Professional Documents
Culture Documents
Catalysis: Dih 59%C C
Uploaded by
Shelly 09Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Catalysis: Dih 59%C C
Uploaded by
Shelly 09Copyright:
Available Formats
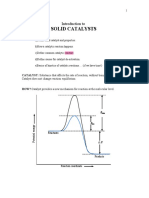
CATALYSIS AW
A catalysis may increase or decrease the rate of reaction.
S u b r e e wih altou te Yate neacken akhouk g e t l
9kel cewumed
CATALYSIS
HOMOGENEOUS HETEROGENEOUS
Reactant.and.catalyst Reactant and catalystare
are
in same phase in different phase
| Download The Lecture Notes From PW App
foronge rer Cabalyjo
Ni@ Vegebe
gRleelo
dih59%C Gh0, + (G MaD% *)
Sucr c
Download The Lecture Notes From PW App
PROMOTERS AND INHIBITORS/POISONS
Promoters are substances that enhance the activity of a catalyst while
poisons decrease the activity of a catalyst.
For example:
I n Haber's process for manufacture of ammonia, molybdenum acts as a
promoter for iron which is used as a catalyst.
N (g)+ 3H () NH, (g)
(co) Mo (prtnsto)
O Carbon monoxide or H,Sact as þoison for Fecatalyst in Haber process
for manufacture of NH,
T Download The Lecture Notes From PW App
AUTO INDUCED
CATALYSIS CATALYSIS
One ofthe product When a chemical
of reaction begins to reaction enhances
actsas a catalyst therate of another
chemical reaction.
CH CooG CHg ot t GHo sodium sul4 LiH
(Este) Eg. hydrolysis of (catmst Oxidation of Na sO,
ester in acidic influences the
medium. oxidation of
Na,AsO Sadium azdewfe
|Download The Lecture Notes From PW App
General characteristics of catalysts W
(i) A catalyst remains unchanged in mass and chemical composition but can
changetheir physical state.
(i) Only a very small amount of catalystissufficient tocatalyse a reaction.
(ii) A catalystdoes notinitiateareaction.
(iv) Whenacatalyst is a solid, it is usually more efficient when used in finely
divided form. anlypt shid need Suece maT, RoR|
(v)Generally catalyst does notchange the naturéof products.
Xi) A catalyst does notchange the equilibrium state of a reversible reaction but
helps to decrease time toachievetheequilibrium state or position of equilibrium.
(vii) Catalysts aregenerallyspecificin nature.
(vii) Catalyst can changerate constantof the reaction.
(ix) Catalysts participatein mechanism ofreaction. AS
(x) Catalyst does not changeenthalpy of reaction, free energy and entropy because
they are state function.
I Download The Lecture Notes From PW App
Adsorption Theory of Heterogeneous Catalysis W
Adsorption of
reacting molerules
Reacting
u l e s
Adsoption of
Catayat aurface havin
Vences
reacting molecules
Denorption of
product molecules
A-B
Catalyst A-B Intermedsate
Prd et
Download The Lecture Notes From PW App
Important features of solidcatalysts
(a) Activity
The activity of a catalyst depends upon the strength of chemisorption to a large
extent. The reactants must get adsorbed reasonably strongly on to the catalyst to
become active. However, they must not get adsorbed so strongly that they are
immobilised and other reactants are leftwith nospace on the catalyst's surface
foradsorption.
(b) Selectivity
The selectivity of a catalyst is its ability to direct a reaction to yield a particular
product selectively, when under the same reaction conditions many products are
possible. Selectivity of different catalysts for same reactantsis different.
Co 3G-CrD
0-Gr
CH
N
0
C o t ah,
chMD
LJVelkaradJ
Download The Lecture Notes From PW App
SHAPE SELECTIVE CATALYSIS BY ZEOLITES
The catalyticreaction that depends upon the porestructure ofthecatalyst and
sizeof reactant and productmolecules is called shape selectivecatalysis.
Zeolites are a good shape-selective catalyst because they have honeycomblike
structure.
Zeolites are microporous aluminosilicates with threedimensional network of
silicates in which some siliconatoms are replaced by aluminium.
The size of pores varies between 260 pm and 740 pm. Thus only those
molecules can beadsorbed in these pores whose size is small enough to enter
these cavities and also leave easily. It will not adsorb those molecules which are
too big to enter.
Sicate i. Sio, (Moshiecte)
Zeolites are being very widely used as catalystin petrochemical industries
for cracking of hydrocarbons and isomerizaton.
