Professional Documents
Culture Documents
Guidelines For Human Embryonic Stem Cell Research
Uploaded by
Cindy ZuritaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Guidelines For Human Embryonic Stem Cell Research
Uploaded by
Cindy ZuritaCopyright:
Available Formats
See discussions, stats, and author profiles for this publication at: https://www.researchgate.
net/publication/7741757
Guidelines for human embryonic stem cell research
Article in Nature Biotechnology · August 2005
DOI: 10.1038/nbt0705-793 · Source: PubMed
CITATIONS READS
43 59
2 authors:
Jonathan Moreno Richard O Hynes
University of Pennsylvania Massachusetts Institute of Technology
48 PUBLICATIONS 1,253 CITATIONS 440 PUBLICATIONS 80,649 CITATIONS
SEE PROFILE SEE PROFILE
Some of the authors of this publication are also working on these related projects:
Do you really want to know? View project
All content following this page was uploaded by Jonathan Moreno on 27 December 2017.
The user has requested enhancement of the downloaded file.
C O M M E N TA R Y
Guidelines for human embryonic stem cell
© 2005 Nature Publishing Group http://www.nature.com/naturebiotechnology
research
Jonathan D Moreno & Richard O Hynes
Responding to a lack of US government leadership on the conduct of human embryonic stem cell research,
a National Academies panel has proposed the creation of new institutional oversight committees.
The limited federal role in funding and moni-
toring research involving human embryonic
stem (hES) cells has raised concerns among the
scientific community that the field is progress-
ing without adequate guidance. In response,
the US National Academies set up a commit-
tee, which we cochaired, to develop guide-
lines for the appropriate conduct of hES cell
research. The committee’s report, released in
April 2005, includes both substantive standards
for research and procedures for oversight. It
divides hES cell research into three categories:
research that does not require review beyond
current regulatory mandates, research that
requires more extensive review and research
that is not permissible at this time. We urge
the scientific community and its supporting
institutions to adopt these voluntary guidelines
in order to establish a common framework for
this important field.
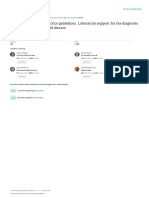
Committee co-chairs Jonathan Moreno and Richard Hynes and committee member Marcia Imbrescia at
The need for guidelines the release of the National Academies report.
As the study of hES cells accelerates worldwide,
federal funding for this research area in the
United States has been severely limited by ethi- in the absence of universal rules for conduct conduct of hES cell research. The guidelines
cal controversy. As a consequence, the normal of the research. In most states, there are no were released April 26, 2005, after eight months
leadership role of the US National Institutes of regulations; in some, the research is illegal, of deliberations, many meetings and a two-day
Health in supporting health-related research either in whole or in part; and, in others, leg- public workshop (http://www.nas.edu/).
and providing the oversight that comes with islation has explicitly legalized research efforts The committee, composed of scientists, phy-
federal funding has been absent. Investigation and even provided public funding. Thus, the sicians, lawyers, ethicists, a social scientist and
of hES cells is being funded increasingly by research is proceeding actively but under a a patient advocate, actively sought to solicit
individual states and by private foundations confusing patchwork of regulations. This situ- a broad range of opinions from the interna-
ation is particularly inappropriate for an area tional scientific and lay public through public
Jonathan D. Moreno is at the Center for of investigation that, although offering great notices. Among those from whom we received
Biomedical Ethics at the University of Virginia. promise, raises ethical issues. comments were stem cell biologists, bioethi-
Richard O. Hynes is at the Massachusetts In response to scientists’ concerns about the cists, members of the President’s Council on
Institute of Technology and is a Howard lack of federal oversight, a committee of the Bioethics, officials of industry and patient
Hughes Investigator. National Research Council and the Institute advocacy groups, and representatives of the US
e-mail: JDM8N@hscmail.mcc.virginia.edu of Medicine of the National Academies under- Food and Drug Administration and the federal
and rohynes@mit.edu took to formulate guidelines for the appropriate Office for Human Research Protections. We
NATURE BIOTECHNOLOGY VOLUME 23 NUMBER 7 JULY 2005 793
C O M M E N TA R Y
also reviewed relevant US reports and inter- IRBs will continue to review aspects of possibility that hES cells could contribute to the
national guidelines, policies and procedures, research that involve human subjects, such as germ line in such chimeras. Finally, no animal
including those of the United Kingdom and gamete or blastocyst donors, or patients who embryonic stem cells should be transplanted
Canada. Fourteen external reviewers from vari- are to receive stem cell therapy. A rigorous into a human blastocyst.
