Professional Documents
Culture Documents
The Dept Experiment:: Polarzation Transfer, Known As DEPT. It Gives The Number of
Uploaded by
Lootus Flower0 ratings0% found this document useful (0 votes)
1 views12 pagesOriginal Title
13C-DEPT
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
1 views12 pagesThe Dept Experiment:: Polarzation Transfer, Known As DEPT. It Gives The Number of
Uploaded by
Lootus FlowerCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 12
THE DEPT EXPERIMENT:
• Another technique called Distortionless Enhancement by
Polarzation Transfer, known as DEPT. It gives the number of
hydrogens attached to a given carbon atom.
• We have three experiment of DEP.
• DEPT-45, only carbon atoms that bear an attached
hydrogen will produce a peak.
• (DEPT-90) shows peaks only for those carbon atoms that
are part of a methine (CH) group. In a DEPT-135 spectrum,
methine and methyl carbons give rise to positive peaks,
whereas methylene carbons appear as inverse peaks.
Quaternary carbons, which have no attached hydrogens are
not recorded in a DEPT experiment.
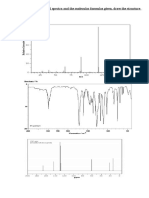
A sample DEPT plot for isopentyl acetate is shown in
the following figure
•The lowest trace in the figure is the usual broad-band-decoupled
13C spectrum. The second trace from the bottom (called a DEPT-
45) in which the only signals detected are those that arise from
protonated carbons. The third trace (called a DEPT-90) shows
those carbons that bear a single hydrogen are seen. The
uppermost trace is called DEPT-135. shows two phases depending
on whether the number of attached hydrogens is an odd or an
even number. Signals arising from CH or CH3 groups will give
positive peaks, while signals arising from CH2 groups will form
negative (inverse) peaks.
• The DEPT-135 spectrum of isopentyl acetate
shows in a positive phase a tallest signal
corresponding to two methyl groups at 22.3 ppm,
shorter signal of the acetyl group at 20.8 ppm
and the methine is still smaller peak at 24.9 ppm.
Negative peaks at 37.1 and 63.0 ppm
corresponding to 2 methylene groups. The
deshielded one is OCH2. The carbonyl carbon (5)
does not appear in the DEPT spectrum since it
has no attached hydrogen atoms.
• The proton-decoupled l3C NMR spectrum of
citronellol shows the peaks at 131 ppm assigned
to carbon 7, while the taller peak at 125 ppm
must arise from carbon 6, which has an attached
hydrogen. The DEPT spectrum of citronellol
shows the positive peak at 125 ppm to carbon 6.
Peak at 131 ppm is missing in the DEPT
spectrum, since carbon 7 has no attached
hydrogens.
•The peak at 61 ppm is negative in the DEPT-135
spectrum indicating that it is due to a methylene
group and it is deshielded by OH.
13CNMR spectrum of citronellol
DEPT-135 spectrum of citronellol
• The three methyl carbons appear at the highest
values of magnetic field and give positive peaks
in the DEPT-135 spectrum.
• We can assign the peak at 17 ppm to carbon, 8
and the peak at 19 ppm to carbon 10 The peak at
25 ppm is actually two peaks, appearing by
coincidence at the same value of chemical shift.
• The DEPT-135 spectrum shows clearly that one
of the peaks is positive (corresponding to the
methyl carbon at C9) and the other is negative
(corresponding to the methylene carbon at C5).
•The positive peak remains must correspond to
the methine position at C3 (30 ppm). The two
remaining negative peaks (at 37 and 40 ppm) are
assigned to the methylene carbons at C4 and C2.
