-related
hypertrophied/dilated in others - Recurrence with re-exposure
- Dilated tubules with flattened - Some patients have increased serum
epithelium may be filled with casts IgE (late phase reaction of type I
resembling thyroid colloid hypersensitivity)
(thyroidization)
- Others have mononuclear / like crystals in the tubular lumens or in the
granulomatous reaction (type IV interstitium
delayed hypersensitivity) Urate deposits evoke a mononuclear
Drugs function as haptens and covalently response containing foreign body giant cells
bind to some plasma membrane or (tophus)
extracellular component of tubular cells Tubular obstruction by the urates causes
- Modifed self-antigens become cortical atrophy and scarring
immunogenic Clinically subtle
- Resultant injury due to IgE or cell- o Nephrolithiasis
mediated immunity Uric acid stones are present in 22% patients
o Morphology with gout and 42% of those with secondary
Interstitium: edema and infiltration by hyperuricemia
mononuclear cells (mainly lymphocytes and 2. Hypercalcemia and Nephrocalcinosis
macrophages) o Hyperparathyroidism, multiple myeloma, vitamin
Inflammation more prominent in the D intoxication, metastatic cancer, or excess
medulla – inciting agent is concentrated calcium intake (milk-alkali syndrome) →
With methicillin and thiazides, interstitial formation of calcium stones and deposition in
non-necrotizing granulomas may be seen the kidney (Nephrocalcinosis)
Tubulitis (lymphocytic infiltration) o Defect: inability to concentrate urine
Glomeruli normal (except in some cases Other tubular defects may also occur
caused by NSAIDs – concurrent MCD and CKD with progression
nephrotic syndrome) 3. Autosomal Dominant Tubulointerstitial Kidney
o Clinical Features Disease (ADTKD)
Recovery after drug withdrawal expected o Previously Medullary Cystic Kidney Disease
but may take months o Genetic Mutations
Occasionally, necrotic papillae are excreted MUC1 Mucin 1 Distal
and may cause gross hematuria or renal nephrons
colic due to ureteric obstruction UMOD Uromodulin Thick
Causes of Papillary Necrosis ascending
Analgesic nephropathy limb of LoH
DM REN Preprorenin JGA
Sickle cell disease HNF1-beta HNF1-beta
UT obstruction 4. Light Chain Cast Nephropathy (Myeloma Kidney)
- Caused by ischemia of medullary o Bence Jones proteinuria and cast nephropathy
vessels o Ig light chains are directly toxic to epithelial cells
o NSAID nephropathy o Bence Jones proteins combine with urinary
d/t inhibition of COX-dependent PG- glycoprotein (Tamm Horsfall protein) under
synthesis acidic conditions to form large histologically
Syndromes distinct tubular casts that obstruct the tubular
- AKI – decreased PG synthesis lumens and induce inflammation
(ischemia_ o Other associations
- Acute hypersensitivity interstitial
AL Amyloidosis (MC lambda)
nephritis
Light chain deposition disease (MC kappa)
- Acute interstitial nephritis and MCD
Hypercalcemia and hyperuricemia
- Membranous nephropathy
o Morphology
D. Other Tubulointerstitial Diseases
Bence Jones tubular casts appear as pink to
1. Urate Nephropathy
blue amorphous masses
o Acute uric acid nephropathy
- Sometimes concentrically laminated
Precipitation of uric acid crystals (mainly at
and often fractured
collecting ducts due to its acidic pH) →
- Fill and distend the tubular lumens
nephron obstruction → AKI
- Some are surrounded by
Occurs in patients with leukemia or
multinucleated giant cells
lymphoma undergoing chemo (tumor lysis
Adjacent interstitial tissue shows
syndrome)
inflammation and fibrosis
o Chronic urate (gouty) nephropathy
Occasionally, casts rupture the tubules,
Rarely occurs in protracted forms of evoking a granulomatous inflammatory
hyperuricemia reaction
Monosodium urate crystals deposit in the o Clinical Features
acidic milieu of distal tubules and collecting
CKD or AKI
ducts → form distinct birefringent needle-
Precipitating factors: dehydration, changes that may resemble renal ablation
hypercalcemia, acute infection, and injury
treatment with nephrotoxic antibiotics o Clinical Features
Bence Jones proteinuria in 70% individuals Rarely cause renal insufficiency except in:
with multiple myeloma - African descent
5. Bile Cast Nephropathy - Severe BP elevation
o Impaired renal function in patients with severe - Other underlying diseases (DM)
acute or advanced chronic liver disease 5% hypertensive patients experience