An important zeolitecatalyst used in petroleum industry is|ZSM-5( Zeolite
o f Sieve Molecular Porosity5).
It convertsalcohol directly into gasoline by dehydrating them to give a
mixture of hydrocarbons.
ZSM-5
Mcahor
Download The Lecture Notes From PW App
ENZYME CATALYSIS
(i) Enzymes are complex nitrogenous organic compounds which are
produced from I+ving8plants and animals.
(ii) They are actually protein molecules of high molecular mass and
form colloidalsolutions in water. hzywrs biocahlyet
(ii) They catalysebiochemicalreactions.
Exumples of enzymatic reactions
Enzyme Source inzymatic reaction
Invertase Yeast SucroseGlucose and fructose
CpHO(ag) +H,o= CHO (aq) + CHiO (aq)
Cane sugar aatsugely Glee Frcto
Pymase Glucose Ethyl alcobol and carbon dioxide
CHO () +H,0() yma GHOH+ co,
Ge
nayl Akoo
Diastase Lalt Starch Mallose
Diastae a CpHO (aqa
(CHO), (aq) + nH,O )
Stac Msite
I Download The Lecture Notes From PW App
Matase Yeast MaltoseGfacose
CypHO1 (aq) + H,O(0) 2CH:0, (aq)
Mate
Urease Soyabean UreaAmmonia and carbon dioxide
NHL,CONIL g)+H,O)2) +(coss
Pepsin Stomcth ProteisAmino acids
Lacto bacilli Curd Milk Cund
Characteristics of enzyme catalysis :
(i) Highly efficient :One molecule of an enzyme may transform one million
molecules of the reactant per minute.
(ii) Highly specific nature: One catalyst cannot catalyse more than one
reaction.
(ii) Highly active under optimymtemperature (298 K to 310K)
(iv) Highly activeunder optimum pll (5 to 7)
(v) Increasing activity in presence of cofactor (Na", Mn", Co", Cu" etc.) and
coenzymes.
(vi) Influence of inhibitors and poisons
Download The Lecture Notes From PW App
Mechanism of enzyme catalysis (key lock theory):
There are number of active centres of definite shape present on the surface of
colloidal particles of enzymes. The molecules of the reactant (substrate),
which have complementary shape fit into these cavities just like a key fits
into a lock. On account of the presence of active groups, an activated complex
is formed which then decomposes to yield the product.
ES E+P
S
E-S
Comp
Enzyine SuDstrate Enzyme-Substrate Enzyne Product
catalyst) treactants) complex
E + Pnduct
....-1 J T. .. AL-A. r.- nur A
Catalysts in Industry
Process Catalyst
1Haber's process for the manufacture of Finely dhdded iron, molybdenum as promoter:
ammonia conditlons: 200 barpressure and 123-73K temp-
eralure. Now-a-days. a mixture of iron oxide,
N,g+310 +2NH,(
potasstum oxide and alumina 1s used._
2 Ostwald's process for the manufacture tos
Platnised asbestos:
of nitric acid. temperature 573K.
4NH,g9+50,g) 4NOg +6H,0(g
2NO(g 0,(g +2NO,(g
4NO, 2H,00) +0,g4HNO,faq)|
Contact process for the manufacture
V2O
Platnised asbestos or vanadtum pentoxide (V,0:
of sulphurlc acsd. temperature 673-723K.
250,gO49 - 250,
So,g H,SO,lag HS,O,0
aleum
HS,0,0) + H,O0) + 2H,So.laq)
|Download The Lecture Notes From PW App
COLLOIDAL SOLUTION
Thomas Graham (1861) studied the process of
substances through a parchment paper or an an+malmembrane and
diffusion of dissolved
dividet the substances intotwo classes-
IHCrystalloid (YColloid
But this classification soon proved to be wrong since a crystalloid could
behave as a colloid under diferent conditions and vice-versa.
For example: NaCl behaves as a crystalloid in.aqueous medium and
behaves as a colloidin benzenemedium, whereas soap behaves as a
typicalcolloid in water and behaves as a crystalloid in alcohol.