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- C-13 NMR and DEPTDocument41 pagesC-13 NMR and DEPTV G Viju Kumar100% (1)
- XXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973From EverandXXIVth International Congress of Pure and Applied Chemistry: Plenary and Main Section Lectures Presented at Hamburg, Federal Republic of Germany, 2–8 September 1973No ratings yet
- Triplet (HB, 8H) - As Predicted, A Singlet Signal Around 6.9 PPM Belongs To Aromatic Methine (2H)Document4 pagesTriplet (HB, 8H) - As Predicted, A Singlet Signal Around 6.9 PPM Belongs To Aromatic Methine (2H)Anonymous f8P42082UNo ratings yet
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionFrom EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionNo ratings yet
- Spectroscopy ManualDocument21 pagesSpectroscopy Manualanthor100% (2)
- Organic Chemistry Study Guide: Key Concepts, Problems, and SolutionsFrom EverandOrganic Chemistry Study Guide: Key Concepts, Problems, and SolutionsRating: 3.5 out of 5 stars3.5/5 (10)
- Handout4 Cyclopentadienyl LigandDocument6 pagesHandout4 Cyclopentadienyl LigandMior AfiqNo ratings yet
- Chem 317 - Lab 7 - Arene II ReportDocument15 pagesChem 317 - Lab 7 - Arene II Reportapi-251758870100% (4)
- C13 NMRDocument11 pagesC13 NMRShahzadNo ratings yet
- Carbon Nuclear Magnetic Resonance (13C-NMR) SpectrosDocument3 pagesCarbon Nuclear Magnetic Resonance (13C-NMR) SpectrosBhushan AmruteNo ratings yet
- Report NMRDocument13 pagesReport NMRsarahNo ratings yet
- NOTE - Aldehyde and KetoneDocument29 pagesNOTE - Aldehyde and KetoneDeevanesh GengatharanNo ratings yet
- Structure Determination: Mass SpectrometryDocument3 pagesStructure Determination: Mass SpectrometrySharmeen HelalNo ratings yet
- Methoxyphenol (Although 3-Allyl-4-Methoxyphenol Can't Be Excluded Either) - Thus, The ProtonDocument5 pagesMethoxyphenol (Although 3-Allyl-4-Methoxyphenol Can't Be Excluded Either) - Thus, The ProtonAnonymous f8P42082UNo ratings yet
- Chapter 3Document64 pagesChapter 3Danson FanNo ratings yet
- 22 and Applications of C NMR: Subject ChemistryDocument13 pages22 and Applications of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- Carbon 13 SpectrosDocument53 pagesCarbon 13 SpectrossaheedvkNo ratings yet
- OrganicDocument93 pagesOrganicPatel MswaziNo ratings yet
- CH637 Final1key f02Document13 pagesCH637 Final1key f02Fahad RashidNo ratings yet
- Interpreting C NMRDocument21 pagesInterpreting C NMRMuhamad ZakyNo ratings yet
- Organic Chemistry: AlkanesDocument39 pagesOrganic Chemistry: AlkanesYu DhaNo ratings yet
- Nuclear Over Hauser Enhancement (NOE)Document18 pagesNuclear Over Hauser Enhancement (NOE)Fatima AhmedNo ratings yet
- Carbon - 13 NMR: Nuclear Magnetic Resonance SpectrosDocument35 pagesCarbon - 13 NMR: Nuclear Magnetic Resonance SpectrosSri Rezeki SamosirNo ratings yet
- CHMBD 449 - Organic Spectral: AnalysisDocument40 pagesCHMBD 449 - Organic Spectral: AnalysisIleana ManciuleaNo ratings yet
- c13ppt 150515121301 Lva1 App6892Document16 pagesc13ppt 150515121301 Lva1 App6892kiran yaseenNo ratings yet
- Arene-Molybdenum Lab ReportDocument7 pagesArene-Molybdenum Lab Reportapi-245391028100% (1)
- Chemguide - Answers: H-1 NMR: High ResolutionDocument2 pagesChemguide - Answers: H-1 NMR: High ResolutionKhondokar TarakkyNo ratings yet
