Professional Documents
Culture Documents
Method Development For Visible Spectrophotometric Analysis of Ibuprofen in Pharmaceuticals
Method Development For Visible Spectrophotometric Analysis of Ibuprofen in Pharmaceuticals
Uploaded by
Cristian-Catalin GavatOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Method Development For Visible Spectrophotometric Analysis of Ibuprofen in Pharmaceuticals
Method Development For Visible Spectrophotometric Analysis of Ibuprofen in Pharmaceuticals
Uploaded by
Cristian-Catalin GavatCopyright:
Available Formats
The 9th IEEE International Conference on E-Health and Bioengineering - EHB 2021
Grigore T. Popa University of Medicine and Pharmacy, Web Conference, Romania, November 18-19, 2021
Method Development for Visible
Spectrophotometric Analysis of Ibuprofen in
Pharmaceuticals
Cristian Catalin Gavat1 and Afrodita Doina Marculescu2
Affiliation 1: Biomedical Sciences Department, Faculty of Medical Bioengineering, University of Medicine and Pharmacy
GRIGORE T. POPA, UMF, Iasi, Romania, ccgavat70@yahoo.com
Affiliation 2: Biochemistry Department, Faculty of General Medicine, University of Medicine and Pharmacy GRIGORE T.
POPA, UMF, Iasi, Romania, afroditadoina@yahoo.com
Abstract— Ibuprofen is a prominent member of the group of Spectrophotometric analysis of Ibuprofen in the Visible
non-steroidal anti-inflammatory drugs (NSAIDs), with good anti- range (VIS) has always been an important concern in
inflammatory action, a very effective analgesic, with increased pharmaceutical research. This paper, has developed,
antipyretic effect. The aim of this research was to exactly improved, and optimized a spectrophotometric method, based
quantify pure Ibuprofen content in tablets of a pharmaceutical, on literature data [6], which has been successfully applied for
by a spectrophotometric analysis method in the Visible range. quantitative analysis of Ibuprofen from pharmaceuticals and
Ibuprofen was quantitatively converted to a bright orange dye subjected to statistical analysis [7]. The standards and rules of
with a yellowish shade, by a color reaction with alpha- the European and International Pharmacopoeias, taken over by
naphthylamine in the presence of sodium nitrite, in an absolute
the Romanian Pharmacopeia X-th Edition, attests that for an
ethanol medium. Following the analysis, it was found 397.952
milligrams of pure Ibuprofen content / film-coated tablet of the
officially declared content of active substance on
pharmaceutical product. This value was very close to Ibuprofen pharmaceuticals, of 100 mg and above 100 mg, the maximum
content declared by the pharmaceutical manufacturer (400 allowed percentage deviation (percentage error) is no more
milligrams), with a mean deviation of only 0.512 percent from the than 5% [8].
officially declared amount of active substance. The value found
fits perfectly within the normal limits provided by the European II. MATERIALS AND METHODS
and International Pharmacopoeias standards, taken over by the
Romanian Pharmacopoeia, 10th Edition. The spectrophotometric A. Materials
analysis method was then successfully subjected to statistical
analysis. The equipment and materials used for this study consisted
Keywords— Ibuprofen; spectrophotometric analysis; anti- of a Spectrophotometer UV-Visible CE 1021, CECIL®
inflammatory action; film-coated tablet; statistical analysis Bandwidth: 8 nm and 1 cm glass cuvettes, with Deuterium
lamp disconnect key (on model CE1021) used for performing
I. INTRODUCTION the measurements; digital analytical balance with electronic
display Kern ABS / ABJ with 4 decimals; crystalline
Ibuprofen, a propionic acid derivative, is a non-steroidal extremely pure standard of ibuprofen powder, supplied by
anti-inflammatory drug part of the NSAIDs family. It is a very Sigma-Aldrich ®; a stock solution of ibuprofen, 1000 µg/mL
effective analgesic with a good antipyretic effect. It also has a
(0.1 g %), prepared from the crystalline pure standard; a
strong anti-inflammatory action. Ibuprofen was introduced on
standard working solution 150 µg/mL obtained directly from
the marketplace in 1969, as a better alternative to Aspirin.