Serum bilirubin markedly elevated → bile malignant hypertension
cast formation (cholemic nephrosis) in distal - Rapidly rising BP – if untreated, leads
nephron segments to death within 1-2 years
o Casts can extend to proximal tubules → direct - SBP > 200, DBP > 120
toxicity and obstruction - Renal failure (malignant
Yellow-green to red-pink casts +/0 sloughed nephrosclerosis)
cells or cellular debris - Retinal hemorrhages and exudates +/-
o Reversibility depends on severity and duration of papilledema
liver dysfunction - Some are associated with
E. Vascular Diseases microangiopathic hemolytic anemia
1. Nephrosclerosis and hemolytic uremic syndrome
o Sclerosis of renal arterioles and small arteries (common factor: endothelial injury)
o Strongly associated with hypertension (both a 2. Renal Artery Stenosis
cause and a consequence) o Pathogenesis
o Lesions Increased production of renin from the
Medial and intimal thickening ischemic kidney → RAAS activation
- Response to hemodynamic changes, o Clinical Features
aging, genetic defects Resemble essential hypertension
Hyalinization of arteriolar walls caused by A bruit may be heard
extravasation of plasma proteins through Increased plasma or renal vein renin
injured endothelium and by increased Response to ACEi
deposition of BM matrix Arteriography – needed for localization
o Thickened walls → narrowed lumens → focal o Morphology
parenchymal ischemia → glomerulosclerosis and 70% cases caused by narrowing of the renal
chronic tubulointerstitial injury → reduced artery origin due to an atheromatous
functional renal mass plaque
o Morphology - Men, advancing age, DM
Normal or reduced kidney size (110-130 g) - Concentric plaque with superimposed
- Due to cortical scarring and shrinking thrombus
Cortical surfaces have a fine even Fibromuscular dysplasia
granularity resembling grain leather - Women, 3rd-4th decade
- Subcapsular scars with sclerotic Arteriolosclerosis: non-ischemic > ischemic
glomeruli and tubular dropout kidney (response to high pressure)
alternating with normal parenchyma Diffuse ischemic atrophy
Hyaline arteriolosclerosis → luminal - Reduced size
narrowing - Crowded glomeruli, atrophic tubules,
Interlobular and arcuate arteries show interstitial fibrosis, and focal
medial hypertrophy, replication of internal inflammatory infiltrates
elastic lamina, and increased 3. Thrombotic Microangiopathies
myofibroblastic tissue in the intima o Thrombi
(fibroelastic hyperplasia) RBC shear → microangiopathic hemolytic
Vascular narrowing causes patchy ischemic anemia
atrophy Occlusion → ischemia
- Foci of tubular atrophy and interstitial Platelet consumption → thrombocytopenia
fibrosis o Includes Hemolytic Uremic Syndrome and
- Glomerular alterations (collapse of Thrombotic Thrombocytopenic Purpura
GBM, collagen deposition within the Typical HUS (epidemic, classic, diarrhea-
Bowman space, periglomerular positive)
fibrosis, and total sclerosis) - Consumption of food contaminated by
Ischemic changes affecting large areas of bacteria producing Shiga-like toxins
parenchyma can produce wedge-shaped Atypical HUS (non-epidemic, diarrhea-
infarcts or regional scars with histologic negative)
- Inherited mutations / autoantibodies - Breaks down C3 convertase to protect
targeting complement-regulatory cells from uncontrolled complement
proteins activation
- Endothelial injury Other: Factor I, CD46
TTP – inherited/acquired deficiency of o Other associated conditions
ADAMTS13 (vWF regulator) Antiphospholipid syndrome
o Pathogenesis Postpartum renal failure
Endothelial Injury (HUS) Renal vascular diseases
- Platelet activation and thrombosis Chemo and immunosuppressants
within microvascular beds (mitomycin, cyclosporine, cisplatin,
- Decreased PGI2 and NO, increased gemcitabine, VEGF antagonists)
endothelin Kidney irradiation
Platelet aggregation (HUS) o Poorer prognosis due to underlying conditions
- Induced by very large multimers of TTP
vWF accumulating due to deficient o Pentad: fever, neurologic symptoms,
ADAMTS13 – bind surface microangiopathic hemolytic anemia,
glycoproteins and activate platelets thrombocytopenia, and renal failure
spontaneously o ADAMTS13 deficiency mostly due to
autoantibodies (some due to mutations)
o > in women, < 40 y/o
o Morphology o Dominant feature: CNS involvement