ous disciplines provided important critiques of donor consent process must be in place, and Although the guidelines are directed at US
the penultimate draft. all donors must give explicit, informed consent investigators and institutions, they specify that
for use of their materials in hES cell research. international collaboration is permissible if the
Summary of the recommendations To avoid financial conflicts of interest, no pay- ESCRO committee at the US institution finds
A key part of our recommendations is that ments beyond direct expenses should be made that the collaborating institution provides pro-
© 2005 Nature Publishing Group http://www.nature.com/naturebiotechnology
all hES cell research protocols be reviewed by for gametes or blastocysts, either to the donors tections consistent with the guidelines.
a new institutional panel, an embryonic stem or to the in vitro fertilization clinic. Researchers Finally, our report recommends that a
cell research oversight (ESCRO) committee. may not propose that more blastocysts be cre- national independent body be constituted
Although we recognized the implications of ated by in vitro fertilization clinics than are to review periodically whether the guidelines
recommending another level of bureaucracy, we intended for reproductive purposes. Only after should be updated in light of evolving public
concluded that the burden in this case was justi- reproductive efforts have ceased may potential attitudes and unforeseen advances in stem
fied given the novel and controversial nature of donors be asked to consent to the research use cell science. Although a National Academies
hES cell research and the complexities likely to of leftover blastocysts. Many other protections report cannot assign such responsibilities to
be associated with organizing its oversight. must also be in place for donors, including con- the Academies, they would seem to be one
The ESCRO committee does not replace fidentiality and protection of privacy. possible host of this important public over-
other research compliance bodies, such as The guidelines rule out certain experiments. sight activity.
institutional review boards (IRBs), whose Following a recommendation from a previous
purview is oversight of research on human National Academies report, nuclear transfer The road ahead
subjects, but is intended to complement and must not be used in attempts to reproduce a In the weeks since their publication, the guide-
help coordinate the involvement of IRBs and human being, and no human embryos used in lines have been endorsed by numerous aca-
other pertinent bodies, including animal care research should be grown in culture for longer demic and research organizations. Although
and use committees, biosafety committees and than 14 days or the formation of the primitive our recommendations are not legally binding,
radiation safety committees. ESCRO commit- streak. There is no scientific rationale for the it is our hope and expectation that all interested
tees should include experts in biology and production of a human via nuclear transfer, parties, including investigators, their institu-
stem cell research, legal and ethical experts, and the 14-day standard has been widely recog- tions, private industry and journal editors, will
as well as representatives of the public. The nized since the UK’s Warnock Report of 1984. voluntarily adhere to them and assist in enforc-
ESCRO committee will be the key point of Research will require the creation of chi- ing them. For example, the guidelines could
contact for all investigators working with hES meras—the products of mixing human and be incorporated by reference into university
cells; they should register their lines with their animal cells—both for investigations of the research rules and industry contracts, founda-
institution’s ESCRO committee even if their developmental potential of stem cells (embry- tions could specify adherence to the guidelines
research is limited to in vitro studies not subject onic or adult) or their derivatives and to satisfy as a condition for awarding of grants and jour-
to review by existing human subjects or ani- FDA requirements before clinical applications. nal editors could require written assurance of
mal care committees. Institutions should also Chimeras can involve transfer of human stem compliance by authors before accepting papers
establish (severally or collectively) stem cell cells and their derivatives into postnatal animals for publication.