Gastric discomfort, nausea, and vomiting effects, though less the stock solution Other solutions used consisted of alpha-
intense than in the case of aspirin or indomethacin, are still the naphthylamine 0.04% and a sodium nitrite NaNO2 4%
most common side effects [1-4]. It is the most commonly used solution. Ibuprofen Grindex® film-coated tablets,
and most frequently prescribed NSAID. It is a non-selective B. Methods
inhibitor of cyclo-oxygenase-1 (COX-1) and Cyclooxygenase-
2 (COX-2). Although its anti-inflammatory properties may be The wavelength corresponding to the maximum absorption
weaker than those of some other NSAIDs like Piroxicam, the of the bright orange azo-dye obtained with a yellowish shade,
Coxib family, Indomethacin, Ketorolac, it has a prominent, the absorption spectrum shown in Fig. 1 was drawn for a
strong analgesic action and an increased antipyretic role [1-5]. separate standard solution of Ibuprofen, 8 µg/mL (0.0008%),
which was prepared directly from the standard working
IEEE 978-1-6654-4000-4/21/$31.00 ©2021 IEEE
solution, 150 µg/mL (0.015%). The maximum absorption
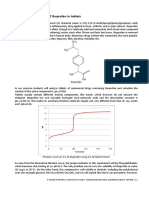
wavelength was found to be at λ max. = 458 nm (Fig. 1).
Fig. 2. Chemical reaction pathway of Ibuprofen assigned to the synthesis of a
bright orange dye with a yellowish shade
The absorbances of ten standard solutions (0.75 µg-mL –
15.00 µg/mL) were measured. These solutions have been
Fig. 1 Absorption spectrum of a bright orange dye with a yellowish shade prepared directly from the standard working solution 150
obtained from ibuprofen
µg/mL according to Table I. Final solutions volume in each
Specific absorptivity or specific extinction represented the graduated test tube was 20 mL, after making up to the mark
with absolute ethanol (TABLE I).
absorbance (as a measure of the absorption of the selected
electromagnetic radiation) of a solution layer with a thickness TABLE I. Obtaining the set of standard ibuprofen solutions from standard
of 1 cm and a concentration of 1% (g / 100 mL) It was working solution
calculated and the result obtained was shown below. mL Ibuprofen Required reagents added to standard solutions
standard working mL alpha- mL sodium
mL absolute
solution, naphthylamine, nitrite, NaNO2, 4
ethanol
150 µg/mL 0.04 % %
0.1 0.5 0.3 19.1
0.2 0.5 0.3 19.0
0.3 0.5 0.3 18.9
0.4 0.5 0.3 18.8
0.6 0.5 0.3 18.6
0.8 0.5 0.3 18.4
1.0 0.5 0.3 18.2
1.2 0.5 0.3 18.0
1.6 0.5 0.3 17.6
2.0 0.5 0.3 17.2
B.1. Ibuprofen sample preparation and calculation procedure.
Average weight of a pharmaceutical tablet was mc =
Analysis method description. Ibuprofen, 2- (4-isobutyl- 0.5661 g = 566.1 mg. The official active substance content of
phenyl) propanoic acid, a non-steroidal anti-inflammatory drug pure ibuprofen on the pharmaceutical tablet was 400 mg. a =
derived from propionic acid, present as an active substance in a 0.0516 g of ibuprofen powder and passed with 10 mL of
pharmaceutical product called Ibuprofen Grindex®, reacted absolute ethyl alcohol in a Berzelius beaker. The obtained
completely with an α-naphthylamine 0.04 % solution, in the solution was transferred into a volumetric flask of volume V =
presence of sodium nitrite NaNO2 4% aqueous solution, by 100 mL. A volume of v = 0.3 mL was measured, into a
heating 5 minutes at 50-60°C, followed by a forced cooling for graduated test tube of volume VP = 20 mL and α-
10 minutes on an ice bath, that led to the quantitative naphthylamine solution 0.04% a NaNO2, 4% were added (table
production of a bright orange colored compound with a II). It was heated on a water bath to 50-60 ⁰C at constant
yellowish shade (an azo-dye), which was obtained in an temperature for 5 minutes. The sample solution was kept on an
amount perfectly equivalent to Ibuprofen present in the studied ice bath for 10 minutes and then filled with absolute ethanol to
sample. By spectrophotometric analysis of the obtained azo- the mark. (TABLE II). Mean sample absorbance was Ap =
dye at the wavelength corresponding to its absorption 0.273.
maximum λ max. = 458 nm, Ibuprofen in the analyzed, sample
was directly spectrophotometrically measured, in relation to the TABLE II. PREPARATION OF THE SAMPLE IBUPROFEN SOLUTION
absolute ethanol p.a. as a control (Fig. 2).