Acute Renal involvement only in 50% patients
- patchy / diffuse cortical necrosis and o Tx: plasma exchange
subcapsular petechiae 4. Other Vascular Disorders
- Glomerular capillaries are distended o Atherosclerotic Ischemic Renal Disease
and occluded by thrombi o Atheroembolic Renal Disease
- Mesangiolysis Embolization of atheromatous plaques from
- Interlobular arteries and arterioles aorta or renal artery into intrarenal vessels
often show occlusive thrombi Occur especially after surgery
Chronic Emboli – (+) cholesterol crystals appearing
- Common in atypical HUS as rhomboid clefts
- Cortical scarring; similar to malignant Commonly insignificant unless renal
hypertension function is already compromised
- Glomeruli mildly hypercellular and o Sickle Cell Nephropathy
have marked thickening of capillary Disease – homozygous; trait – heterozygous
walls associated with BM splitting Abnormalities: hematuria and decreased
(double contour or tram track) concentrating ability (hyposthenuria)
- Onion skinning – increased layers of Sickling accelerated in the hypertonic
cells and connective tissue in artery hypoxic milieu of the renal medulla
and arteriole walls Other possible findings: patchy papillary
- Acute ischemic infarction necrosis, proteinuria
Typical HUS o Renal Infarcts
o Mostly occurs after intestinal infection with Mostly due to embolism from mural
strains of E. coli (O157:H7) that produce Shiga- thrombosis in the LA and LV due to MI
like toxins (also Shigella dysenteriae) (other sources: vegetative endocarditis,
o Contaminated ground meat (also in drinking aortic aneurysm, aortic atherosclerosis)
water, raw milk, and person to person Most often clinically silent, but sometimes
transmission) with CVA pain, RBC urine showers, and
o Most at risk: children and older adults hypertension if with arterial narrowing
o Prodrome of influenza or diarrhea → sudden Morphology
onset bleed (hematemesis, melena), severe - White anemic appearance due to lack
oliguria, hematuria of collateral blood supply
Associated with microangiopathic hemolytic - Within 24 h, infarcts become sharply
anemia, thrombocytopenia, neurologic demarcated, pale yellow-white areas
changes, and hypertension that may contain small irregular foci of
o Endothelial activation and apoptosis hemorrhagic discoloration
Atypical HUS - Usually ringed by a zone of intense
o >50% have an inherited deficiency of hyperemia
complement regulatory proteins
Most common: Factor H
- Wedge-shaped infarcts (base against o Both alleles of the involved genes have to be
cortical surface and apex pointing nonfunctional for the disease to develop
towards medulla) o Bilateral; initially involve few nephrons (renal
- Progress to fibrous scarring → function retained until 4th-5th decade of life)
depressed pale gray-white V-shaped Genetics and Pathogenesis
scars o PKD1 and PKD2 mutation
PKD1 gene
IV. Congenital and Developmental Anomalies - Located on chromosome 16p13.3
10% people are born with significant malformations - Encodes polycystin-1 – expressed in
of the urinary system tubular epithelial cells (distal nephron)
Hereditary or acquired during gestation - Mutation in 85% cases
1. Agenesis of the Kidney - Likelihood of developing renal failure
o Bilateral agenesis – incompatible with life; often increases with aging
in stillborn infants and associated with other PKD2 gene
congenital disorders - Located on chromosome 4q21
o Unilateral agenesis – rare; compatible with life if - < severe disease (older age of onset,
without other abnormalities; undergoes late development of renal failure)
compensatory hypertrophy but some progress to - Polycystin-2 – expressed in all
glomerular sclerosis, leading to CKD segments of the renal tubules and in
2. Hypoplasia many extrarenal tissues; Ca2+-
o Failure of the kidneys to develop to a normal size permeable cation channel
o Unilateral > bilateral o Cilia-centrosome complex of tubular epithelial
o True renal hypoplasia (no scars, < 6 pyramids) cell defects (changes in mechanosensing, Ca 2+
observed in low-birth-weight infants and may flux, and signal transduction)
contribute to increased lifetime risk of CKD Polycystin 1 and 2 are localized to the
3. Ectopic Kidneys primary cilium
o Within the pelvis or just above the pelvic brim Altered tubular epithelial growth and
o Usually normal or slightly smaller; otherwise differentiation lead to abnormal ECM, cell