banks that keep careful records of all aspects or into developing embryos. The guidelines We encourage all those who may participate
of cell culture and source and assure that all state that an ESCRO committee should review in work involving hES cells to familiarize them-
donor consent and confidentiality rules have any proposals to place hES cells into an animal. selves with the guidelines and to commit them-
been satisfied. Uniform, industry-wide stan- Particular consideration and oversight will be selves to the standards they contain. This will
dards should be adopted by each bank. required when it is possible that hES cells could establish a consistent set of rules for the ethi-
The report recommends that ESCRO com- contribute in a major, organized way to brain cal conduct of this important area of scientific
mittees review all proposals for research involv- structures. This is perhaps more likely in the research and provide both a stable scientific
ing hES cells from all sources. These sources case of transfer into early stages such as blasto- environment and assurance to the public that
include unused blastocysts stored at in vitro cysts, and such experiments will require more the research is being conducted with appropri-
fertilization clinics, blastocysts generated careful oversight. Chimeras should not be cre- ate oversight. Failure to implement a common
explicitly for the purpose of generating hES ated if there are alternatives for achieving the standard would not only make interinstitu-
cells (not eligible for federal funding but legal same scientific goal. No hES cells should be put tional cooperation more difficult but would
in several states) and blastocysts created by into nonhuman primate blastocysts, and no ani- also undermine public confidence that the
nuclear transfer (again not eligible for federal mal into which hES cells have been introduced scientific community is proceeding with care
funding but legal in several states). should be allowed to breed, to avoid the unlikely in this sensitive field.
794 VOLUME 23 NUMBER 7 JULY 2005 NATURE BIOTECHNOLOGY
View publication stats
You might also like
- Responsible Conduct of Research Adil e Shamoo and 5ac6f8041723ddcd8f2a73eaDocument17 pagesResponsible Conduct of Research Adil e Shamoo and 5ac6f8041723ddcd8f2a73eaJayaprakashNo ratings yet
- Trust in the system: Research Ethics Committees and the regulation of biomedical researchFrom EverandTrust in the system: Research Ethics Committees and the regulation of biomedical researchNo ratings yet
- Ethics in Stem Cell Research and TherapyDocument6 pagesEthics in Stem Cell Research and TherapySwathi sampathkumarNo ratings yet
- Academic Research Record Keeping Best Practices.10Document6 pagesAcademic Research Record Keeping Best Practices.10TimKellerNo ratings yet
- Un-Ethical Review Why It Is Wrong To Apply The Medical Model of Reasearch Governance To Human GeographyDocument20 pagesUn-Ethical Review Why It Is Wrong To Apply The Medical Model of Reasearch Governance To Human GeographyGut0No ratings yet
- OOK Eview: Responsible Conduct of ResearchDocument4 pagesOOK Eview: Responsible Conduct of ResearchJayaprakashNo ratings yet
- Protein Drops' May Seed Brain Disease: Cellular Droplets Promote Vital Biochemistry-But May Dangerously SolidifyDocument2 pagesProtein Drops' May Seed Brain Disease: Cellular Droplets Promote Vital Biochemistry-But May Dangerously SolidifyGreg GluesniffNo ratings yet
- Zarzeczny 2009Document6 pagesZarzeczny 2009Matias MarinNo ratings yet
- Nature Magazine 7125 - 2007-01-18Document104 pagesNature Magazine 7125 - 2007-01-18Roberto KlesNo ratings yet