Reagents added to the sample solution
Sample mL sodium
solution of mL alpha-
nitrite, mL absolute
naphthylamine,
Ibuprofen NaNO2, 4 % ethanol
0.04 % solution
Grindex® (mL) aqueous
solution
0.3 0.5 0.3 18.9 System Precision. For system precision investigation, a
target standard solution of 1.50 μg./ mL was chosen from the
standard solutions set (from Table I) and processed under
B.2. Statistic analysis.. The aim was to determine the
established experimental conditions. Standard deviation (SD)
following parameters: the linearity of the proposed method,
and RSD% were calculated. RSD ≤ 5% (TABLE V).
detection limit (LD), quantitation limit (LQ), stability of the
standard solutions, and system precision that consisted of the TABLE V. SYSTEM PRECISION ANALYSIS
standard solutions containing pure Ibuprofen and the UV-VIS
spectrophotometer utilized for measurements, as a whole. System precision, as a whole, that consists of the standard
solutions containing pure Ibuprofen and the UV-VIS
Linearity of the proposed method. The linearity of an Number of spectrophotometer
analysis process consisted of the ability to lead to results experimental Mean Relative
trials The Standard
directly proportional to the concentration of an analyte in a measured standard
Standard mean deviation
absorbance deviation
given sample, within a given range (0.75 μg/mL-15.00 solution A(λ)
value (SD)
(RSD %)
μg/mL). The correlation coefficient had to be R > 0.999 and 1. of 0.271
linear regression coefficient R2 ≥ 0.999. Microsoft Office 2.
Ibuprofen,
0.270
Excel 2016 software was used (TABLE III). 1.50 0.1146 0.003050 2.6614
3. 0.270
μg/mL
4. 0.268
TABLE III. STATISTICAL PARAMETERS OF THE LINEARITY 5. 0.269
Regression Statistics
Observations
Linear Standard
(measured Correlation Adjusted R
absorbance coefficient
regression
square
error of the III. RESULTS
coefficient linear
values) (Multiple (adjusted a. Calculation of Ibuprofen concentration Cp (μg/mL)
R square, regression
R) R2)
R2) (SE) taken into study, from the calibration graph (Fig. 3).
10 0.999676 0.999352 0.999271 0.004974
Detection limit (LD) and quantitation limit (LQ),
Detection limit (LD) was the smallest amount of analyte that
could be detected in a sample compared to a blank. It was
evaluated using the formula: LD = 3. SE/ slope (A). SE has
represented standard error of the regression line. The
Quantitation limit (LQ) was given by the lowest analyte
concentration in a sample, which could be quantified. Its value
was calculated as follows: LQ = 10. SE / slope (B).
Stability of the prepared standard solutions A standard
solution with a concentration of 5.0 μg/mL was chosen from
the set of ten standard solutions prepared (from Table I). This
solution was investigated for 32 hours at λ = 458 nm from the
time of its preparation, at various intervals, in normal storage Fig. 3 Calibration graph obtained for Ibuprofen standard solutions (0.75
μg/mL -15.00 μg/mL)
conditions. Relative standard deviation (coefficient of
variation) RSD %: RSD % = (SD. 100)/ X Average (C). For From the regression line y = 0.039 x + 0.0608 (Fig, 3), y =
accurate measurements, RSD ≤ 5%. (TABLE IV). Ap = 0.273 and x = Cp (μg/mL). Thus, Cp (μg/mL), = (0.273 –
0.0608)/0.039 (1). Cp = 5.441 μg/mL pure Ibuprofen. Ap =
TABLE IV. STABILITY ANALYSIS OF IBUPROFEN STANDARD SOLUTIONS 0.273 = mean absorbance of the sample solution.
Stability over time of the standard solutions containing b. Calculation of Ibuprofen pure content (X µg) from VP
Number of pure Ibuprofen = 20 mL solution existing in the graduated test tube,
experimental Mean Relative depending on CP (µg/mL) found above: CP µg →1 mL
trials Time The Standard
(measured
measured
mean deviation
standard solution, so in VP = 20 mL solution were X = 20. CP µg of
absorbance deviation Pure Ibuprofen (2). X = 20. 5.441. So, X = 108.82 µg of
in hours) value (SD)
A(λ) (RSD %)
Ibuprofen.
