Professional Documents
Culture Documents
Atomic Structure Notes for Class XI Chemistry
Uploaded by
Sora RoseOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Atomic Structure Notes for Class XI Chemistry
Uploaded by
Sora RoseCopyright:
Available Formats
M.E.
S INDIAN SCHOOL, DOHA -QATAR
NOTES -2020-2021
Section: BOYS’ &GIRLS’ Date :22-05-2021
Class & Div.: XI (All divisions) Subject: CHEMISTRY
Lesson / Topic: ATOMIC STRUCTURE
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
Atom :
• It is the smallest particle of matter that takes part in a chemical reaction.
• Most of the elements are monatomic, oxygen, hydrogen, nitrogen and halogens are
diatomic Phosphorous is tetra atomic and sulphur is octa atomic
Sub atomic particles : Electron , Proton and Neutron
• Electron : Discovered by J.J Thomson by studying cathode rays and name g electron
is given by Stony
• It is that fundamental particle which carries 1 unit negative charge (1.6 x 10 -19 c) and has
a mass nearly equal to 1/1837 th of that hydrogen atom(9.11x10-31 kg)
• Proton : Discovered by E.Goldstein by studying cannal rays/anode rays .
• It is the fundamental particle which carries 1 unit Positive charge (1.6 x 10 -19 c) and has
a mass nearly equal to of that hydrogen atom(1.672× 10–27kg)
• Neutron : Discovered by James Chadwick by bombardment of alpha rays on beryllium .
2He
4
+ 4Be9 → 6C12 + 0n1
• It is that fundamental particle which carries neutral charge and has a mass nearly equal
to of that hydrogen atom(1.672× 10–27kg)
A
Representation of atom : ZX
Where : A -> Mass number, Z →Atomic number, X →Symbol of atom
• Atomic Number : It is represented by Z. The number of protons present in the nucleus of an
atom is called atomic number of an element. It is also known as nuclear charge.
• For neutral atom : Number of proton = Number of electron
• For cation : Number of electron = Z – (charge on atom)
• For anion: Number of electron = Z + (charge on atom)
• Mass Number : It is represented by capital A. The sum of number of neutrons and protons
is called the mass number of the element. It is also known as number of nucleons because
neutron & proton are present in nucleus. A = number of protons + number of neutrons
F 061, Rev 01, dtd 10th March 2020
1 Structure of atom
PRACTICE :
1. Find number of protons, eiectrons and neutrons in the following :
12 23
6C , 11Na , F- (Z=9 , A=19)
2. If no. of protons in X–2 is 16. then no. of e– in X+2 will be–
(a) 14 (b) 16 (c) 18 (d) None
Isotopes : Given by Soddy
• They are the atoms of a same element having same atomic number (Z) but different
mass number (A)
• They have same nuclear charge (Z) but different number of neutrons (A–Z)
• Isotopes have same chemical property but different physical property.
• 6C
12
6C
13
6C
14
✓ e=6 e=6 e=6
✓ p=6 p=6 p=6
✓ n=6 n=7 n=8
Isobars : Given by Aston
• They are the atoms of different element having same mass number (A) but different
atomic number (Z)
• They have different number of Electrons, Protons & Neutrons but sum of number of
neutrons & Protons ((number of nucleons) remains same.
• For Eg. 19 K40 20 Ca40
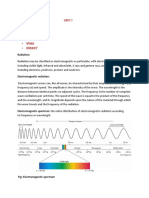
Electromagnetic waves (EM waves) or Radiant Energy/Electromagnetic radiation
▪ It is the energy transmitted from one body to another in the form of waves and these
waves travel in the space with the same speed as light ( 3 × 10 8 m/s) and these waves
are known as Electromagnetic waves or radiant energy.