So new classification was given based on the size of solute particles.
|Download The Lecture Notes From PW App
You might also like
- anapurna M: Operator ManualDocument62 pagesanapurna M: Operator ManualAnonymous gQcTl4bot867% (3)
- Characteristics of Catalytic ReactionsDocument4 pagesCharacteristics of Catalytic ReactionsAsmaa Ahmed100% (2)
- Vietnam Pesticide Use QuestionnaireDocument13 pagesVietnam Pesticide Use QuestionnaireSharmadevan SundrasegaranNo ratings yet
- Lecture 10 Catalysis IntroDocument21 pagesLecture 10 Catalysis IntroRahim KalathilNo ratings yet
- Catalyst HO 181Document7 pagesCatalyst HO 181Mohammad KhNo ratings yet
- Lecture I New - Introduction To Heterogeneous Reactions 1Document81 pagesLecture I New - Introduction To Heterogeneous Reactions 1chalachewNo ratings yet
- التكنولوجيا الحيوية التطبيقية - الاسبوع السادسDocument21 pagesالتكنولوجيا الحيوية التطبيقية - الاسبوع السادسhsjsg jdhekNo ratings yet
- Chapter 1Document74 pagesChapter 1PMNo ratings yet
- Chapter 5 CatalysisDocument31 pagesChapter 5 CatalysisHien HoangNo ratings yet
- CH 15Document51 pagesCH 15IshNo ratings yet
- Chapter 10 for Students 增加生活小常識Document43 pagesChapter 10 for Students 增加生活小常識jessNo ratings yet
- Introduction To Catalysis Cp4Document26 pagesIntroduction To Catalysis Cp4Godfrey Eric MuendoNo ratings yet
- Chapter 1Document74 pagesChapter 1Sasmilah KandsamyNo ratings yet
- Auliya Rahman - PPT CBRDocument8 pagesAuliya Rahman - PPT CBRAuliaRahmanNo ratings yet
- Catalysis : 9.1. Catalysis-A General IntroductionDocument6 pagesCatalysis : 9.1. Catalysis-A General IntroductionSatwik ChoudhuryNo ratings yet
- Lecture 1 CatalysisDocument28 pagesLecture 1 CatalysisMo MobarkNo ratings yet
- Introduction To Heterogeneous Catalysis: Letcture-1Document14 pagesIntroduction To Heterogeneous Catalysis: Letcture-1hamoodahNo ratings yet
- Vietnam-ECat Introduction 1Document8 pagesVietnam-ECat Introduction 1Hoai LinhNo ratings yet
- Outline:: Homogenous Catalysis General and Acid/base Catalysis Acidity Function Bronsted Relation and Enzyme ReactionDocument36 pagesOutline:: Homogenous Catalysis General and Acid/base Catalysis Acidity Function Bronsted Relation and Enzyme Reactionuma villashini100% (1)
- Duhok Polytechnique University-Petrochemical Department 2018 / 2019 Catalysis DR Farhad M. Ali 2018/2019Document6 pagesDuhok Polytechnique University-Petrochemical Department 2018 / 2019 Catalysis DR Farhad M. Ali 2018/2019MUHAMMAD AKRAMNo ratings yet
- CHE505 ChapterOne Part3 HIM PDFDocument28 pagesCHE505 ChapterOne Part3 HIM PDFShammil AshmanNo ratings yet
- Chemical Reaction EngineeringDocument3 pagesChemical Reaction EngineeringGayathri GanesanNo ratings yet
- 63697Document18 pages63697Thirunavuk KarasuNo ratings yet
- DehydrogenationDocument6 pagesDehydrogenationOmar SaeedNo ratings yet
- PDFDocument20 pagesPDFThirunavuk KarasuNo ratings yet
- CSTR ManualDocument11 pagesCSTR ManualMelly FulaNo ratings yet
- Catalysis: by Prof. Abhinav Shukla 9096905695Document12 pagesCatalysis: by Prof. Abhinav Shukla 9096905695Noor AL HudaNo ratings yet
- QB PDFDocument18 pagesQB PDFShivani0% (1)
- Catalyst Synthesis TechniquesDocument19 pagesCatalyst Synthesis Techniquescoolcupidguy100% (1)
- Introduction of Catalyst مقدمةDocument30 pagesIntroduction of Catalyst مقدمةIbrahimNo ratings yet
- Introduction To Catalyst Science & Technology Lab. By: Year Mr. Mohammed MahmoudDocument21 pagesIntroduction To Catalyst Science & Technology Lab. By: Year Mr. Mohammed Mahmoudsoran najebNo ratings yet