1. 0 0.271
2. 2 0.270 c. Ibuprofen pure content estimation (Y µg) from the
3. 4 0.270 initial solution existing in V = 100 mL volumetric flask,
4. 6 0.268 0.2671 0.003871 1.4493
depending on X (µg) value: If X µg Ibuprofen → v = 0.3 mL
5. 12 0.269 sample solution, then in V = 100 mL volumetric flask were Y
6. 18 0.266
= (V. X) / v µg pure Ibuprofen (3). Y = (100. 108.82) / 0.3.
7. 24 0.263
Thus, Y = 36273.33 µg pure Ibuprofen
8. 32 0.260
d. Ibuprofen pure content evaluation (Y1 µg transformed IV. CONCLUSIONS
in mg), calculated on a pharmaceutical tablet, as a final It was found an amount of 397.952 mg of pure Ibuprofen
result, reported to mc = 0.5661 g = 566.1 mg: It is known content / film-coated tablet. This value was very close to
that: Y (µg) pure Ibuprofen → a = 0.0516 g ibuprofen powder, Ibuprofen content declared by the pharmaceutical
then in mc = 0.5661 g average weight of a pharmaceutical manufacturer (400 milligrams), with a mean percentage
tablet were: Y1 = (mc. Y) / a Ibuprofen (4). Y1 = (0.5661 deviation of only 0.512 % from the officially declared amount
36273.33) / 0.0516. Thus, Y1 = 397952.173 µg of pure of active substance and felt within the normal limits (˂ 5%).
Ibuprofen / pharmaceutical tablet = 397.952 mg of pure
Ibuprofen/ pharmaceutical tablet, as a final result (TABLE The applied analysis method was linear over the entire
VI). chosen concentration range of 0.75 μg / mL -15.00 μg / mL.
R2 = 0.999352 R2 ≥ 0.9990 and the correlation coefficient R =
TABLE VI. CALCULTATION OF IBUPROFEN PURE CONTENT BY FILM-COATED 0.999676, R > 0.9990 have been fit perfectly in the normal
TABLET
range of values. LD = 0,383 μg/ mL and LQ = 1,275 μg/ mL.
Aqueous solution of 5.0 µg/mL Ibuprofen was particularly
Calculated parameters stable and could be stored at least 32 hours, the value of the
Analyzed (µg) (mg) relative standard deviation RSD = 1.4493%, RSD ≤ 5%. The
Mean Sample
sample solution Ibuprofen Ibuprofen
of Ibuprofen
measured solution
content / content /
system composed by the standard solutions of Ibuprofen and
absorbance concentration UV-Vis Spectrophotometer presented statistically a very good
film-coated film-coated
(AP) (µg/mL)
tablets tablets precision assigned by the standard relative deviation value,
Ibuprofen 0.273 5.441 397952.173 397.952 which was RSD = 2.6614%, RSD ≤ 5%. The proposed method
Grindex® tablets can be successfully applied in practice to quantitative
Ibuprofen analysis from different pharmaceutical tablet forms,
e. Percentage content (Z%) estimation of pure ibuprofen according to the actual European and international standards.
in film-coated tablet, depending on Y1 from above expressed
in mg and reported to the pure official content of ibuprofen ACKNOWLEDGMENT
on the pharmaceutical tablet, that was 400 mg: This study is simply an objective scientific research paper
For 100 % content → 400 mg of pure Ibuprofen, then for that does not aim to confirm or deny the official results
Y1 (mg) Ibuprofen it was: Z = (Y1 .100) / 400 % = (Y1 / 4) obtained by the pharmaceutics manufacturing company, nor
% (5). It was concluded that: Z = 397.952 / 4 = 99.488 %. to cause any damage to its image. We, the authors, declare no
Thus, Z = 99.488 % of Pure Ibuprofen percentage content / conflict of interests.
pharmaceutical tablet.
Statistical analysis results. Linearity of the method. REFERENCES
Spectrophotometric analysis of Ibuprofen has shown very [1] Tripathi KD., “Non steroidal anti inflammatory drugs and anti pyretic
good linearity, regression coefficient value was R2 = 0.9994 analgesics”. In: Essentials of medical pharmacology. 5th edit., Jaypee
Brothers, New Delhi, 2003. p. 176.
(fig. 4). R2 ≥ 0.9990. The standard error of the regression line
[2] Abrahm P, KI KD, “Nitro-argenine methyl ester, a non selective
(SE) was SE = 0.004974, which had a corresponding, highly inhibitor of nitric oxide synthase reduces ibuprofen-induced gastric
low value (Table III). mucosal injury in the rat”, Digestive Diseases, 2005, 50 (9), pp. 1632-
1640.