unremarkable except if with ureteral tortuosity proliferation, and fluid secretion which
or kinking results to cyst formation
Obstruction to urinary flow → infection Morphology
predisposition o Gross: kidneys are bilaterally enlarged (some can
4. Horseshoe Kidneys weigh up to 4 kg)
o Common (1 in 500-1000 autopsies); fusion of the o External surface appears to be composed solely
upper (10%) or lower (90%) poles – continuous of a mass of cysts (3-4 cm d) with no intervening
across the midline anterior to the great vessels parenchyma
Microscopic exam reveals intervening
V. Cystic Diseases of the Kidney functional nephrons
o Cysts filled with clear serous fluid or turbid red-
brown hemorrhagic fluid
o Enlargement may lead to calyx and pelvis
encroachment, producing pressure defects
o Variable lining epithelia
Clinical Features
o Asymptomatic until renal insufficiency develops
o In others, hemorrhage or dilation may produce
pain
o Blood clot excretion causes renal colic
o Enlarged kidneys on abdominal palpation –
dragging sensation
o Disease may begin with insidious onset
A. Autosomal Dominant (Adult) Polycystic Kidney Disease hematuria followed by other CKD features
Generalities o Progression > in blacks, sickle cell trait, males,
o Multiple expanding cysts of both kidneys that and hypertensives
ultimately destroy renal parenchyma and cause o Extrarenal congenital anomalies
renal failure 40% have polycystic liver disease – often
o 1 in 400-1000 live births asymptomatic; from biliary epithelium
o 5-10% of ESRD cases – requiring RRT < cysts in spleen, pancreas, and lungs
Intracranial berry aneurysms – altered
expression of Polycystin in vascular smooth
muscle; at circle of Willis; SAH can cause o Tubular BM disruption → tubular atrophy and
death interstitial fibrosis
Mitral valve prolapse in 20-25% patients o Cause of eventual renal insufficiency: cortical
o Patients may survive for many years with tubulointerstitial damage
azotemia slowly progressing to uremia o Variants
Cause of Death % Sporadic non-familial
Heart disease 40 Familial juvenile (MC) – AR
Infection 25 Renal-retinal dysplasia
Ruptured berry aneurysm or 15 o MC genetic cause of ESRD in children and young
hypertensive ICH adults
B. Autosomal Recessive (Childhood) Polycystic Kidney o Present first with polyuria and polydipsia
Disease Also sodium wasting and tubular acidosis
Most commonly perinatal or neonatal presentation o Can be syndromic
o Serious manifestations MC present at birth o Progresses to ESRD in 5-10 years
(death due to renal failure) o Mutations: NPHP-1 to 11, JBTS-2, 3, 9, 11
Genetics and Pathogenesis Proteins (+) in the primary cilia
o MC PKHD1 mutation NPHP-2 – inversin – mediates L-R patterning
Chromosome 6p21-p23 during embryogenesis
Highly expressed in adult and fetal kidney, o Morphology
also liver and pancreas Small kidneys – contracted granular
Encodes fibrocystin – also at primary cilium surfaces
of tubular cells Medullary cysts
- Lined by flattened or cuboidal
epithelium
Morphology - Surrounded by inflammatory cells or
o Kidneys enlarged with a smooth external fibrous tissue
appearance Cortex: widespread atrophy and thickening
o Cut section: numerous small cysts in the cortex of tubular BM with interstitial fibrosis
and medulla – spongelike appearance Glomerular structure preserved
o Dilated elongated channels are present at right D. Multicystic Renal Dysplasia
angles to the cortical surface Sporadic; kidney enlarged, irregular with varying cyst
o Cylindrical > saccular dilation of collecting size
tubules o Lined by flattened epithelium
o Cysts have a uniform lining of cuboidal cells Characteristic histology: presence of islands of
(originate from collecting ducts) undifferentiated mesenchyme, often with cartilage,
o Liver also has cysts – associated with portal and immature collecting ducts
fibrosis and proliferation of portal bile ducts Most often associated with ureteropelvic obstruction,
Clinical Features ureteral agenesis or atresia
o Patients who survive infancy may develop a Unilateral or bilateral
peculiar hepatic injury characterized by bland o Unilateral – mimic neoplasm; good prognosis
periportal fibrosis and proliferation of well- once removed as opposite kidney is normal
differentiated biliary ductules (congenital hepatic o Bilateral – renal failure later on
