Professional Documents
Culture Documents
FABRIZIO VINO SORIA GALVARRO - Precipitation-Reactions Worksheet
Uploaded by
FabriV9Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
FABRIZIO VINO SORIA GALVARRO - Precipitation-Reactions Worksheet
Uploaded by
FabriV9Copyright:
Available Formats
Precipitation Reactions
A precipitation reaction is a chemical reaction between two soluble salts where one of the products is an insoluble
salt (a precipitate).
Examples
lead nitrate + sodium iodide lead iodide + sodium nitrate
(soluble) (soluble) (insoluble) (soluble)
Describe what you observed when the lead nitrate solution was added to the sodium iodide solution. How did you
know a reaction had occurred? How did you know an insoluble salt had been formed?
The lead nitrate turned yellow after we added potassium iodine. The change in color showed us that
……………………………………………………………………………………………………………………………………………………………………………………..
a reaction has occurred. We know that insoluble salt has formed because we mixed two substances
and a solution has formed at the botton of the
……………………………………………………………………………………………………………………………………………………………………………………..
……………………………………………………………………………………………………………………………………………………………………………………..
A precipitation reaction can be carried out between silver nitrate and sodium chloride.
Fill in the flow chart to show how you could obtain the insoluble salt.
Silver nitrate and sodium chloride
are mixed and passed Put the solution in a
through a filter paper
to a beaker petri dish Water will disoslve
and insoluble salt will
be left
Fill in the gaps in the chemical equation.
silver chloride
silver nitrate + sodium chloride ……………………………………………….. sodium nitrate
+ …………………………………………….
Use the solubility rules in the table below to work out which of the two products is the insoluble salt (precipitate).
silver chloride
Insoluble salt ……………………………………………………….
Soluble Insoluble
All sodium, potassium and ammonium salts none
All nitrates none
Most chlorides, bromides and iodides Lead chloride, bromide and iodide
Silver chloride, bromide and iodide
Most sulfates Barium, calcium and lead sulfates
Sodium, potassium and ammonium carbonates Most carbonates
Sodium, potassium and ammonium hydroxides Most hydroxides
You might also like
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5806)
- Writing PDFDocument3 pagesWriting PDFFabriV9No ratings yet
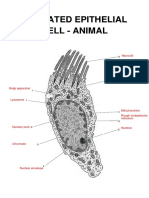
- Sebastian Wu Wu - A Typical Animal CellDocument2 pagesSebastian Wu Wu - A Typical Animal CellFabriV9No ratings yet
- Sex Fill in The BlanksDocument2 pagesSex Fill in The BlanksFabriV9No ratings yet
- Homeostasis and Body TemperatureDocument3 pagesHomeostasis and Body TemperatureFabriV9No ratings yet
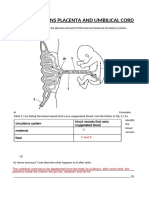
- Igcse Questions Placenta and Umbilical CordDocument3 pagesIgcse Questions Placenta and Umbilical CordFabriV9No ratings yet
- Biometric Security: Advantages DisadvantagesDocument3 pagesBiometric Security: Advantages DisadvantagesFabriV9No ratings yet
- Igcse Hormones and Contraceptive MethodsDocument2 pagesIgcse Hormones and Contraceptive MethodsFabriV9No ratings yet
- Internal and External Communication: Revision AnswersDocument5 pagesInternal and External Communication: Revision AnswersFabriV9100% (3)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (589)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (842)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Q3 Week 4 Claim and Types of Claims: I. ObjectiveDocument8 pagesQ3 Week 4 Claim and Types of Claims: I. ObjectiveJhe Hann BurcaNo ratings yet
