Professional Documents
Culture Documents
Books
Uploaded by
Collin LongCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Books
Uploaded by
Collin LongCopyright:
Available Formats
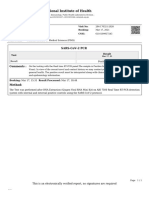
Coronavirus (COVID-19)
SARS-CoV-2 Virus
Dynamic DNA Laboratories
2144 East Republic Road, B204
Springfield, MO 65804
Phone: (417) 319-1047
Laboratory Director: Elaine Allgood, MD
CLIA: 26D2106631
dynamicdnalabs.com
PROVIDER INFORMATION PATIENT INFORMATION SPECIMEN INFORMATION
Site: State of Missouri - SE (Park Name: COLLIN JOSEPH LONG Lab Accession Number: 22-02EF3
Hills) 10239 HWY JJ Specimen Type: Anterior Nare
Physician: George Turabelidze VALLES MINES, MO 63087-0000 Date Collected: 1/19/2022 10:14 AM
Address: 407 Pennsylvania Ave Suite 202 Date Received: 1/20/2022 8:51 AM
Joplin, MO 64801 Phone: 573-664-6860 Date Processed: 1/20/2022 3:02 PM
DOB: 12/03/2001 Date Reported: 1/20/2022 3:03 PM
Phone: Gender: Male
NPI: 1750496246 MRN:
Test Performed Result
SARS-CoV-2 RT-PCR Positive SARS-CoV-2
Methodogy:
Laboratory specimens associated with this report were analyzed using molecular technologies. Total nucleic acids were extracted from the submitted
sample and analyzed using a combination reverse transcription of viral RNA and PCR amplification using real-time reverse transcriptase PCR (rRT-PCR).
Internal controls run simultaneously with patient samples ensure the correct operation of the extraction and RT-PCR steps of this assay.
Inconclusive:
An inconclusive result indicates that viral DNA was detected in the specimen, but at a concentration below the threshold required for a SARS-CoV-2
positive call. Such a result may occur due to low concentrations of SARS-CoV-2 target RNA or cross-reactivity with another strain of coronavirus.
Invalid:
An invalid result indicates that insufficient human genetic material was detected in the specimen. Such a result may occur due to improper sample
collection or sample degradation due to improper transport or storage.
Disclaimer:
This test was developed and its performance characteristics determined by Dynamic DNA Laboratories, LLC. It has not been cleared or approved by the
U.S. Food and Drug Administration and is operated as a laboratory developed test. Results should be used in conjunction with clinical findings, and should
not form the sole basis for a diagnosis or treatment decision. Negative results do not preclude SARS-CoV-2 infection and should not be used as the sole
basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information.
Dynamic DNA follows federal and state requirements for both notification of results and any confirmatory testing that is required by another agency.
Inquiries:
Please contact Dynamic DNA Laboratories at 417.319.1047 or info@dynamicdnalabs.com for assistance with this report. Please be aware that Dynamic
DNA cannot provide heathcare guidance directly to patients. Patients are directed to speak with their primary care physician regarding this information.
References:
https://www.cdc.gov/coronavirus/2019-ncov/index.html
This document contains private and confidential Final Report
heath information protected by state and federal law.