fibrosis) E. Acquired Cystic Disease
Hepatic disease – predominant clinical Patients with ESRD who have undergone prolonged
concern in older children (portal dialysis develop numerous cortical and medullary
hypertension with splenomegaly) renal cysts
C. Cystic Diseases of the Renal Medulla o 0.1-4 cm d, clear fluid, lined by hyperplastic or
1. Medullary Sponge Kidney flattened tubular epithelium, and contain
o Multiple cystic dilations of the medullary calcium oxalate crystals
collecting ducts o Form as a result of obstruction of tubules by
o Occurs in adults; incidental finding interstitial fibrosis or by oxalate crystals
radiographically as renal function is normal o MC asymptomatic but seem bleed, causing
o Gross: papillary ducts dilated, +/- small cysts hematuria
Lined by cuboidal > transitional epithelium o 100x increased risk of RCC
(-) cortical scarring unless with F. Simple Cysts
superimposed pyelonephritis Single or multiple, MC at cortex; 1-5 cm but may
2. Nephronophthisis reach 10 cm
o Variable number of medullary cysts, MC Translucent, lined by gray glistening smooth
concentrated at corticomedullary junction membrane and filled with clear fluid
o Microscopic: membranes composed of 1 layer of Progressive blunting of pyramidal apices
cuboidal or flattened cuboidal epithelium (may Advanced: thin-walled cystic transformation
be atrophic) with striking parenchymal atrophy, total
Common post-mortem findings without clinical obliteration of the pyramids, and cortical
significance thinning
o Hemorrhage may cause sudden distension and Clinical Features
pain o Acute obstruction – provoke pain attributed to
o Calcification of hemorrhage may give rise to distention of the collecting system or renal
bizarre radiographic shadows capsule
Difference from renal tumors: cysts have Early sx MC d/t underlying cause (eg renal
smooth contours, often avascular, and give colic with ureteral calculi, bladder sx with
fluid signals on UTZ BPH)
o Unilateral complete/partial hydronephrosis –
VI. Urinary Tract Obstruction (Obstructive Uropathy) may remain silent for a long time as the
Increased susceptibility to infection and stone unaffected kidney can maintain adequate
formation function
o If unrelieved, leads to permanent renal atrophy May be seen first on imaging incidentally
(hydronephrosis or obstructive uropathy) UTZ – useful in the dx of obstructive
o Often treatable uropathy
Obstruction – sudden/insidious, partial/complete, o Bilateral partial obstruction
unilateral/bilateral, intrinsic/extrinsic, may occur at Earliest manifestation: polyuria and
any level; common causes: nocturia
o Congenital anomalies Some develop dTA, renal salt wasting,
o Urinary calculi secondary renal calculi, and chronic TIN
o BPH with scarring and atrophy of the papilla and
o Tumors medulla
o Inflammation Hypertension common
o Sloughed papillae / blood clots
o Pregnancy
o Uterine prolapse and cystocele
o Complete bilateral obstruction
o Functional disorders
If rapid onset, results in oliguria/anuria –
Hydronephrosis – dilation of the renal pelvis and
incompatible with survival unless
calyces associated with progressive atrophy of the
obstruction is relieved
kidney due to urine outflow obstruction
With relief, post-obstructive diuresis occurs
o Even with complete obstruction, GFR persists as
(can be massive; urine rich in NaCl)
the filtrate subsequently diffuses back into the
renal interstitium and perirenal spaces –
VII. Urolithiasis
returned to lymphatic and venous systems
Epidemiology
Continued filtration causes marked dilation
o Men > women, peak age of onset 20-30 y/o
of affected calyces and pelvis
o Familial predisposition (eg IEM – cystinuria,
o High pressure at pelvis transmitted back through
primary hyperoxaluria)
collecting ducts into the cortex
Etiology and Pathogenesis
Renal atrophy
o Main types of calculi
Compression of medulla renal vasculature
Calcium (70%)
o Tubular precedes glomerular defects
Triple / struvite (15%) – magnesium
o Interstitial inflammation → fibrosis
ammonium phosphate
Morphology
Uric acid (5-10%)
o Sudden and complete obstruction → mild
Cystine (1-2%)
dilation of the pelvis and calyces
o An organic mucoprotein matrix is present in all
o Subtotal or intermittent obstruction →
calculi (1-5% of stone weight)
progressive dilation (hydronephrosis)