- Caulfield Et Al 2008Document6 pagesCaulfield Et Al 2008Ava GodhardtNo ratings yet
- Informed Consent in The Genomics EraDocument4 pagesInformed Consent in The Genomics EraMaria BernalNo ratings yet
- Beyond Informed Consent: Zulfiqar A. BhuttaDocument8 pagesBeyond Informed Consent: Zulfiqar A. BhuttaHestyNo ratings yet
- Stem Cell ReportsDocument9 pagesStem Cell ReportsANZE YOURGANo ratings yet
- Elek Tro For EnsisDocument14 pagesElek Tro For Ensisdella blatamaNo ratings yet
- bmj00593 0041Document3 pagesbmj00593 0041Canache DamianNo ratings yet
- Bookshelf_NBK17483Document134 pagesBookshelf_NBK17483Decan TofikNo ratings yet
- Bureaucracies of Mass DeceptionDocument16 pagesBureaucracies of Mass DeceptionPauline Ochoa LeónNo ratings yet
- Foreword: Human Stem Cell Research and Regenerative MedicineDocument16 pagesForeword: Human Stem Cell Research and Regenerative Medicineapi-28783347No ratings yet
- Novel Techniques For The Prevention of Mitochondrial DNA Disorders CompressedDocument98 pagesNovel Techniques For The Prevention of Mitochondrial DNA Disorders CompressedKP DAVAO CITYNo ratings yet
- SchorePediatricsinReview05 PDFDocument14 pagesSchorePediatricsinReview05 PDFVioleta-Nella MocanuNo ratings yet
- Research Ethics Course GuideDocument28 pagesResearch Ethics Course GuidemubbasherashrafNo ratings yet
- Meeting Highlights Session DescriptionsDocument11 pagesMeeting Highlights Session Descriptionsproud2bemeNo ratings yet
- Ethics and The Welfare of The Physics Profession: Kate Kirby Frances A. HouleDocument6 pagesEthics and The Welfare of The Physics Profession: Kate Kirby Frances A. HouleMaria RitaNo ratings yet
- Inv Futuras en Meditacion y ParanormalidadDocument30 pagesInv Futuras en Meditacion y ParanormalidadIgnacio PisanoNo ratings yet
- Early Child Assessment PDFDocument311 pagesEarly Child Assessment PDFLucía BustamanteNo ratings yet
- Conducting Research On The InternetDocument6 pagesConducting Research On The InternetJosé Manuel Gaete FiscellaNo ratings yet
- Research Ethics and Stem CellsDocument1 pageResearch Ethics and Stem Cellsad3shofNo ratings yet
- How To Write A Thesis Statement On Stem Cell ResearchDocument8 pagesHow To Write A Thesis Statement On Stem Cell ResearchAngel Evans100% (2)
- Ethical Challenges of Research-Behavioral and Social Sciencies Research PDFDocument66 pagesEthical Challenges of Research-Behavioral and Social Sciencies Research PDFcamelia_10i746No ratings yet
- Conceptualización de Los COEDocument9 pagesConceptualización de Los COEEsteban Cáceres PinillaNo ratings yet
- Pone 0156961Document15 pagesPone 0156961Nở TrầnNo ratings yet
- Paper7 1Document18 pagesPaper7 1Journal of Humanities and Social Sciences StudiesNo ratings yet
- INgber 2022 ReviewDocument25 pagesINgber 2022 ReviewAtul MohanNo ratings yet
- Developing a Framework to Identify Research Gaps from Systematic ReviewsDocument6 pagesDeveloping a Framework to Identify Research Gaps from Systematic ReviewsunderwinefxNo ratings yet
- Lab Exercises That Help Substantiate The Cell Theory: A Lesson On CellsDocument15 pagesLab Exercises That Help Substantiate The Cell Theory: A Lesson On CellsJhaii Sumi-og BerongesNo ratings yet
- Cell Turnover and Adult Tissue Homeostasis: From Humans To PlanariansDocument24 pagesCell Turnover and Adult Tissue Homeostasis: From Humans To PlanariansFritzienico BaskoroNo ratings yet
- Stem Cell ProjectDocument15 pagesStem Cell Projectonepiece678912No ratings yet
- The Ethics of Internet ResearchDocument19 pagesThe Ethics of Internet Researchagus maulanaNo ratings yet