If you have received this report in error, please call
(417) 319-1047
Page 1 of 1
You might also like
- Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedDocument1 pageCovid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedGEr JrvillaruElNo ratings yet
- Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedDocument1 pageCovid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedRodel OrtegaNo ratings yet
- Covid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not DetectedDocument1 pageCovid-19 Test Result Summary: Sars-Cov-2 Viral Rna - Not Detectedpogito ramosNo ratings yet
- MithunDocument1 pageMithunMithun MukherjeeNo ratings yet
- Covid-19 RT-PCR: Test Results PanelDocument1 pageCovid-19 RT-PCR: Test Results PanelPatricia Cottle-SalyerNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedDocument1 pageSars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedKaoruTecsonNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedDocument1 pageSars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedKaoruTecsonNo ratings yet
- Test Name Result Bio. Ref. Range Unit Method: Nasopharyngeal and Oropharyngeal SwabDocument2 pagesTest Name Result Bio. Ref. Range Unit Method: Nasopharyngeal and Oropharyngeal SwabGovind Arun KamatNo ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRDRSM QAUNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not DetectedDocument1 pageSars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not DetectedLorainne MarceloNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedDocument1 pageSars-Cov-2 (Causative Agent of Covid-19 Viral Rna Not DetectedKaoruTecsonNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not DetectedDocument1 pageSars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not Detectedabbey jane mallillinNo ratings yet
- Velasco, Crestita VelosoDocument1 pageVelasco, Crestita VelosoAdan NunungNo ratings yet
- SR4750118 1Document1 pageSR4750118 1ac9467593No ratings yet
- Report-132010930002928 Master ADITYAGADGIL 20mar2021 151347Document3 pagesReport-132010930002928 Master ADITYAGADGIL 20mar2021 151347AtulNo ratings yet
- Molecular Diagnostics: Assay Name Result Sars Cov-2 (Real Time RT-PCR)Document2 pagesMolecular Diagnostics: Assay Name Result Sars Cov-2 (Real Time RT-PCR)AdibNo ratings yet
- 19/jun/2021 06:14PM 32 Yrs/Male 19/jun/2021 12:08PM Dr. G.H. 01190178Document3 pages19/jun/2021 06:14PM 32 Yrs/Male 19/jun/2021 12:08PM Dr. G.H. 01190178Nitin GuptaNo ratings yet
- Madhan - 642161200148401 2Document2 pagesMadhan - 642161200148401 2madhanNo ratings yet
- Covid ReportDocument1 pageCovid ReportGourima BabbarNo ratings yet
- MR Lokesh Wadhey - 9300401789Document2 pagesMR Lokesh Wadhey - 9300401789Aks WadheNo ratings yet
- Check Out This File: COV-350851-1-SARS-CoV-2 - 2019-nCoV-1632039566Document1 pageCheck Out This File: COV-350851-1-SARS-CoV-2 - 2019-nCoV-1632039566Joana Marie DomingoNo ratings yet
- Molecular Biology Sars-Cov-2 (Covid 19) Detection by Real Time PCRDocument2 pagesMolecular Biology Sars-Cov-2 (Covid 19) Detection by Real Time PCRMithileshNo ratings yet
- Lab E Express SDN BHD 137-01, Jalan Bestari 1/5, Taman Nusa Bestari Iskandar Puteri JohorDocument2 pagesLab E Express SDN BHD 137-01, Jalan Bestari 1/5, Taman Nusa Bestari Iskandar Puteri JohorSilvia SilviaNo ratings yet
- Molecular Diagnostics: Assay Name Result Sars Cov-2 (Real Time RT-PCR)Document2 pagesMolecular Diagnostics: Assay Name Result Sars Cov-2 (Real Time RT-PCR)Nifaal E ANo ratings yet
- National Institute of Health: Sars-Cov-2 PCRDocument1 pageNational Institute of Health: Sars-Cov-2 PCRirfan shabbbirNo ratings yet
- Mr. Ravtej Singh: Test Description Observed Value Biological Reference RangeDocument2 pagesMr. Ravtej Singh: Test Description Observed Value Biological Reference Rangevasu jamwalNo ratings yet
- Order Information Ghigliotti Ramos, Ohana T: CommentsDocument1 pageOrder Information Ghigliotti Ramos, Ohana T: CommentsThaiz RamosNo ratings yet
- Order Information Iwama, Moises: CommentsDocument1 pageOrder Information Iwama, Moises: CommentsLuis IwamaNo ratings yet