- Probevorlesung Hapke Hand-OutDocument14 pagesProbevorlesung Hapke Hand-OutALL2WINNo ratings yet
- Adv. Chemical Reaction Engineering L1Document22 pagesAdv. Chemical Reaction Engineering L1pradnyaNo ratings yet
- Activation Catalytic SpanishDocument1 pageActivation Catalytic SpanishHector Martinez HernandezNo ratings yet
- Catalyst ActivityDocument14 pagesCatalyst ActivityMUHAMMAD AKRAMNo ratings yet
- CH 6701 Cre IiDocument230 pagesCH 6701 Cre IiVaibhav Gupta100% (1)
- Modeling of A Radialflow Movingbed Reactor For Dehydrogenation of IsobutaneDocument7 pagesModeling of A Radialflow Movingbed Reactor For Dehydrogenation of IsobutaneForcus onNo ratings yet
- Dynamic Modelling of Glucose Oxidation W PDFDocument8 pagesDynamic Modelling of Glucose Oxidation W PDFTysir SarhanNo ratings yet
- Catalyst DefinitionDocument17 pagesCatalyst DefinitionTRIBRATA BASKORONo ratings yet
- Heterogeneous Catalytic Chemistry by ExampleDocument7 pagesHeterogeneous Catalytic Chemistry by ExampleAbdullah18No ratings yet
- Chapter 1 - Reaction Kinetics of HeterogenousDocument68 pagesChapter 1 - Reaction Kinetics of HeterogenousFhan Sani SeowNo ratings yet
- Selection of Catalysts - General RequirementsDocument3 pagesSelection of Catalysts - General RequirementsUroosa ShaikhNo ratings yet
- Catalytic Reactor Design (Fourth Year)Document126 pagesCatalytic Reactor Design (Fourth Year)Ahmed AbdullaNo ratings yet
- 4.isomerization Process (Will Be Tough by UOP)Document93 pages4.isomerization Process (Will Be Tough by UOP)An Lê Trường100% (1)
- 3 - Mechanism and Kinetics of Deactivation of Catalyst and Its RegenerationDocument31 pages3 - Mechanism and Kinetics of Deactivation of Catalyst and Its RegenerationVikash ChandravanshiNo ratings yet
- Catalysis-Inorganic All NotesDocument292 pagesCatalysis-Inorganic All Notesdavidedamiani5No ratings yet
- Chapter 10 For StudentsDocument42 pagesChapter 10 For Students陳祖德No ratings yet
- Catalyst: Ostwald (1895) Redefined A Catalyst As, "A Substance Which ChangesDocument14 pagesCatalyst: Ostwald (1895) Redefined A Catalyst As, "A Substance Which ChangesRahul ReddyNo ratings yet
- Lectura 4. Structured Packings For Multiphase Catalytic ReactorsDocument32 pagesLectura 4. Structured Packings For Multiphase Catalytic ReactorsTRIANA FORERO GABRIEL RICARDONo ratings yet
- Catalytic Cracking of PetroleumDocument12 pagesCatalytic Cracking of Petroleummessy munozNo ratings yet
- Catalyst Science & Technology Lab. By: Year Dr. Farhad & Mr. MohammedDocument12 pagesCatalyst Science & Technology Lab. By: Year Dr. Farhad & Mr. MohammedMUHAMMAD AKRAMNo ratings yet
- Apresentação IntegrinasDocument27 pagesApresentação IntegrinasFrancisco AndradeNo ratings yet
- Pre Lab 8Document10 pagesPre Lab 8Aaliyah MosesNo ratings yet
- Homogeneous, Heterogeneous, and Enzymatic CatalysisDocument5 pagesHomogeneous, Heterogeneous, and Enzymatic CatalysisLEIDY JOHANA PALACIOS SOLERNo ratings yet
- Characteristics of Catalysts: Prepared By: Mahaswari JogiaDocument16 pagesCharacteristics of Catalysts: Prepared By: Mahaswari JogiaIka Silvia AnggraeniNo ratings yet
- Three Catalysts Tango With Olefins: News & ViewsDocument3 pagesThree Catalysts Tango With Olefins: News & ViewsAnahí TessaNo ratings yet
- Heterogenous Catalyst ReactionDocument31 pagesHeterogenous Catalyst ReactionVishal GuptaNo ratings yet
- Catalysis: Srujal RanaDocument45 pagesCatalysis: Srujal RanavandanNo ratings yet
- Kinetics and Modeling of Fatty Alcohol Ethoxylation in An Industrial Spray Loop ReactorDocument10 pagesKinetics and Modeling of Fatty Alcohol Ethoxylation in An Industrial Spray Loop ReactorGrizzlybeer Van DyckNo ratings yet
