Professional Documents
Culture Documents
Any Theft: Law and Molar Volume
Uploaded by
AYA SABAH FAREEDOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Any Theft: Law and Molar Volume
Uploaded by
AYA SABAH FAREEDCopyright:
Available Formats
2022-2-3 11.
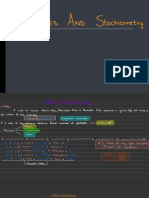
3 Gas volume and the Ideal Gas law
* 21-12+02 21-120
2×2×1 -12×16
2×2×1=49 16×2=32g
369
=
Gray Lassacs law
'
* -
are gases and molar Volume .
Molar volume of gases the volume of of gas and it equals to 22.4L at STP
* }, -
wine
any
Theftandird molar
Volume at gases .
0.350
* I M = 22.41
,
29=44.81 10.5M = 11.51
*
Side 8D 1-1*502 302 +4 Heo
a. In = 0.06851 13=1.93 V= ?
0.350×12×3 ?
22.4--1 M =
7.9×103
16×2 "
b- M -2.21L 0.986 7.9×10-3 7-2×10
M=Zj2y-
- ✗ =
=
22µg , ,,
? = 22.4
* Practice Page 370 "
↳ He -1502 302+41-120 1) 21-12+02=21-120 3) 3. Nor H2O ZHNO } NO
gj?÷
* 0.0171 * → + +
→
=
0.0171
1216,1¥ 0.0125 5=1.74721 4. 55
F- 9.1L 708×1-3=2361
02.23¥
✗
✗ ✗ =
=
0.0125 ✗ 16×2 -0.4
>
" " " " at as constant -
o.odzoam.t.mn
* Ideal Gas law , py ,
NRF
\>
a Temperature ( k)
nun .
of moles
You might also like
- Reaction Kinetics Answers PDFDocument55 pagesReaction Kinetics Answers PDFManish GotameNo ratings yet
- ES 230 Lecture 14 PDFDocument4 pagesES 230 Lecture 14 PDFJessie F. SermanNo ratings yet
- Instructor's Manual to Accompany CALCULUS WITH ANALYTIC GEOMETRYFrom EverandInstructor's Manual to Accompany CALCULUS WITH ANALYTIC GEOMETRYNo ratings yet
- Stem - Physics 2 CGDocument15 pagesStem - Physics 2 CGVictoria Mabini100% (7)
- Sheet Pile Analysis Sheet v1.07Document1 pageSheet Pile Analysis Sheet v1.07SES DESIGNNo ratings yet
- Sharp MX2650-6070 PWB Parts GuideDocument57 pagesSharp MX2650-6070 PWB Parts Guiderob.sheldrakeNo ratings yet
- Elements of Microcontroller Ecosystem, Power Supply For ESDocument38 pagesElements of Microcontroller Ecosystem, Power Supply For ESAmbuj Singh KushwahaNo ratings yet
- KindinDocument2 pagesKindinmur tazaNo ratings yet
- Minortestsep 5Document25 pagesMinortestsep 5chirag birlaNo ratings yet
- Class NotesDocument6 pagesClass NotesBakkashreya SriNo ratings yet
- Riveted JointsDocument21 pagesRiveted JointsPRAVAKAR BOGATINo ratings yet
- Engineering MathematicsDocument14 pagesEngineering MathematicsBayu SaputraNo ratings yet
- Taller Nuclear ProducidaDocument1 pageTaller Nuclear ProducidaximenaNo ratings yet
- Tarea I ResistenciaDocument15 pagesTarea I ResistenciaLeonel José AlvaradoNo ratings yet
- Assignment 1Document2 pagesAssignment 1Ethan ChanNo ratings yet
- Chapter 12 - ComputationalDocument12 pagesChapter 12 - ComputationalBen SzapiroNo ratings yet
- Trojkstg: MoneyDocument2 pagesTrojkstg: MoneyPrzemek OblińskiNo ratings yet
- Generating Patterns #2Document1 pageGenerating Patterns #2Broom botNo ratings yet
- TrigonometriaDocument10 pagesTrigonometriaBesenyői-Szabó EszterNo ratings yet
- Física II Lab01Document2 pagesFísica II Lab01GIANCARLOS KEVIN HUATUCO SOVERONo ratings yet
- 未命名的筆記本Document1 page未命名的筆記本henry03306No ratings yet
- Parcial 2Document2 pagesParcial 2Rangel Xavier Gonzalez DiazNo ratings yet
- Talleres de Principios de Análisis QuímicoDocument11 pagesTalleres de Principios de Análisis QuímicoJuan Pablo Aparicio LeyvaNo ratings yet
- r ρ l lg d lg 2× 3: elv sol v v v vDocument2 pagesr ρ l lg d lg 2× 3: elv sol v v v vDallas GabrielNo ratings yet