- Sars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRDocument2 pagesSars-Cov-2 (Covid 19) Detection (Qualitative) by Real Time RT PCRVijay KNo ratings yet
- Covid-19 Qualitative Real Time PCR:: DR - SELFDocument1 pageCovid-19 Qualitative Real Time PCR:: DR - SELFRajdeep DeyNo ratings yet
- Molecular Analysis For Qualitative Detection of Sars-Cov-2.: Negative Negative Negative PassDocument4 pagesMolecular Analysis For Qualitative Detection of Sars-Cov-2.: Negative Negative Negative PassmeezNo ratings yet
- This Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureDocument1 pageThis Is A Computer Generated Form and If Issued Without Any Alteration, This Does Not Require A SignatureIssa LlamasNo ratings yet
- Covid-19 Qualitative Real Time PCR:: DR - SELFDocument1 pageCovid-19 Qualitative Real Time PCR:: DR - SELFSUBHADIPNo ratings yet
- Clinical Genomics Laboratory: Test ResultDocument1 pageClinical Genomics Laboratory: Test ResultPeds Lim PagayatanNo ratings yet
- KPJ Perdana Specialist Hospital Lot PT.37 & PT.600, Seksyen 14, Jalan Bayam, Kota Bahru, KelantanDocument2 pagesKPJ Perdana Specialist Hospital Lot PT.37 & PT.600, Seksyen 14, Jalan Bayam, Kota Bahru, Kelantananuarhussaini.abdullatifNo ratings yet
- Department of Molecular Biology Covid-19 Virus Qualitative PCRDocument2 pagesDepartment of Molecular Biology Covid-19 Virus Qualitative PCRpooja sharmaNo ratings yet
- SR3802595Document1 pageSR3802595om agencyNo ratings yet
- Clinical Genomics Laboratory: Test ResultDocument1 pageClinical Genomics Laboratory: Test ResultPeds Lim PagayatanNo ratings yet
- ANKIT VERMA (DOB - 22-06-1997) INDIAN-Male25 Years-41572 - 230422 - 051746Document2 pagesANKIT VERMA (DOB - 22-06-1997) INDIAN-Male25 Years-41572 - 230422 - 051746Ankit VermaNo ratings yet
- Patientreport - Ayu Mazlina Binti Mohd Kassim - 0220303499Document1 pagePatientreport - Ayu Mazlina Binti Mohd Kassim - 0220303499thundercats mkNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19) Viral Rna Detected (+)Document1 pageSars-Cov-2 (Causative Agent of Covid-19) Viral Rna Detected (+)Dominica PalacioNo ratings yet
- Rensselaer Polytechnic Institute: Patient InformationDocument1 pageRensselaer Polytechnic Institute: Patient InformationTahmidAzizAbirNo ratings yet
- Real Time Qualitative RT-PCR Detection of 2019-nCOV RNA / COVID-19 RNADocument1 pageReal Time Qualitative RT-PCR Detection of 2019-nCOV RNA / COVID-19 RNAHemendra RaiNo ratings yet
- Covid-19 Qualitative Real Time PCR:: DR - SELFDocument1 pageCovid-19 Qualitative Real Time PCR:: DR - SELFBibhas MajumderNo ratings yet
- Department of Serology Covid-19 Antigen Test: Covid 19 Ag Test, Test Name Result Unit Bio. Ref. Range MethodDocument1 pageDepartment of Serology Covid-19 Antigen Test: Covid 19 Ag Test, Test Name Result Unit Bio. Ref. Range MethodPradeep VunnamNo ratings yet
- Sars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not Detected (-)Document1 pageSars-Cov-2 (Causative Agent of Covid-19) Viral Rna Not Detected (-)jeffry billanNo ratings yet
- Medical Officer KPJ Puteri Specialist Hospital No. 33, Jalan Tun Razak (Susur 5) Johor BahruDocument2 pagesMedical Officer KPJ Puteri Specialist Hospital No. 33, Jalan Tun Razak (Susur 5) Johor BahruLynn LynzzNo ratings yet
- RTPCR of AbhigyanDocument3 pagesRTPCR of AbhigyanAbhigyan TiwariNo ratings yet
- Vargas, Juana Dr. Thornton, Karen 17675278Document1 pageVargas, Juana Dr. Thornton, Karen 17675278ahmedNo ratings yet
- Untitled 12Document2 pagesUntitled 12Natasa PrelevicNo ratings yet
- Department of Molecular Virology: COVID-19 (Corona) VirusDocument1 pageDepartment of Molecular Virology: COVID-19 (Corona) VirusMaaz SiddiquiNo ratings yet
- Covid-19 Qualitative Real Time PCR:: DR - Pradip Kumar DasDocument1 pageCovid-19 Qualitative Real Time PCR:: DR - Pradip Kumar Dasdebabrata maitraNo ratings yet
- De La Cuesta, Joseph Adrian - Negative Covid TestDocument3 pagesDe La Cuesta, Joseph Adrian - Negative Covid TestRegi PonferradaNo ratings yet