- Ethical Issues in Stem Cell Research: Bernard Lo and Lindsay ParhamDocument10 pagesEthical Issues in Stem Cell Research: Bernard Lo and Lindsay ParhamasfwegereNo ratings yet
- Artigo - Carcaterizações HApDocument7 pagesArtigo - Carcaterizações HApAmanda AlvesNo ratings yet
- Week 1- Lecture 2Document21 pagesWeek 1- Lecture 2M HossainNo ratings yet
- Safeguarding The Future of Human Gene EditingDocument1 pageSafeguarding The Future of Human Gene EditingvvvNo ratings yet
- How_ethics_committees_and_requirements_are_structuredDocument12 pagesHow_ethics_committees_and_requirements_are_structuredahiljo127No ratings yet
- Forum of Ethics Review Committees - Sri LankaDocument78 pagesForum of Ethics Review Committees - Sri Lankazhei43No ratings yet
- Embryonic Stem Cell Research Thesis StatementDocument5 pagesEmbryonic Stem Cell Research Thesis Statementcolagiglibridgeport100% (2)
- Strausfeld Et Al 2016 Introduction To Homology and Convergence in Nervous System EvolutionDocument6 pagesStrausfeld Et Al 2016 Introduction To Homology and Convergence in Nervous System EvolutionRaymundo Lopez NNo ratings yet
- Stem Cell Ethics: An Important Consideration in Tissue Engineering AdvancementsDocument5 pagesStem Cell Ethics: An Important Consideration in Tissue Engineering Advancementsaufar syehanNo ratings yet
- Ethical Review of Physiotherapy ResearchDocument6 pagesEthical Review of Physiotherapy ResearchAlexandra NadinneNo ratings yet
- 2008 010 Reevesetal Qualitativeresearchmethodologies EthnographyDocument4 pages2008 010 Reevesetal Qualitativeresearchmethodologies EthnographyPablo PeñarandaNo ratings yet
- EmergingTechnologies Assignment1Document4 pagesEmergingTechnologies Assignment1Roseann BihagNo ratings yet
- 23 Bio SignalsDocument6 pages23 Bio Signalssquirrel_chaserNo ratings yet
- Nature Magazine 7123 - 2007-01-04Document119 pagesNature Magazine 7123 - 2007-01-04Roberto KlesNo ratings yet
- Institutional Ethics Committees: Policy StatementDocument9 pagesInstitutional Ethics Committees: Policy Statementanwini kumNo ratings yet
- Promoting Good Scientific PracticeDocument2 pagesPromoting Good Scientific PracticenicckNo ratings yet
- Opportunities For Early Intervention Based On Theory, Basic Neuroscience, and Clinical ScienceDocument13 pagesOpportunities For Early Intervention Based On Theory, Basic Neuroscience, and Clinical ScienceRama BayuNo ratings yet
- 2011 Revisión Sistemática de Razones ArgumentosDocument6 pages2011 Revisión Sistemática de Razones ArgumentosCamilo Andrés Salas SandovalNo ratings yet
- Estso BatoDocument8 pagesEstso BatoJesusTigroTgmcNo ratings yet
- 03 Na CB GuidelinesDocument152 pages03 Na CB GuidelinesPutri Zahra ArdiyanitaNo ratings yet
- 1 s2.0 S016517811930602X MainDocument6 pages1 s2.0 S016517811930602X MainIim Roatul HamidahNo ratings yet
- Implantation of Depth Electrodes in Children Using Varioguide Frameless Navigation SystemDocument8 pagesImplantation of Depth Electrodes in Children Using Varioguide Frameless Navigation SystemCindy ZuritaNo ratings yet
- Handbook On The Neuropsychology of EpilepsyDocument366 pagesHandbook On The Neuropsychology of EpilepsyLaia GasullNo ratings yet
- Fernández-Rodríguez 2016 Review Real Brain Controlled WheelchairsDocument16 pagesFernández-Rodríguez 2016 Review Real Brain Controlled WheelchairsCindy ZuritaNo ratings yet
- Flanagan Et Al 2014 EEG-fMRI Focal Epilepsy - Local Activation and Regional NetworksDocument11 pagesFlanagan Et Al 2014 EEG-fMRI Focal Epilepsy - Local Activation and Regional NetworksCindy ZuritaNo ratings yet