- AntacidsDocument14 pagesAntacidsVaida MatulevičiūtėNo ratings yet
- CH6711 CRE Lab Manual 3.3.2020 1 To 8 Experiments - Extract - 1-8,12-19Document16 pagesCH6711 CRE Lab Manual 3.3.2020 1 To 8 Experiments - Extract - 1-8,12-19Vignesh Raja.PNo ratings yet
- P Block2012 457Document143 pagesP Block2012 457Abhishek Bansal100% (1)
- Class 12 - Genetics NotesDocument1 pageClass 12 - Genetics NotesDimas HernadyNo ratings yet
- 10 Fish Amino AcidDocument2 pages10 Fish Amino AcidCute Guardian AngelNo ratings yet
- Tencile StrengthDocument11 pagesTencile StrengthYdzel Jay Dela TorreNo ratings yet
- Eight Super FoodsDocument34 pagesEight Super FoodsJumaroli100% (3)
- Absorber Design: Sizing A Packed TowerDocument24 pagesAbsorber Design: Sizing A Packed TowerMbarouk Shaame MbaroukNo ratings yet
- CE215 GeoLab Manual - Determination of Specific GravityDocument2 pagesCE215 GeoLab Manual - Determination of Specific GravityJoy MondalNo ratings yet
- A Procedure To Measure The Antiradical EfficiencyDocument7 pagesA Procedure To Measure The Antiradical EfficiencyLucila Sanchez BoadoNo ratings yet
- Material Safety Data Sheet: 1. Product & Company IdentificationDocument7 pagesMaterial Safety Data Sheet: 1. Product & Company Identificationlurab3991No ratings yet
- STP Presentation by JeffconDocument45 pagesSTP Presentation by JeffconCHIN FelleNo ratings yet
- Digestion and Absorption of CarbohydratesDocument25 pagesDigestion and Absorption of CarbohydrateskhadijaNo ratings yet
- Performance Report: Critical Deepwater Frac-Pack Recovery and Successful Workover Attributed To DIPRO SystemDocument2 pagesPerformance Report: Critical Deepwater Frac-Pack Recovery and Successful Workover Attributed To DIPRO SystemRuben Waldir Segarra MoralesNo ratings yet
- Patriot 5510Document2 pagesPatriot 5510Forum Pompierii0% (1)
- Individual Paper Proposal For Biochar Literature ReviewDocument2 pagesIndividual Paper Proposal For Biochar Literature ReviewraiiinydaysNo ratings yet
- Thermodynamics For Chemists, GlasstoneDocument533 pagesThermodynamics For Chemists, GlasstoneRowie Carpio100% (2)
- Pedot:pss/go Nanocomposite For Indoor Co2 SensorDocument9 pagesPedot:pss/go Nanocomposite For Indoor Co2 SensorIJAR JOURNALNo ratings yet
- CH 3. Mass Relations in Chemistry - StoichiometryDocument12 pagesCH 3. Mass Relations in Chemistry - Stoichiometryewewwe weweweweNo ratings yet
- 2.1.2 AlkanesDocument25 pages2.1.2 Alkaneslocus448450No ratings yet
- Catalogue Surfactants PDFDocument16 pagesCatalogue Surfactants PDFMukesh KumarNo ratings yet
- Effect of Filler Concentration On Breakdown of IsotacticDocument15 pagesEffect of Filler Concentration On Breakdown of Isotacticdev sNo ratings yet
- Concrete Plant OperationsDocument28 pagesConcrete Plant OperationsJuned Hamid Khan100% (2)
- Comparative Study of Natural and Synthetic IndicatorsDocument4 pagesComparative Study of Natural and Synthetic IndicatorsJeff BEzosNo ratings yet
- AFW NewDocument4 pagesAFW NewJoão BaffiniNo ratings yet
- Catalog FittingsDocument18 pagesCatalog FittingsBe-ToolNo ratings yet
- Novoperm Yellow M2R 70 A High Quality Pigment For Lead-Free Industrial Paints and Powder CoatingsDocument2 pagesNovoperm Yellow M2R 70 A High Quality Pigment For Lead-Free Industrial Paints and Powder CoatingsMaximiliano MackeviciusNo ratings yet
- MSDS Lem Tikus Papan 2018Document4 pagesMSDS Lem Tikus Papan 2018abdul ropiNo ratings yet
- GroutingDocument4 pagesGroutingDrPadipat ChaemmangkangNo ratings yet