- Negative: What Does It Mean To Have A Test Result?Document2 pagesNegative: What Does It Mean To Have A Test Result?robertoNo ratings yet
- Ninti Bisht RTPCRDocument1 pageNinti Bisht RTPCRShaikh RoshanNo ratings yet
- AnthonyDocument1 pageAnthonyJ D PatelNo ratings yet
- Dinesh RamDocument1 pageDinesh RamchandanNo ratings yet
- ArvindbhaiDocument1 pageArvindbhaiJ D PatelNo ratings yet
- SARS-CoV-2 Viral Outbreak Investigation: Laboratory Perspective: Clinical Updates in COVID-19From EverandSARS-CoV-2 Viral Outbreak Investigation: Laboratory Perspective: Clinical Updates in COVID-19Rating: 3 out of 5 stars3/5 (1)
- SARS-CoV-2 Viral Outbreak Investigation: Laboratory PerspectiveFrom EverandSARS-CoV-2 Viral Outbreak Investigation: Laboratory PerspectiveNo ratings yet
- YellowstoneDocument1 pageYellowstoneOana GalbenuNo ratings yet
- John Sisler CISSP Study GuideDocument126 pagesJohn Sisler CISSP Study GuideAnthonyNo ratings yet
- Convection Concentric Annulus Vertical Cylinders Filling Porous MediaDocument17 pagesConvection Concentric Annulus Vertical Cylinders Filling Porous MediakarthikeyanNo ratings yet
- Final TestDocument10 pagesFinal TestbennyNo ratings yet
- Sudip Praposal - 1Document20 pagesSudip Praposal - 1Usha BbattaNo ratings yet
- History of Flash Part - 2Document7 pagesHistory of Flash Part - 2YOGESHWER NATH SINGHNo ratings yet
- 7 +Royal+Court+Affairs,+Sultanate+of+OmanDocument12 pages7 +Royal+Court+Affairs,+Sultanate+of+OmanElencheliyan PandeeyanNo ratings yet
- Angewandte: ChemieDocument13 pagesAngewandte: ChemiemilicaNo ratings yet
- Siemens Internship ReportDocument84 pagesSiemens Internship Reportujjawalbhojawala100% (1)
- VTA28-G5: Fuel OptimizedDocument3 pagesVTA28-G5: Fuel OptimizedIslam HemdanNo ratings yet
- Action Analysis For Animators by Chris WebsterDocument409 pagesAction Analysis For Animators by Chris WebsterThomas Yandex100% (8)
- Navy Supplement To The DOD Dictionary of Military and Associated Terms, 2011Document405 pagesNavy Supplement To The DOD Dictionary of Military and Associated Terms, 2011bateljupko100% (1)
- 412 X 7 Va CJ CSDocument1 page412 X 7 Va CJ CSRajesh KumarNo ratings yet
- Texto CuritibaDocument1 pageTexto CuritibaMargarida GuimaraesNo ratings yet
- Superposition and Statically Indetermina - GDLCDocument25 pagesSuperposition and Statically Indetermina - GDLCAnonymous frFFmeNo ratings yet
- The Wild T1 TheodoliteDocument61 pagesThe Wild T1 TheodoliteCJLara100% (1)
- Fentanyl - Wikipedia, The Free EncyclopediaDocument13 pagesFentanyl - Wikipedia, The Free EncyclopediaKeren SingamNo ratings yet
- Complete Processing Lines For Extruded Pet FoodDocument13 pagesComplete Processing Lines For Extruded Pet FoodденисNo ratings yet
- Sw34 Religion, Secularism and The Environment by NasrDocument19 pagesSw34 Religion, Secularism and The Environment by Nasrbawah61455No ratings yet
- Top Coat-200 - Data PDFDocument4 pagesTop Coat-200 - Data PDFLiliana GeorgianaNo ratings yet
- Maha Shivratri: (Shiv Avtaran, Incarnation of God)Document4 pagesMaha Shivratri: (Shiv Avtaran, Incarnation of God)Varsha RoyNo ratings yet
- Vehicle Intercom Systems (VIS)Document4 pagesVehicle Intercom Systems (VIS)bbeisslerNo ratings yet
- 9701 w09 QP 21Document12 pages9701 w09 QP 21Hubbak KhanNo ratings yet
- Bahir Dar University BIT: Faculity of Mechanical and Industrial EngineeringDocument13 pagesBahir Dar University BIT: Faculity of Mechanical and Industrial Engineeringfraol girmaNo ratings yet
- Chapter 10 - The Mature ErythrocyteDocument55 pagesChapter 10 - The Mature ErythrocyteSultan AlexandruNo ratings yet
- LPG GasDocument39 pagesLPG Gasv prasanthNo ratings yet
- Ni Elvis ManualDocument98 pagesNi Elvis ManualZhi YiNo ratings yet
- ME 352 Design of Machine Elements: Lab ReportDocument5 pagesME 352 Design of Machine Elements: Lab ReportKeshav VermaNo ratings yet
- LighthouseDocument4 pagesLighthousejaneborn5345No ratings yet
- Latihan To: Nilai: 7.4 Benar: 37 Salah: 13Document17 pagesLatihan To: Nilai: 7.4 Benar: 37 Salah: 13glensNo ratings yet