- A2 Chemistry Revision NotesDocument13 pagesA2 Chemistry Revision NotesJobe Bryer50% (4)
- Notes 14C CNMRDocument5 pagesNotes 14C CNMRTux BsdNo ratings yet
- Learning Outcome: Reaction of Alkyl Halides With Organometals Reduction of Alkyl HalidesDocument46 pagesLearning Outcome: Reaction of Alkyl Halides With Organometals Reduction of Alkyl HalidesSàtz ÑÖÑïtNo ratings yet
- Tutorial 5 RingkasanDocument21 pagesTutorial 5 RingkasanHana NisrinaNo ratings yet
- Orgo Spectroscopy KeyDocument6 pagesOrgo Spectroscopy KeysikandarmirzaNo ratings yet
- Unknown 3 SmaugDocument8 pagesUnknown 3 Smaugapi-248990050No ratings yet
- ChemistryDocument70 pagesChemistryKieran SangheraNo ratings yet
- Final Exam KeyDocument12 pagesFinal Exam KeykitthiNo ratings yet
- C NMR Spectroscopy Worksheet (30 Points) Due 2/24/11 in LectureDocument4 pagesC NMR Spectroscopy Worksheet (30 Points) Due 2/24/11 in LectureNurillahi Febria LeswanaNo ratings yet
- HW 9Document5 pagesHW 9Suryakant Pandey0% (1)
- Analytical TechniquesDocument6 pagesAnalytical TechniquesahumanbeinginearthNo ratings yet
- 5.13 Drill Problems For 9/17/03-9/24/03: O O O H ZNCL Cucl H O O O H HDocument2 pages5.13 Drill Problems For 9/17/03-9/24/03: O O O H ZNCL Cucl H O O O H HEvaBravoNo ratings yet
- Alkanes, Alkenes and AlkynesDocument65 pagesAlkanes, Alkenes and AlkynesTeresita CamachoNo ratings yet
- 13-C NMR-09Document27 pages13-C NMR-09M Nur M. MahmudNo ratings yet
- Aldehydes and KetonesDocument7 pagesAldehydes and KetonesAshok PradhanNo ratings yet
- Reactive Intermediates in Organic Chemistry Structure, Mechanism, and Reactions by Maya Shankar SinghDocument9 pagesReactive Intermediates in Organic Chemistry Structure, Mechanism, and Reactions by Maya Shankar SinghSaman AkramNo ratings yet
- Mass SpectrometryDocument40 pagesMass SpectrometryvitrekNo ratings yet
- HydrocarbonDocument33 pagesHydrocarbonaleenashaji.abraham1No ratings yet
- Spectroscopy and ChromatographyDocument7 pagesSpectroscopy and ChromatographyPa GesNo ratings yet
- ALKANES2Document41 pagesALKANES2Shiki Asagami BrunestedNo ratings yet
- Week 3 Alkanes and CycloalkanesDocument69 pagesWeek 3 Alkanes and Cycloalkanesjojojhinno rosalesNo ratings yet
- Grade 12 Chemistry Organic Chemistry I HydrocarbonsDocument92 pagesGrade 12 Chemistry Organic Chemistry I Hydrocarbonsraadumar02No ratings yet
- 13C NMRDocument40 pages13C NMRKrishna BurakaNo ratings yet
- I. Given Data and Known Spectral Parameters: Gc-MsDocument9 pagesI. Given Data and Known Spectral Parameters: Gc-Msapi-303230363No ratings yet
- Introduction To Orgnic ChemistryDocument27 pagesIntroduction To Orgnic ChemistryladybugNo ratings yet
- NMR N M R: Uclear Agnetic EsonanceDocument33 pagesNMR N M R: Uclear Agnetic EsonanceCarolina AlpucheNo ratings yet
- Alkanes: IB Chemistry Topic 10.2Document20 pagesAlkanes: IB Chemistry Topic 10.2Ravi RanjanNo ratings yet
- Organic Chemistry 1Document110 pagesOrganic Chemistry 1Mahmoud RslanNo ratings yet
- Example 1:: Types of Compounds The Alkanes 2-MethylpentaneDocument8 pagesExample 1:: Types of Compounds The Alkanes 2-MethylpentanekalloliNo ratings yet
- CyclopentadieneDocument27 pagesCyclopentadieneNilmani SinghNo ratings yet