- Brain Development Using Neurophysiologic and Behavioral Measures ThomasDocument8 pagesBrain Development Using Neurophysiologic and Behavioral Measures ThomasCindy ZuritaNo ratings yet
- Addison - 2005 - Wavelet Transform and ECG - A Review - Physiol MeasDocument46 pagesAddison - 2005 - Wavelet Transform and ECG - A Review - Physiol MeasCindy ZuritaNo ratings yet
- Fathers Involvement During Pregnancy As Described by Expectant Fathers and MothersDocument10 pagesFathers Involvement During Pregnancy As Described by Expectant Fathers and MothersCindy ZuritaNo ratings yet
- Mendoza-Angeles Et Al - 2007 - Slow Waves During Sleep in Crayfish - A Time Frecuency AnalysisDocument8 pagesMendoza-Angeles Et Al - 2007 - Slow Waves During Sleep in Crayfish - A Time Frecuency AnalysisCindy ZuritaNo ratings yet
- Buzsaky y Moser 2013 Theta Entorrinal CXDocument22 pagesBuzsaky y Moser 2013 Theta Entorrinal CXCindy ZuritaNo ratings yet
- Abreviaciones Padecimientos en Medicina-InglesDocument24 pagesAbreviaciones Padecimientos en Medicina-InglesCindy ZuritaNo ratings yet
- Prenatal and Postpartum Depression in Fathers and Its Association PDFDocument9 pagesPrenatal and Postpartum Depression in Fathers and Its Association PDFCindy ZuritaNo ratings yet
- Prenatal and Postpartum Depression in Fathers and Its Association PDFDocument9 pagesPrenatal and Postpartum Depression in Fathers and Its Association PDFCindy ZuritaNo ratings yet
- A Neural Substrate For Atypical Low-Level Visual Processing in Autism Spectrum DisorderDocument12 pagesA Neural Substrate For Atypical Low-Level Visual Processing in Autism Spectrum DisorderCindy ZuritaNo ratings yet
- Bartsch & Wulff - 2015 - The Hippocampus in Aging and DiseaseDocument16 pagesBartsch & Wulff - 2015 - The Hippocampus in Aging and DiseaseCindy ZuritaNo ratings yet
- Bartsch & Wulff - 2015 - The Hippocampus in Aging and DiseaseDocument16 pagesBartsch & Wulff - 2015 - The Hippocampus in Aging and DiseaseCindy ZuritaNo ratings yet
- Plastic Id Ad, Del Musculo Al CerebroDocument31 pagesPlastic Id Ad, Del Musculo Al CerebroCindy ZuritaNo ratings yet
- Zelazo, Frye, 1998 CCC Development EF ChildhoodDocument7 pagesZelazo, Frye, 1998 CCC Development EF ChildhoodCindy ZuritaNo ratings yet
- Autism Spectrum Disorder - Correlation Between Behaviors EEG Abnormalities - and SeizuresDocument6 pagesAutism Spectrum Disorder - Correlation Between Behaviors EEG Abnormalities - and SeizuresCindy ZuritaNo ratings yet
- RH AssementDocument13 pagesRH AssementMerwan KemalNo ratings yet
- Origin of Aids DiseaseDocument13 pagesOrigin of Aids DiseaseRahul SharotriNo ratings yet
- Ps41 2013 Guidelines On Acute Pain ManagementDocument5 pagesPs41 2013 Guidelines On Acute Pain ManagementPratamaAnandaNo ratings yet
- Concept MapDocument2 pagesConcept MapCasas, Jo-an Pauline A.No ratings yet
- COVID-19: Breaking Down A Global Health Crisis: Annals of Clinical Microbiology and AntimicrobialsDocument36 pagesCOVID-19: Breaking Down A Global Health Crisis: Annals of Clinical Microbiology and AntimicrobialsEdo RezaprasgaNo ratings yet
- Nursing Informatic S in EuropeDocument27 pagesNursing Informatic S in EuropeGlenn DanoNo ratings yet
- NIOH Research PaperDocument9 pagesNIOH Research PaperKodeChandrshaekharNo ratings yet
- Sciencefairreserchpaper LogankushnerDocument5 pagesSciencefairreserchpaper Logankushnerapi-347582278No ratings yet
- Guest Lecture 3Document3 pagesGuest Lecture 3Jim XieNo ratings yet
- HRTDocument62 pagesHRTArpita ArpitaNo ratings yet
- The Care Process of Diabetic Foot Ulcer Patients: A Qualitative Study in IranDocument5 pagesThe Care Process of Diabetic Foot Ulcer Patients: A Qualitative Study in IranSofyan IndrayanaNo ratings yet