- The Dynamics of Hate Speech and Counter Speech in The Social Media - English 1Document16 pagesThe Dynamics of Hate Speech and Counter Speech in The Social Media - English 1A MNo ratings yet
- Pioneer QX 747 BrochureDocument6 pagesPioneer QX 747 BrochureArmando JimenezNo ratings yet
- PRP ConsentDocument4 pagesPRP ConsentEking InNo ratings yet
- Catalogo PDFGeneratorDocument3 pagesCatalogo PDFGeneratoredwraulNo ratings yet
- A5E43455517 6 76 - MANUAL - SITOP Manager - en USDocument252 pagesA5E43455517 6 76 - MANUAL - SITOP Manager - en USdasdNo ratings yet
- Reliability & Validity: Nathan A. Thompson PH.DDocument5 pagesReliability & Validity: Nathan A. Thompson PH.DJames SaupanNo ratings yet
- Oglej Case Study 4300 1Document20 pagesOglej Case Study 4300 1api-533010905No ratings yet
- Learning Unit 1 (Chap. 11)Document15 pagesLearning Unit 1 (Chap. 11)sbusisoNo ratings yet
- Keys To The Ultimate Freedom PDFDocument245 pagesKeys To The Ultimate Freedom PDFdebtru100% (2)
- En User Manual Singleviu 22 201801Document40 pagesEn User Manual Singleviu 22 201801Josko PrnjakNo ratings yet
- Maryada Purushotham RamDocument1 pageMaryada Purushotham RamNithin SridharNo ratings yet
- L2 AND L3 Switch DifferenceDocument6 pagesL2 AND L3 Switch DifferenceVivek GhateNo ratings yet
- Mobile Tower and Mobile Phone Radiation Hazards - Prof Girish Kumar - June 2013Document50 pagesMobile Tower and Mobile Phone Radiation Hazards - Prof Girish Kumar - June 2013Neha Kumar100% (1)
- First Mass in The PhilippinesDocument8 pagesFirst Mass in The PhilippinesMj RepilNo ratings yet
- ASV Program Guide v3.0Document53 pagesASV Program Guide v3.0Mithun LomateNo ratings yet
- A Literary Voyage Into The Unconscious: A Philosophical Approach To The Psychological Novel in Woolf's Mrs. Dalloway (1925)Document10 pagesA Literary Voyage Into The Unconscious: A Philosophical Approach To The Psychological Novel in Woolf's Mrs. Dalloway (1925)Muhammad IbrahimNo ratings yet
- Biogeochemical CycleDocument2 pagesBiogeochemical Cyclekoko BunchNo ratings yet
- Project Management Quality Management PlanDocument9 pagesProject Management Quality Management PlanPriya100% (1)
- CVS Series E 1 6 Inch March 2021 1Document16 pagesCVS Series E 1 6 Inch March 2021 1iqmpslabNo ratings yet
- Aura and Chakra 3Document88 pagesAura and Chakra 3Theodore Yiannopoulos100% (2)
- Aceleradores Serie 6900Document2 pagesAceleradores Serie 6900jdavis5548No ratings yet
- Cubex 6200 Spec Sheet PDFDocument4 pagesCubex 6200 Spec Sheet PDFtharciocidreira0% (1)
- Guidelines Adhd AdultDocument19 pagesGuidelines Adhd AdultJavier Cotobal100% (1)
- Hsep-05 - Communication, Participation & ConsultationDocument6 pagesHsep-05 - Communication, Participation & ConsultationScha AffinNo ratings yet
- VungleDocument14 pagesVunglegaurdevNo ratings yet
- MTH 212 OdeDocument18 pagesMTH 212 OdeJames ojochegbNo ratings yet
- IR Sensor Infrared Obstacle Sensor Module Has Builtin IR Transmitter and IR Receiver That Sends Out IR Energy and Looks ForDocument12 pagesIR Sensor Infrared Obstacle Sensor Module Has Builtin IR Transmitter and IR Receiver That Sends Out IR Energy and Looks ForRavi RajanNo ratings yet
- PARATIE EN - Advanced-Modelling-2014 PDFDocument50 pagesPARATIE EN - Advanced-Modelling-2014 PDFJPachasNo ratings yet
- Datasheet Ls 7222Document4 pagesDatasheet Ls 7222Martín NestaNo ratings yet