- Identification of Unknown CompoundsDocument50 pagesIdentification of Unknown CompoundsFilemonEndhyPutraKesumaNo ratings yet
- Module 2Document12 pagesModule 2AbhijeetNo ratings yet
- Khairat Al-Emar Co. For Oil Services: Material Safety Data SheetDocument6 pagesKhairat Al-Emar Co. For Oil Services: Material Safety Data SheetqwaszxcdeNo ratings yet
- SPE Turkey Can Bakiler PDFDocument42 pagesSPE Turkey Can Bakiler PDFZhunio BenavidesNo ratings yet
- Prria MemoDocument4 pagesPrria MemoRebecca C. LewisNo ratings yet
- Common Forms OF MedicationDocument19 pagesCommon Forms OF MedicationJonica AngNo ratings yet
- Chlorine Dioxide: Chlorophenol Red Method Method 8065 0.01 To 1.00 MG/L Clo (LR)Document6 pagesChlorine Dioxide: Chlorophenol Red Method Method 8065 0.01 To 1.00 MG/L Clo (LR)Oudah AliNo ratings yet
- Edwards CP25K Cold Cathode Gauge Sensor ManualDocument18 pagesEdwards CP25K Cold Cathode Gauge Sensor Manualঅর্ণব কোলেNo ratings yet
- Nadcap 2020Document4 pagesNadcap 2020amirkhakzad498No ratings yet
- B31.3 Course Handout IntroDocument0 pagesB31.3 Course Handout IntroNeily LiuNo ratings yet
- Exhibitor ListDocument5 pagesExhibitor ListAanchal DasNo ratings yet
- Iso AnnealingDocument2 pagesIso AnnealingPurushottam Sutar100% (1)
- Design and Analysis of Shock AbsorberDocument12 pagesDesign and Analysis of Shock AbsorberSILAMBARASANNo ratings yet
- Mineralogi 1Document90 pagesMineralogi 1baihaqiNo ratings yet
- Archer Pipe Support StandardDocument125 pagesArcher Pipe Support StandardjeddijNo ratings yet
- En 50216-10Document16 pagesEn 50216-10Mahmoud ShaheenNo ratings yet
- Welding Purge Panel WPP Series: Features and BenefitsDocument2 pagesWelding Purge Panel WPP Series: Features and BenefitsAlexNo ratings yet
- Food Chemistry: M.S. Altaki, F.J. Santos, M.T. GalceranDocument6 pagesFood Chemistry: M.S. Altaki, F.J. Santos, M.T. GalceranAberuNo ratings yet
- TB400 Painting and Corrosion ProtectionDocument21 pagesTB400 Painting and Corrosion ProtectionAliZenatiNo ratings yet
- Saic Q 1048Document1 pageSaic Q 1048Gian Carlo100% (1)
- Chapter 13: SolutionsDocument18 pagesChapter 13: SolutionsBSNo ratings yet
- Vermicomposting GuideDocument34 pagesVermicomposting GuideRobin RheaumeNo ratings yet
- EHV Cables Laying MethodDocument22 pagesEHV Cables Laying MethodSudharsanan Sitrarasu100% (2)
- Bulan AgustusDocument22 pagesBulan AgustusaristhanovyraNo ratings yet
- Risk Assesment For Sand Blasting and PaintingDocument6 pagesRisk Assesment For Sand Blasting and PaintingRochdi Bahiri100% (10)
- Concentration Term Jee Main Selected 2Document3 pagesConcentration Term Jee Main Selected 2aebafbigiNo ratings yet
- 162 - Post Graduate Diploma Fire Safety and Disaster Management SyllabusDocument10 pages162 - Post Graduate Diploma Fire Safety and Disaster Management SyllabusDebayanbasu.juNo ratings yet
- Bio T4 DLP KSSM Chapter 13 Homeotasis and The Human Urinary SystemDocument66 pagesBio T4 DLP KSSM Chapter 13 Homeotasis and The Human Urinary SystemNurasyikin SaidinNo ratings yet
- FlotacionDocument2 pagesFlotacionJose Ariel TorrezNo ratings yet
- Axis TurbineDocument7 pagesAxis TurbineBhertrand GomesNo ratings yet
- Lactic AcidDocument54 pagesLactic AcidchadewiNo ratings yet
- EML 3701 Quiz 2 SP2017 SolutionDocument3 pagesEML 3701 Quiz 2 SP2017 Solutionthez100% (3)