Detection limit (LD) and quantitation limit (LQ) were
[3] Bradbury F. ,“How important is the role of the physician in the correct
calculated with formulas (A) and (B) written above. LD = use of a drug? An observational cohort study in general practice”.
0,383 μg/ mL and LQ = 1,275 μg/ mL, have been within the International Journal of Clinical Practice, 2004, 144, pp.: 27-32.
normal limits. Stability of the standard solutions. Following [4] Chavez M.L, DeKorte C.J., “Valdecoxib: A review”, Clinical Therapy,
the calculation, it was found that the value of the SD standard 2003, 25 (3), pp. 817-851.
deviation = 0.003871, and the relative standard deviation was [5] Carlile D.J., Hakooz N., Bayliss M.K., Houston J.B., “Microsomal
calculated with the formula (C) as follows: RSD = (0.003871. prediction of in vivo clearance of CYP2C9 substrates in humans”,
100) / 0.2671 = 1.4493%. Thus, RSD = 1.4493%. It was British Jorunal of Clinical Pharmacology, 1999, 47 (6), pp. 625-35.
found, according to table IV, that the solutions were stable [6] Abdel Aziz Wahbi, Ekram Hassan, Dalia Hamdy, Essam Khamis Andus,
Magda Barary, “Spectrophotometric Methods for the Determination of
during at least 32 hours from their preparation. Ibuprofen in Tablets”, Pakistan Journal of Pharmaceutical Sciences,
System Precision estimation The absorbances 2005, 18 (4), pp. 1-6.
corresponding to the chosen standard solution 1.50 μg/ mL [7] Vasile Dorneanu, Maria Stan, Marcel Florin Musteaţă, “Chimie
Analitica”, Grigore T. Popa Ed., Iasi, 2003, pp. 164-331.
proved to be very close to each other (Table VI). According to
formula (C) the relative standard deviation RSD = (0.003050. [8] *** “Farmacopeea Romana”, Editia a X-a, Editura Medicala, Bucuresti,
1993, pp. 977-1293.
100) / 0.1146 = 2.6614%. Thus, RSD = 2.6614%, was within
the normal limits, RSD ≤. 5% (Table V).
You might also like
- Top 300 Drugs Pocket Reference Guide (2021 Edition)From EverandTop 300 Drugs Pocket Reference Guide (2021 Edition)Rating: 5 out of 5 stars5/5 (1)
- Ukelele 3.2 TutorialDocument54 pagesUkelele 3.2 TutorialJeffersonNo ratings yet
- Lab Report SQC7008 ParabensDocument8 pagesLab Report SQC7008 ParabenstamilarasiganasanNo ratings yet
- Draughts PersonDocument44 pagesDraughts PersonAmila Rangana Karunarathne100% (3)
- Art Ibuprofen STUDIADocument12 pagesArt Ibuprofen STUDIAMartincu AlinaNo ratings yet
- 2021 PDFDocument8 pages2021 PDFAsif Fareed QaisraniNo ratings yet
- Editorajpcr,+32 AJPCR 35690 RADocument9 pagesEditorajpcr,+32 AJPCR 35690 RAtaikhoan217No ratings yet
- Albendazol 2Document1 pageAlbendazol 2Chatgpt goaNo ratings yet
- Ibuprofen Oral Suspension 19-07-2023Document4 pagesIbuprofen Oral Suspension 19-07-2023filesvariosNo ratings yet
- Pi AminolebanDocument1 pagePi AminolebanPatricia DalanNo ratings yet
- Pak J Pharm Sci 2012 25 4 751 756Document6 pagesPak J Pharm Sci 2012 25 4 751 756pbs4yvxjndNo ratings yet
- HPLC AlADocument4 pagesHPLC AlAsahtehesabmNo ratings yet
- NUTRACEUTICALS LABORATORY Lab 2Document10 pagesNUTRACEUTICALS LABORATORY Lab 2Phú NguyễnNo ratings yet
- Contoh SoalDocument3 pagesContoh SoalDR. D NOTENo ratings yet
- Dissertation Report On: Dr. Ekta Menghani (Assistant Professor) Lakshya Garg 16BBIN016Document30 pagesDissertation Report On: Dr. Ekta Menghani (Assistant Professor) Lakshya Garg 16BBIN016Aditya SharmaNo ratings yet
- Formulation and Evaluation of Pregabalin PDFDocument7 pagesFormulation and Evaluation of Pregabalin PDFRobbyAlivianNo ratings yet