- Tuition MathsDocument60 pagesTuition MathsNavien sivarajanNo ratings yet
- ChemistryDocument2 pagesChemistryeve kidonNo ratings yet
- Ejemplos Diseño Muros SDPWS-NCh1198Document8 pagesEjemplos Diseño Muros SDPWS-NCh1198Ignacio A. GonzalezNo ratings yet
- Solución 2o Parcial - 1S2022Document12 pagesSolución 2o Parcial - 1S2022Josue LemusNo ratings yet
- Time SeriesDocument7 pagesTime SeriesVy TrầnNo ratings yet
- .Tl/-Ili2-Oemdmetodaluigamer, Seisezesolreriste-: Sistema - 1912%+9133 61Document6 pages.Tl/-Ili2-Oemdmetodaluigamer, Seisezesolreriste-: Sistema - 1912%+9133 61Hajdeu MaximNo ratings yet
- תרגול 7Document2 pagesתרגול 7ofek kolaNo ratings yet
- תרגול גנטיקה נח שמלה סופיDocument3 pagesתרגול גנטיקה נח שמלה סופיNoah ChemlaNo ratings yet
- Tugas Nci DeibyDocument7 pagesTugas Nci DeibyRisma TirangkaNo ratings yet
- TF06 P11 Final CorDocument5 pagesTF06 P11 Final Corthekrump0% (1)
- Esti Param 29Document5 pagesEsti Param 29Walter Varela RojasNo ratings yet
- Moles and StoichiometryDocument46 pagesMoles and StoichiometryInspector Chulbul PandayNo ratings yet
- Analisis Plastis Baja 2Document10 pagesAnalisis Plastis Baja 2khusnul hakimNo ratings yet
- Les Faisal 20 November 2022Document8 pagesLes Faisal 20 November 2022faisal adhiNo ratings yet
- Analisis Regresi SederhanaDocument3 pagesAnalisis Regresi Sederhanasri nur aulyaNo ratings yet
- Ejemplo Diseño A TensionDocument11 pagesEjemplo Diseño A TensionLucia MurilloNo ratings yet
- Ch8 SecondOrder (140362) PDFDocument19 pagesCh8 SecondOrder (140362) PDFNongPhatNo ratings yet
- Sheet Pile Analysis Sheet v1.07-18.1Document2 pagesSheet Pile Analysis Sheet v1.07-18.1SES DESIGN100% (1)
- FCXT-BFSDSC-GCW - TN Hardest First: Go GodDocument8 pagesFCXT-BFSDSC-GCW - TN Hardest First: Go GodsmvnbbjpwrNo ratings yet
- Sahil JEEDocument5 pagesSahil JEESandeep TyagiNo ratings yet
- Exercise 5Document19 pagesExercise 5Benjamin KjelbyNo ratings yet
- Hw8 PDFDocument6 pagesHw8 PDFDisanee SuphawatdisakulNo ratings yet
- Assignment 5Document8 pagesAssignment 5Alex-Jaden DurandNo ratings yet
- Suhu Dan Teori Kinetik GasDocument8 pagesSuhu Dan Teori Kinetik GasCarrisa Ferly WidjayaNo ratings yet
- MA3006 Tutorials (7 To 10) AnswersDocument12 pagesMA3006 Tutorials (7 To 10) AnswersWilliam LuaNo ratings yet
- k1 Jawapan Trial Negeri Sembilan 2021Document27 pagesk1 Jawapan Trial Negeri Sembilan 2021jimmy palikatNo ratings yet
- Soil Dynamics HW 5Document5 pagesSoil Dynamics HW 5jobaydaNo ratings yet
- Pavement Assignment 7 - M6201074Document7 pagesPavement Assignment 7 - M6201074Kongsak AkkharawongwhatthanaNo ratings yet
- FenoliDocument3 pagesFenolitiberiu costinNo ratings yet
- Atoms, Molecules and StoichiometryDocument10 pagesAtoms, Molecules and StoichiometrySoma Chowdhury RosyNo ratings yet
- Tarea+3 +Version+Preliminar+2-2023Document12 pagesTarea+3 +Version+Preliminar+2-2023sebastian.venegas.castroNo ratings yet
- Examen Control2Document3 pagesExamen Control2ricardo martinez albertoNo ratings yet
- Exercise I4Document6 pagesExercise I4Royal StxrNo ratings yet
- Tarea #4: Jose Daniel Alejandro Guzman 201718954Document8 pagesTarea #4: Jose Daniel Alejandro Guzman 201718954Daniel GuzmanNo ratings yet
- Tswelnoctm"8": R2 (Raislamientol Re)Document1 pageTswelnoctm"8": R2 (Raislamientol Re)Rebeca GarzaNo ratings yet