o Most important determinant in stone formation:
o Kidney may be slightly to massively enlarged
urinary concentration of stone constituent –
depending on degree and duration of
exceed solubility (supersaturation)
obstruction
Other determinants: changes in urine pH,
o Progression
decreased urine volume, presence of
Simple dilation of the pelvis and calyces
bacteria, deficiency of inhibitors (eg
Interstitial inflammation
pyrophosphate, diphosphonate, citrate,
Cortical tubular atrophy with marked
glycosaminoglycans, osteopontin,
diffuse interstitial fibrosis
nephrocalcin)
1. Calcium Oxalate stones
o 5%: hypercalcemia and hypercalciuria
(hyperparathyroidism, diffuse bone disease,
sarcoidosis)
o 55%: hypercalciuria without hypercalcemia
Absorptive – hyperabsorption of calcium
from the intestine
Renal – intrinsic impairment in renal tubular
reabsorption of calcium
Idiopathic
o 20%: hyperuricosuric calcium nephrolithiasis
Nucleation of calcium oxalate by uric acid
crystals in the collecting ducts
o 5%: hyperoxaluria
o Other associations
Hypocitraturia
Idiopathic calcium stone disease
o Radiopaque
2. Magnesium ammonium phosphate stones
o Formed mainly after infection by urea-splitting
bacteria (Proteus, staphylococci) – convert urea
to ammonia
o Alkaline urine causes precipitation of MAP salts
o Staghorn calculi formation common
3. Uric acid stones
o Common in hyperuricemia (gout, leukemia)
However, > 50% patients with uric acid
calculi have neither hyperuricemia nor
increased urine excretion of uric acid
- Excrete urine at pH < 5.5
o Radiolucent
4. Cystine stones
o Genetic defects in renal reabsorption of amino
acids (including cystine) → cystinuria
o Form at low urine pH
Morphology
o Unilateral in 80% patients
o Favored sites of formation: renal calyces, pelvis,
and in the bladder
o If formed in the renal pelvis, they tend to remain
small (2-3 mm)
o Smooth or irregular contour
o Often many stones are found within 1 kidney
o Rare: progressive accretion of salts lead to
development of branching structures – staghorn
calculi
Create a cast of the pelvic and calyceal
system
Clinical Features
o Asymptomatic or produce severe renal colic and
abdominal pain or may cause significant renal
damage
o Large stones – manifest as hematuria
o Stones predispose to superimposed infection
Obstruction
Trauma
You might also like
- Gene Related DiseaseDocument3 pagesGene Related Diseasevivek govardhanamNo ratings yet
- Pathology of Urinary SystemDocument384 pagesPathology of Urinary SystemNzau MuangeNo ratings yet
- Pathology GlomerulonephritisDocument4 pagesPathology GlomerulonephritisGerardLum100% (2)
- Jaundice An Emergency Department Approach To Diagnosis and ManagementDocument24 pagesJaundice An Emergency Department Approach To Diagnosis and ManagementAmali FikriahNo ratings yet
- Varicose Vein and Its Homeopathic Cure DR Bashir Mahmud ElliasDocument8 pagesVaricose Vein and Its Homeopathic Cure DR Bashir Mahmud ElliasSanjay MisraNo ratings yet
- Differential Diagnosis of Glomerular DiseasesDocument2 pagesDifferential Diagnosis of Glomerular DiseasesMaryam Fadah100% (1)
- NEPHROTIC SYNDROME - HamidDocument20 pagesNEPHROTIC SYNDROME - HamidAbdul Hamid OmarNo ratings yet
- Inflammation - Case StudiesDocument55 pagesInflammation - Case StudiesDr Suvarna Nalapat100% (3)
- Glomerulonephritis 2019Document31 pagesGlomerulonephritis 2019EsoklailNo ratings yet
- EOs PDFDocument91 pagesEOs PDFArushee BhatnagarNo ratings yet
- Glomerulonephritis Medical Student Lecture 2Document67 pagesGlomerulonephritis Medical Student Lecture 2ibnbasheer100% (12)
- NCP For Preterm LaborDocument2 pagesNCP For Preterm LaborP Sta Maria75% (4)
- Immunologic (Autoimmune) Disorders: Risk Factors Diagnostic TestsDocument7 pagesImmunologic (Autoimmune) Disorders: Risk Factors Diagnostic Testsfebie pachecoNo ratings yet
- Hospital Acquired Infections PDFDocument4 pagesHospital Acquired Infections PDFMuhammad Mohsin Ali DynamoNo ratings yet
- Nursing Care Plan2 CVADocument4 pagesNursing Care Plan2 CVAhermesdave1No ratings yet
- Midterm Psych NSG Nclex Test BanksDocument19 pagesMidterm Psych NSG Nclex Test BanksAliza AlyyNo ratings yet
- @acute Nephritic SyndromeDocument3 pages@acute Nephritic SyndromeMazlia FarzanaNo ratings yet
- Chapter 7Document2 pagesChapter 7Mychelle MenesNo ratings yet
- Renal DiseaseDocument5 pagesRenal DiseasefeajhanineladagaNo ratings yet