- NCP - PoliomyelitisDocument4 pagesNCP - PoliomyelitisCassey CuregNo ratings yet
- Test Bank For 3 2 1 Code It 7th Edition by Green Full DownloadDocument12 pagesTest Bank For 3 2 1 Code It 7th Edition by Green Full Downloadrobertabergmjnxqrasct100% (35)
- 328 IndexDocument29 pages328 IndexDafi SanNo ratings yet
- Pelvic Inflammatory DiseaseDocument2 pagesPelvic Inflammatory DiseasejayinthelongrunNo ratings yet
- JT 4412/11 AUG/SIN-DPS Flight PlanDocument62 pagesJT 4412/11 AUG/SIN-DPS Flight PlanWahdhan HadiNo ratings yet
- Introduction To PathologyDocument14 pagesIntroduction To PathologyAlfred ChowNo ratings yet
- Long-Term Surgery Graves Disease 20 YearsDocument3 pagesLong-Term Surgery Graves Disease 20 YearsTambunta TariganNo ratings yet
- Use of A Direct Anatomic Post in A Flared Root Canal: A Three Year Follow UpDocument6 pagesUse of A Direct Anatomic Post in A Flared Root Canal: A Three Year Follow UpJoshua ValdezNo ratings yet
- Buergers Disease NCPDocument5 pagesBuergers Disease NCPNikko Dela Cruz100% (2)
- Daftar Pustaka: Head and Neck Surgery 3Rd Edition. Mcgraw-Hill, San Francisco (HalDocument3 pagesDaftar Pustaka: Head and Neck Surgery 3Rd Edition. Mcgraw-Hill, San Francisco (HalBhisma SuryamanggalaNo ratings yet
- Meta Tags - Cosmodontist DentalDocument2 pagesMeta Tags - Cosmodontist Dentalotos mediaNo ratings yet
- Anesthesiaforemergency Abdominalsurgery: Carol Peden,, Michael J. ScottDocument13 pagesAnesthesiaforemergency Abdominalsurgery: Carol Peden,, Michael J. ScottJEFFERSON MUÑOZNo ratings yet
- Copptech: Successful Tests Against Sars-Cov-2Document2 pagesCopptech: Successful Tests Against Sars-Cov-2enologiacomNo ratings yet
- Eming Report Precipitous LaborDocument11 pagesEming Report Precipitous LaborJudeLaxNo ratings yet
- Panna Dhai Maa Subharti Nursing College, Meerut: Seminar On AbortionDocument31 pagesPanna Dhai Maa Subharti Nursing College, Meerut: Seminar On Abortionriya singhNo ratings yet
- The 6th International Conference on Public Health at Best Western Premier HotelDocument20 pagesThe 6th International Conference on Public Health at Best Western Premier HotelDammar DjatiNo ratings yet
- Analgesic Efficacy of Ketoprofen in Postpartum, General Surgery, and Chronic Cancer PainDocument8 pagesAnalgesic Efficacy of Ketoprofen in Postpartum, General Surgery, and Chronic Cancer PainYohanna Lawanda da CostaNo ratings yet
- Notice: Grants and Cooperative Agreements Availability, Etc.: Women Living in Puerto Rico and U.S. Virgin Islands HIV Prevention ProgramDocument8 pagesNotice: Grants and Cooperative Agreements Availability, Etc.: Women Living in Puerto Rico and U.S. Virgin Islands HIV Prevention ProgramJustia.comNo ratings yet
- FNCP PPWBDocument12 pagesFNCP PPWBMatty JolbitadoNo ratings yet
- Gut: the new and revised Sunday Times bestsellerFrom EverandGut: the new and revised Sunday Times bestsellerRating: 4 out of 5 stars4/5 (392)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisFrom EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisRating: 3.5 out of 5 stars3.5/5 (2)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 4 out of 5 stars4/5 (3)
- Tales from Both Sides of the Brain: A Life in NeuroscienceFrom EverandTales from Both Sides of the Brain: A Life in NeuroscienceRating: 3 out of 5 stars3/5 (18)
- Fast Asleep: Improve Brain Function, Lose Weight, Boost Your Mood, Reduce Stress, and Become a Better SleeperFrom EverandFast Asleep: Improve Brain Function, Lose Weight, Boost Your Mood, Reduce Stress, and Become a Better SleeperRating: 4.5 out of 5 stars4.5/5 (15)