- Ejemplo Parcial 1Document7 pagesEjemplo Parcial 1Fernando RuizNo ratings yet
- 1 Fundamentos Básicos Del Equilibrio Químico ZoomDocument14 pages1 Fundamentos Básicos Del Equilibrio Químico ZoomjaclavijopNo ratings yet
- CHEMISTRYDocument1 pageCHEMISTRYAYA SABAH FAREEDNo ratings yet
- CHEMISTRYDocument1 pageCHEMISTRYAYA SABAH FAREEDNo ratings yet
- CHEMISTRYDocument1 pageCHEMISTRYAYA SABAH FAREEDNo ratings yet
- CHEMISTRYDocument1 pageCHEMISTRYAYA SABAH FAREEDNo ratings yet
- Solu HWset 9Document16 pagesSolu HWset 9AmalinaNo ratings yet
- Ariston Spare Part CatalougeDocument19 pagesAriston Spare Part CatalougeNuboogh ErbilNo ratings yet
- Chapter 03Document16 pagesChapter 03t1m0thyNo ratings yet
- 1.6 MomentumDocument4 pages1.6 MomentumkookiemonsterNo ratings yet
- Mechanicsssssss ExamplesDocument9 pagesMechanicsssssss ExamplesJhonrey Joey DesabilleNo ratings yet
- June 2008 MS - M1 EdexcelDocument7 pagesJune 2008 MS - M1 EdexcelKollol KolllolNo ratings yet
- CentroidDocument7 pagesCentroidAnit MollaNo ratings yet
- Measuring Time, Speed and DistanceDocument3 pagesMeasuring Time, Speed and DistanceDaryl OribiadaNo ratings yet
- Project ReportDocument122 pagesProject Reportck13011992No ratings yet
- News - Free Cooling Using Water Economizers PDFDocument8 pagesNews - Free Cooling Using Water Economizers PDFlipi8No ratings yet
- Chapter 12: Three-Phase Transformers: 10/13/2003 Electromechanical Dynamics 1Document15 pagesChapter 12: Three-Phase Transformers: 10/13/2003 Electromechanical Dynamics 1Berthy EtoneNo ratings yet
- Euler Equation For Pump & TurbinesDocument4 pagesEuler Equation For Pump & Turbineschakravarthime100% (2)
- Falling CatDocument39 pagesFalling CatKhairul ZakirahNo ratings yet
- 2940-Article Text-13743-1-10-20200330Document12 pages2940-Article Text-13743-1-10-20200330Luthfi AdyNo ratings yet
- Incropera Ex 2.2 7 Ed.Document2 pagesIncropera Ex 2.2 7 Ed.luanremNo ratings yet
- Different Types of Renewable EnergiesDocument1 pageDifferent Types of Renewable EnergiesJamlick KibuchiNo ratings yet
- Orders of Magnitude of PressueDocument6 pagesOrders of Magnitude of PressueAkilaIyerNo ratings yet
- GRAVITATIONDocument3 pagesGRAVITATIONvaniak01028No ratings yet
- Experiment 1 - Crank and RodDocument3 pagesExperiment 1 - Crank and Rodalextty75% (12)
- Forces Assignment: (57 Marks)Document2 pagesForces Assignment: (57 Marks)Lai Kee KongNo ratings yet
- Triac: SCR Can Be Used To Control Lamps, Motors, or Heaters Etc. However, One of TheDocument9 pagesTriac: SCR Can Be Used To Control Lamps, Motors, or Heaters Etc. However, One of TheMohsin Iqbal Department of Electrical EngineeringNo ratings yet
- ABB Interruptor Diferencial F804Document12 pagesABB Interruptor Diferencial F804Arthur E-n SpinosaNo ratings yet
- 2020 Australian Science Olympiad Exam: PhysicsDocument18 pages2020 Australian Science Olympiad Exam: PhysicsmarettaNo ratings yet
- Sistem Instalasi MekanikalDocument156 pagesSistem Instalasi MekanikalDharmestha DewantoroNo ratings yet
- Paper 2: Energy Efficiency in Thermal Systems Section ADocument6 pagesPaper 2: Energy Efficiency in Thermal Systems Section Amd hasanuzzamanNo ratings yet
- Black Holes and Holography Course NotesDocument71 pagesBlack Holes and Holography Course NotesLeonardoSanhuezaMardonesNo ratings yet