- Formulation and Evaluation of Pregabalin Loaded Eudragit S100 NanoparticlesDocument7 pagesFormulation and Evaluation of Pregabalin Loaded Eudragit S100 NanoparticlesGopalasatheeskumar KNo ratings yet
- IJRTI2005019Document6 pagesIJRTI2005019Daniel Njoto SantosoNo ratings yet
- Determinação Do Teor de Carbacol Com Azul de BromofenolDocument9 pagesDeterminação Do Teor de Carbacol Com Azul de BromofenolAilton GranjaNo ratings yet
- 8B. Acid-Base Titration of Ibuprofen in TabletsDocument2 pages8B. Acid-Base Titration of Ibuprofen in TabletsAhmed Salih100% (1)
- Imbuprofen 1Document4 pagesImbuprofen 1aggelisgeorge8546No ratings yet
- Contoh SoalDocument3 pagesContoh SoalAji PhanonkNo ratings yet
- Pharmaceutics-I - Practical Record - 1st Sem-M.pharmDocument44 pagesPharmaceutics-I - Practical Record - 1st Sem-M.pharmVenkatesh VenkateshNo ratings yet
- Method Development and Validation On Etomidate Injection by RP-HPLCDocument6 pagesMethod Development and Validation On Etomidate Injection by RP-HPLCSriram NagarajanNo ratings yet
- Biochem 313 Prac 5Document8 pagesBiochem 313 Prac 5Anonymous G8WVOfRqV100% (2)
- Determination of Ibuprpfen in Aqueaus Solutions and PharmaceticalDocument9 pagesDetermination of Ibuprpfen in Aqueaus Solutions and PharmaceticalMaria AlvarezNo ratings yet
- Investigation of Linearity, Detection Limit (LD) and Quantitation Limit (LQ) of Active Substance From Pharmaceutical TabletsDocument4 pagesInvestigation of Linearity, Detection Limit (LD) and Quantitation Limit (LQ) of Active Substance From Pharmaceutical TabletsCristian-Catalin GavatNo ratings yet
- EZ1001 Series - Aluminium: Method and Reagent Sheets 02/2019, Edition 3Document7 pagesEZ1001 Series - Aluminium: Method and Reagent Sheets 02/2019, Edition 3Anonymous owMJ21JRzCNo ratings yet
- JPSR 03110401Document5 pagesJPSR 03110401Cypriano NetoNo ratings yet
- Spectrophotometric Determination of CetirizineDocument20 pagesSpectrophotometric Determination of CetirizineAhmad AzmiNo ratings yet
- Methods and ProceduresDocument9 pagesMethods and ProceduresQuebec GC RPhNo ratings yet
- Analytical Method Development For Determination of Eugenol Concentrations in Selected Species of OcimumDocument11 pagesAnalytical Method Development For Determination of Eugenol Concentrations in Selected Species of OcimumsafaniaNo ratings yet
- V35n3a04 PDFDocument7 pagesV35n3a04 PDFricky07No ratings yet
- Analysis FinalDocument34 pagesAnalysis FinalDawit BeharuNo ratings yet
- Method Development and Validation of Esomeprazole Magnesium Trihydrate in Bulk and Formulation by UV Spectroscopic MethodDocument29 pagesMethod Development and Validation of Esomeprazole Magnesium Trihydrate in Bulk and Formulation by UV Spectroscopic MethodSantoshNo ratings yet
- Total Phenolic Content Exp-1Document4 pagesTotal Phenolic Content Exp-1beankit88No ratings yet
- At Enolo LolDocument5 pagesAt Enolo Loldrever2010No ratings yet
- Saponin in Plant EctractDocument5 pagesSaponin in Plant Ectractamelia.k.yusufNo ratings yet
- Analytical Chemistry Laboratory IIIDocument2 pagesAnalytical Chemistry Laboratory IIIAlfin MuhafidhahNo ratings yet
- Method Development and Validation of Afatinib in Bulk and Pharmaceutical Dosage Form by Uvspectroscopic MethodDocument7 pagesMethod Development and Validation of Afatinib in Bulk and Pharmaceutical Dosage Form by Uvspectroscopic MethodBaru Chandrasekhar RaoNo ratings yet
- Kouadri 2018Document8 pagesKouadri 2018Miyyada AichaouiNo ratings yet