- Glomerular Diseases My NotesDocument5 pagesGlomerular Diseases My Notesmalar_km43No ratings yet
- Chapter 8 - Renal DiseaseDocument7 pagesChapter 8 - Renal DiseaseCha GuingabNo ratings yet
- Glomerulonephritis: Prof DR DR Haerani Rasyid, Mkes, SPPD, KGH, SPGK Tim Ginjal Hipertensi Unhas 2019Document68 pagesGlomerulonephritis: Prof DR DR Haerani Rasyid, Mkes, SPPD, KGH, SPGK Tim Ginjal Hipertensi Unhas 2019uzan100% (1)
- Renal DiseasesDocument7 pagesRenal DiseasesXyleene Jency Bien IINo ratings yet
- Summary of Renal Disorders - 9.11.19Document4 pagesSummary of Renal Disorders - 9.11.19Nicole Juliette CCNo ratings yet
- DISC, Drugs, Infection, Thick Basal MembraneDocument5 pagesDISC, Drugs, Infection, Thick Basal MembraneHOPENo ratings yet
- LEC AUBF Renal-Diseases MIDTERMS 02Document3 pagesLEC AUBF Renal-Diseases MIDTERMS 02Jashmine May TadinaNo ratings yet
- Glomerular Disease - Dr. LuDocument8 pagesGlomerular Disease - Dr. LuMACATANGAY, GAELLE LISETTENo ratings yet
- Imunosupresan For Treatment of Nephrotic Syndrome DR Harnavi HarunDocument27 pagesImunosupresan For Treatment of Nephrotic Syndrome DR Harnavi HarunM Ivan Pratama ZebuaNo ratings yet
- Systemic Lupus Erythematosus: PathogenesisDocument5 pagesSystemic Lupus Erythematosus: PathogenesisMiguel Cuevas DolotNo ratings yet
- Renal Diseases - BSMLS OLFUDocument15 pagesRenal Diseases - BSMLS OLFUMitch IbayNo ratings yet
- Nephrotic Syndrome PDF 2Document2 pagesNephrotic Syndrome PDF 2MNo ratings yet
- AUBF Lecture-I FINDocument8 pagesAUBF Lecture-I FINChrissa Mae Tumaliuan CatindoyNo ratings yet
- PATH - Nephritic SyndromeDocument14 pagesPATH - Nephritic SyndromeMuhamad Zul ImanNo ratings yet
- Nephritic SyndromeDocument15 pagesNephritic Syndrome76q88b4yrxNo ratings yet
- Renal Diseases NotesDocument4 pagesRenal Diseases NotesJanine CabreraNo ratings yet
- Chapter 20 - The KidneyDocument24 pagesChapter 20 - The KidneyAgnieszka WisniewskaNo ratings yet
- Hematology CLL, ММ tableDocument4 pagesHematology CLL, ММ tableShur .ENo ratings yet
- Muscle Nerve ColorDocument47 pagesMuscle Nerve ColorRafay BokhariNo ratings yet
- Disease PDFDocument6 pagesDisease PDFJohn Christopher LucesNo ratings yet
- Glomerulonephritis EngDocument43 pagesGlomerulonephritis EngNosirova ManijaNo ratings yet
- Nephritic SyndromeDocument2 pagesNephritic Syndromevalari8069No ratings yet
- UTI Dan Glomerular DiseaseDocument58 pagesUTI Dan Glomerular DiseaseLiana Ika SuwandyNo ratings yet
- SN 1Document9 pagesSN 1lilisNo ratings yet
- PATH - Nephrotic SyndromeDocument11 pagesPATH - Nephrotic SyndromeTeshale TekleNo ratings yet
- Minimal Change DiseaseDocument2 pagesMinimal Change DiseasejoeNo ratings yet
- 7 Renal Disease StudentDocument34 pages7 Renal Disease Studentrbm121415chyNo ratings yet
- Poster ROICAM 2018Document1 pagePoster ROICAM 2018ibrahimNo ratings yet
- BloodDocument129 pagesBloodJoe JosephNo ratings yet
- Nephrotic Nephritic SyndromeDocument15 pagesNephrotic Nephritic Syndromeshefalika mandremNo ratings yet
- Disorder Etiology: Acute GlomerulonephritisDocument1 pageDisorder Etiology: Acute GlomerulonephritisChynna Izzabelle Alcantara AbellanaNo ratings yet
- Anemia of Bone Marrow FailureDocument6 pagesAnemia of Bone Marrow FailureKim Alyssa GoNo ratings yet
- Plasma Cell DyscrasiasDocument10 pagesPlasma Cell DyscrasiasRazib HasanNo ratings yet
- AUBF Lec - Renal DiseasesDocument6 pagesAUBF Lec - Renal Diseasescdsteenkamp18No ratings yet
- Monoclonal Gammopathies of Clinical SignificanceDocument9 pagesMonoclonal Gammopathies of Clinical SignificanceSabrina DaidNo ratings yet
- Glomerular Disease and DiureticsDocument26 pagesGlomerular Disease and DiureticsDapot SianiparNo ratings yet
- Renal DiseaseDocument4 pagesRenal DiseaseApril Lady Faith P. PaundogNo ratings yet
- BMLT Class Note On Anemia ClassificationDocument2 pagesBMLT Class Note On Anemia ClassificationSubhasish BarikNo ratings yet
- General Pathology EdgeDocument2 pagesGeneral Pathology EdgeskNo ratings yet
- Dr. Dhian Endarwati, Spa Maret 2017Document60 pagesDr. Dhian Endarwati, Spa Maret 2017Diany LarasatiNo ratings yet
- Amharic ConversationDocument94 pagesAmharic Conversationtsehay asratNo ratings yet
- Glomerular Diseases: Pathogenesis of Glomerular Diseases Progression of Glomerular DiseasesDocument6 pagesGlomerular Diseases: Pathogenesis of Glomerular Diseases Progression of Glomerular DiseasesSeff CausapinNo ratings yet