- The Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionFrom EverandThe Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionRating: 4 out of 5 stars4/5 (811)
- The Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorFrom EverandThe Other Side of Normal: How Biology Is Providing the Clues to Unlock the Secrets of Normal and Abnormal BehaviorNo ratings yet
- 10% Human: How Your Body's Microbes Hold the Key to Health and HappinessFrom Everand10% Human: How Your Body's Microbes Hold the Key to Health and HappinessRating: 4 out of 5 stars4/5 (33)
- Gut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)From EverandGut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)Rating: 4 out of 5 stars4/5 (378)
- All That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesFrom EverandAll That Remains: A Renowned Forensic Scientist on Death, Mortality, and Solving CrimesRating: 4.5 out of 5 stars4.5/5 (397)
- Who's in Charge?: Free Will and the Science of the BrainFrom EverandWho's in Charge?: Free Will and the Science of the BrainRating: 4 out of 5 stars4/5 (65)
- Undeniable: How Biology Confirms Our Intuition That Life Is DesignedFrom EverandUndeniable: How Biology Confirms Our Intuition That Life Is DesignedRating: 4 out of 5 stars4/5 (11)
- A Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsFrom EverandA Brief History of Intelligence: Evolution, AI, and the Five Breakthroughs That Made Our BrainsRating: 4.5 out of 5 stars4.5/5 (4)
- Good Without God: What a Billion Nonreligious People Do BelieveFrom EverandGood Without God: What a Billion Nonreligious People Do BelieveRating: 4 out of 5 stars4/5 (66)
- The Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceFrom EverandThe Molecule of More: How a Single Chemical in Your Brain Drives Love, Sex, and Creativity--and Will Determine the Fate of the Human RaceRating: 4.5 out of 5 stars4.5/5 (515)
- The Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindFrom EverandThe Consciousness Instinct: Unraveling the Mystery of How the Brain Makes the MindRating: 4.5 out of 5 stars4.5/5 (93)
- Inside of a Dog: What Dogs See, Smell, and KnowFrom EverandInside of a Dog: What Dogs See, Smell, and KnowRating: 4 out of 5 stars4/5 (390)
- Wayfinding: The Science and Mystery of How Humans Navigate the WorldFrom EverandWayfinding: The Science and Mystery of How Humans Navigate the WorldRating: 4.5 out of 5 stars4.5/5 (18)
- This Is Your Brain On Parasites: How Tiny Creatures Manipulate Our Behavior and Shape SocietyFrom EverandThis Is Your Brain On Parasites: How Tiny Creatures Manipulate Our Behavior and Shape SocietyRating: 3.5 out of 5 stars3.5/5 (31)
- Why We Sleep: Unlocking the Power of Sleep and DreamsFrom EverandWhy We Sleep: Unlocking the Power of Sleep and DreamsRating: 4.5 out of 5 stars4.5/5 (2083)
- Crypt: Life, Death and Disease in the Middle Ages and BeyondFrom EverandCrypt: Life, Death and Disease in the Middle Ages and BeyondRating: 4 out of 5 stars4/5 (3)
- A Series of Fortunate Events: Chance and the Making of the Planet, Life, and YouFrom EverandA Series of Fortunate Events: Chance and the Making of the Planet, Life, and YouRating: 4.5 out of 5 stars4.5/5 (62)
- The Dog Who Couldn't Stop Loving: How Dogs Have Captured Our Hearts for Thousands of YearsFrom EverandThe Dog Who Couldn't Stop Loving: How Dogs Have Captured Our Hearts for Thousands of YearsNo ratings yet
- The Invention of Tomorrow: A Natural History of ForesightFrom EverandThe Invention of Tomorrow: A Natural History of ForesightRating: 4.5 out of 5 stars4.5/5 (5)