- Direct Enantioseparation of Adrenergic Drugs Via Thin-LayerDocument9 pagesDirect Enantioseparation of Adrenergic Drugs Via Thin-Layerq52rqhqsybNo ratings yet
- Hospital Training ReviewDocument12 pagesHospital Training ReviewMaheshvari VyavhareNo ratings yet
- JurnalDocument12 pagesJurnalwira guna pratiwiNo ratings yet
- Paracetamol & Ibuprofen SuspensionDocument3 pagesParacetamol & Ibuprofen SuspensionAmik TuladharNo ratings yet
- Method Development and Validation For Simultaneous Estimation of Periondopril Erbumine and Indapamide by RP-HPLC in Pharmaceutical Dosegae FormsDocument6 pagesMethod Development and Validation For Simultaneous Estimation of Periondopril Erbumine and Indapamide by RP-HPLC in Pharmaceutical Dosegae FormsAlexandru GondorNo ratings yet
- Preparation and Characterization of Aloe Vera ExtractDocument7 pagesPreparation and Characterization of Aloe Vera Extractsuganthi ramanNo ratings yet
- Jurnal 1 Asidi AlkalimetriDocument11 pagesJurnal 1 Asidi AlkalimetriSintyaDewiiNo ratings yet
- Organic Compound Proj 2l8zdicDocument6 pagesOrganic Compound Proj 2l8zdicbergedel praktikumNo ratings yet
- Asam UratDocument38 pagesAsam UratRai Fit TimikaNo ratings yet
- Report 1Document11 pagesReport 1LinhNguye100% (1)
- Tsvetkova para IbuDocument4 pagesTsvetkova para Ibuadolfo olmosNo ratings yet
- BisabololDocument8 pagesBisabololNguyễn Trọng BảoNo ratings yet
- GUID - 1 en-USDocument5 pagesGUID - 1 en-USSadaf GondalNo ratings yet
- Spectrophotometric Determination of Aminophenol Isomers in Aqueous Solution Using 1,2-Naphthoquinone-4-Sulphonate ReagentDocument8 pagesSpectrophotometric Determination of Aminophenol Isomers in Aqueous Solution Using 1,2-Naphthoquinone-4-Sulphonate ReagentJoaquin G. MarreroNo ratings yet
- Validated Spectroscopic Method For Estimation of Aceclofenac From Tablet FormulationDocument3 pagesValidated Spectroscopic Method For Estimation of Aceclofenac From Tablet FormulationOmar Nassir MoftahNo ratings yet
- Simple Spectrophotometric Methods For DeterminatioDocument6 pagesSimple Spectrophotometric Methods For DeterminatioMima AzrahNo ratings yet
- ANFAR FIX-Spectrophotometric Method For Assay of Salbutamol in PharmaceuticalDocument6 pagesANFAR FIX-Spectrophotometric Method For Assay of Salbutamol in PharmaceuticaltheaefoliumNo ratings yet
- A Simple and Rapid HPLC UV Method For THDocument8 pagesA Simple and Rapid HPLC UV Method For THsaid ibrahimNo ratings yet
- 01 TKD Tiu CPNSDocument5 pages01 TKD Tiu CPNSRaisa NurhijriyahNo ratings yet
- UV Nr. 4Document9 pagesUV Nr. 4camelia_ioana_14No ratings yet
- Experimental approaches to Biopharmaceutics and PharmacokineticsFrom EverandExperimental approaches to Biopharmaceutics and PharmacokineticsNo ratings yet
- Articol 2 BDIDocument6 pagesArticol 2 BDICristian-Catalin GavatNo ratings yet
- Articol ChisinauDocument7 pagesArticol ChisinauCristian-Catalin GavatNo ratings yet
- Art 1Document7 pagesArt 1Cristian-Catalin GavatNo ratings yet
- MagnesiumDocument14 pagesMagnesiumCristian-Catalin GavatNo ratings yet
- CV GAVAT C.C - Cristian-Catalin Gavat Selected Publications ListDocument4 pagesCV GAVAT C.C - Cristian-Catalin Gavat Selected Publications ListCristian-Catalin GavatNo ratings yet
- Investigation of Linearity, Detection Limit (LD) and Quantitation Limit (LQ) of Active Substance From Pharmaceutical TabletsDocument4 pagesInvestigation of Linearity, Detection Limit (LD) and Quantitation Limit (LQ) of Active Substance From Pharmaceutical TabletsCristian-Catalin GavatNo ratings yet