- Renal DiseaseDocument6 pagesRenal DiseaseyeonjiNo ratings yet
- Medical KidneyDocument13 pagesMedical KidneyJose SirittNo ratings yet
- Renal SystemDocument43 pagesRenal SystemYash SoniNo ratings yet
- Canine Immune-Mediated Hemolytic Anemia: Pathophysiology, Clinical Signs, and DiagnosisDocument10 pagesCanine Immune-Mediated Hemolytic Anemia: Pathophysiology, Clinical Signs, and DiagnosisRiriawan DMNo ratings yet
- Topic 3Document17 pagesTopic 3Jacqueline Tungcul DonatoNo ratings yet
- The Genetic Basis of Haematological CancersFrom EverandThe Genetic Basis of Haematological CancersSabrina TosiNo ratings yet
- Acute GastroenteritisDocument71 pagesAcute GastroenteritisKrisha Marie BadilloNo ratings yet
- Chapter 6Document12 pagesChapter 6Krisha Marie BadilloNo ratings yet
- Cestodes and Trematodes - PDFDocument6 pagesCestodes and Trematodes - PDFKrisha Marie BadilloNo ratings yet
- Vision Source Eye ChartDocument1 pageVision Source Eye ChartKrisha Marie BadilloNo ratings yet
- Intestinal-Nematodes PDFDocument3 pagesIntestinal-Nematodes PDFKrisha Marie BadilloNo ratings yet
- HPV, Cin, Cervical CaDocument106 pagesHPV, Cin, Cervical CaKrisha Marie BadilloNo ratings yet
- Acute Myeloid Leukemia: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)Document154 pagesAcute Myeloid Leukemia: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)Krisha Marie BadilloNo ratings yet
- Covid-19 Response Checklist For Lgus: Executive SummaryDocument7 pagesCovid-19 Response Checklist For Lgus: Executive SummaryKrisha Marie BadilloNo ratings yet
- Micro ParasitesDocument4 pagesMicro ParasitesKrisha Marie BadilloNo ratings yet
- Bone DseDocument2 pagesBone DseKrisha Marie BadilloNo ratings yet
- PolyuriaDocument5 pagesPolyuriaKrisha Marie BadilloNo ratings yet
- Manuskrip Askep PujaaDocument8 pagesManuskrip Askep PujaaArie IchsanNo ratings yet
- TB (Tuberkulosis)Document30 pagesTB (Tuberkulosis)Fanny BudimanNo ratings yet
- Arizona Communicable Disease FlipchartDocument98 pagesArizona Communicable Disease Flipchartapi-510866696No ratings yet
- Skoring Osas AmirahDocument11 pagesSkoring Osas AmirahthtklNo ratings yet
- PsychiartryDocument7 pagesPsychiartryNeeraj SahuNo ratings yet
- Sample of Clinical PortraitDocument3 pagesSample of Clinical PortraitSweetie StarNo ratings yet
- (Answers) Diarrhea I McqsDocument9 pages(Answers) Diarrhea I McqsKero amgedNo ratings yet
- Gestational Diabetes MellitusDocument25 pagesGestational Diabetes MellitusMazlina MaidinNo ratings yet
- Gastroenterology EsophagusDocument2 pagesGastroenterology EsophagusJayanthNo ratings yet
- 8 IX September 2020Document10 pages8 IX September 2020IJRASETPublicationsNo ratings yet
- BIOL122 Exam Format and Case StudyDocument1 pageBIOL122 Exam Format and Case StudyHuge Lovely SmileNo ratings yet
- Laxatives and Anti-DiarrhealDocument28 pagesLaxatives and Anti-DiarrhealimnasNo ratings yet
- CONFIDENTIAL MEDICAL REPORT Viginie Alicia Massie - FEVERDocument2 pagesCONFIDENTIAL MEDICAL REPORT Viginie Alicia Massie - FEVERtahuchubby sesetanNo ratings yet
- Medicine MCQS JULY 2023 SOLVEDDocument15 pagesMedicine MCQS JULY 2023 SOLVEDLijo JoNo ratings yet
- L17. Askep Kritis Dan Gadar Pasien Dengan Ggguan Sistem Pernafan NontraumatikDocument75 pagesL17. Askep Kritis Dan Gadar Pasien Dengan Ggguan Sistem Pernafan NontraumatikSepto KristianaNo ratings yet
- DementiaDocument38 pagesDementiarajikakurupNo ratings yet
- English TM 8Document11 pagesEnglish TM 8Budi AtmikaNo ratings yet
- 31.chronic Diarrhea and MalabsorbtionDocument14 pages31.chronic Diarrhea and MalabsorbtionSajjal AliNo ratings yet
- Benefir PackageDocument115 pagesBenefir PackageSourav KumarNo ratings yet
- Fibroids by EasterDocument7 pagesFibroids by EasterIGA ABRAHAMNo ratings yet
- Oral Station Scenario PDFDocument3 pagesOral Station Scenario PDFlotuss45No ratings yet
- Spiritual Emotional Freedom Technique (SEFT) To Reduce Depression For Chronic Renal Failure Patients Are in Cilacap Hospital To Undergo HemodialysisDocument6 pagesSpiritual Emotional Freedom Technique (SEFT) To Reduce Depression For Chronic Renal Failure Patients Are in Cilacap Hospital To Undergo Hemodialysisyuli astariNo ratings yet
- Initial Management Chest Pain1Document12 pagesInitial Management Chest Pain1Mohd Faizal SimonNo ratings yet