- The Keyboard Remains The Least Ergonomically Designed Computer DeviceDocument5 pagesThe Keyboard Remains The Least Ergonomically Designed Computer DeviceCristian-Catalin GavatNo ratings yet
- s03 Drill Feed - TFX 6.12Document60 pagess03 Drill Feed - TFX 6.12hyserperusacNo ratings yet
- VTU Exam Question Paper With Solution of 18CS33 Analog and Digital Electronics March-2021-Dhanya ViswanathDocument43 pagesVTU Exam Question Paper With Solution of 18CS33 Analog and Digital Electronics March-2021-Dhanya Viswanath1DT20CS072 MANISHA POONIANo ratings yet
- Analisis Sootblower Terhadap Head TransferDocument5 pagesAnalisis Sootblower Terhadap Head TransferRDSetyawanNo ratings yet
- Root Resorption Diagnosis, Classification and Treatment Choices Based On Stimulation FactorsDocument8 pagesRoot Resorption Diagnosis, Classification and Treatment Choices Based On Stimulation FactorsAhmad AssariNo ratings yet
- Assignment 2 - SolutionsDocument14 pagesAssignment 2 - Solutionshassaniqbal84No ratings yet
- Typical Anchor BoltsDocument33 pagesTypical Anchor BoltslucianduNo ratings yet
- Guide Touchstone I Unit 2Document4 pagesGuide Touchstone I Unit 2Luz Maria Batista MendozaNo ratings yet
- All 14607Document23 pagesAll 14607davian wijayaNo ratings yet
- Operations Management: Chapter 4 - ForecastingDocument35 pagesOperations Management: Chapter 4 - ForecastingCristine Aba BoholNo ratings yet
- BS Iso Iec 29794-1-2009Document34 pagesBS Iso Iec 29794-1-2009Олег СоловьевNo ratings yet
- Focus Group APIC Seattle Confidentiality Agreement 031115Document1 pageFocus Group APIC Seattle Confidentiality Agreement 031115Maira HassanNo ratings yet
- 4 Reading-PracticeDocument24 pages4 Reading-PracticeCườiLênNhéNo ratings yet
- Global Roaming Tutorial For LTE Only Operators White Paper V1.0Document41 pagesGlobal Roaming Tutorial For LTE Only Operators White Paper V1.0Azmir MNo ratings yet
- 2010 Fodera Price ListDocument3 pages2010 Fodera Price ListPhil BassNo ratings yet
- Sweet Et Al. Systematic Review Clavicle and Rib FracturesDocument8 pagesSweet Et Al. Systematic Review Clavicle and Rib FracturesRut Herdianti P. EkasiwiNo ratings yet
- Pen NamesDocument21 pagesPen NamesJessa Meneses DucoNo ratings yet
- Ais Fraud-Case StudyDocument3 pagesAis Fraud-Case StudyErica AlimpolosNo ratings yet
- Silverman 1991 - Writing Grant Proposals For Anthropological ResearchDocument6 pagesSilverman 1991 - Writing Grant Proposals For Anthropological ResearchHéctor Cardona MachadoNo ratings yet
- Ansoft HFSS 3D Post Processor and CalculatorDocument20 pagesAnsoft HFSS 3D Post Processor and CalculatorHeba El-SawafNo ratings yet
- Paper 5 Revised PDFDocument576 pagesPaper 5 Revised PDFameydoshiNo ratings yet
- Methods of Solving Number TheoriesDocument404 pagesMethods of Solving Number Theoriesאחמד סלאח כאמל100% (1)
- Embedded Images Non Verbal Reasoning Questions and AnswersDocument27 pagesEmbedded Images Non Verbal Reasoning Questions and AnswersAditya DeshmukhNo ratings yet
- Yem Adm. Finan Manual 2013-15Document60 pagesYem Adm. Finan Manual 2013-15Awol AminNo ratings yet
- Cognizant: Process AssociateDocument3 pagesCognizant: Process AssociateNomadic World TourNo ratings yet
- Electronics PDFDocument165 pagesElectronics PDF3334333No ratings yet
- 370-1 An PremiumDocument178 pages370-1 An PremiumDejan Pušac100% (3)
- Time, Coordinate Systems and Spherical Astronomy: IWAA 2018Document33 pagesTime, Coordinate Systems and Spherical Astronomy: IWAA 2018Jan SkoczNo ratings yet
- 2021 Kia Sorento 116225Document700 pages2021 Kia Sorento 116225Tien Nguyen TatNo ratings yet