▪ Characteristics
1. All electromagnetic radiations travel with the velocity of light.
2. These consist of electric and magnetic fields components that oscillate in
directions perpendicular to each other and perpendicular to the direction in
which the wave is travelling
3. The radiant Energy do not need any medium for propagation
▪ A wave is characterized by following six characterstics
F 061, Rev 01, dtd 10th March 2020
2 Structure of atom
a) Wavelength (Lambda) : It is defined as the distance between two nearest crest or
nearest trough. It is measured in terms of a A° (Angstrom), pm (Picometre), nm
(nanometer), cm(centimetre), m (metre)
1Å = 10–10 m, 1 Pm = 10–12 m, 1nm = 10–9 m, 1cm = 10–2m
b) Frequency (nu )
Frequency of a wave is defined as the number of waves which pass through a point
in one second .
It is measured in terms of Hertz (Hz ), sec–1 , or cycle per second (cps) 1 Hertz = 1
sec–1 = 1 cps. .
c) Velocity (c)
Velocity of a wave is defined as distance covered by a wave in 1 sec
c = ( c(speed of light )=3 × 108 m/s, =frequency , =wave number)
d) Wave number ( nu bar) It is the reciprocal of the wave length. That is number of
waves present
Questions
1. An element atomic number 30 and mass number 66, what will be the number of
protons and neutrons in this atom?
PLANCK'S QUANTUM THEORY
In 1901, Max Planck studied the distribution of the frequencies of radiations emitted from
the hot bodies. He proposed a bold hypothesis.
According to planck's quantum theory :
1) The radiant energy emitted or absorbed by a body not continuously but discontinuously
in the form of small discrete packets of energy and these packets are called quantum.
2) In case of light, the smallest packet of energy is called as 'photon' but in general case
the smallest packet of energy called as quantum.
3) The energy of each quantum is directly proportional to frequency of the radiation i.e.
= h = hc/ Planck’s constant (h)= (The value of h is 6.626 − JS
4) Total amount of energy transmitted from one body to another will be some integral
multiple of energy of a quantum. E = nh Where n is an integer
Photoelectric Effect
• It was observed by Hertz and Lenard.
• The phenomenon of ejection of the electrons from metal surface when a monochromatic
light of proper frequency strikes on it is called Photoelectric Effect.
F 061, Rev 01, dtd 10th March 2020
3 Structure of atom
work function: Minimum energy needed to eject electron=hv0
V0- Threshold frequency
Incident energy = work function + Kinetic energy
hv=hv0 + K.E
Intensity increases number of electrons ejected
Frequency increases the K.E of ejected electron
Black body radiation :
An ideal body is a perfect absorber and emitter of radiation
Demerits of EM radiation :
It fails to explain phenomena like photoelectric effect, black body radiation etc.
Spectrum :
• A spectrum is a series of coloured bands obtained by the dispersion of white light.
• The spectrum of radiation emitted by a substance that has absorbed energy is called an
emission spectrum. It appears as white lines in dark background. Atoms, molecules or
ions that have absorbed radiation are said to be “excited”.
• A continuum of radiation is passed through a sample which absorbs radiation of certain
wavelengths. The missing wavelength which corresponds to the radiation absorbed by the
matter, leave dark spaces in the bright continuous spectrum.
• The study of emission or absorption spectra is referred to as spectroscopy.
• An absorption spectrum is like the photographic negative of an emission spectrum.
• The emission spectra of atoms in the gas phase, on the other hand, do not show a
continuous spread of wavelength from red to violet, rather they emit light only at specific
wavelengths with dark spaces between them. Such spectra are called line spectra or
atomic spectra.
• Each element has a unique line emission spectrum. So line spectrum is called the finger
print of an atom.
LINE SPECTRA OF HYDROGEN :
• When an electric discharge is passed through gaseous hydrogen, the H 2 molecules
dissociate and the energetically excited hydrogen atoms produced emit electromagnetic
radiation of discrete frequencies. The hydrogen spectrum consists of several series of lines
named after their discoverers.
• The line spectra of hydrogen lies in three regions of Electromagnetic Spectrum: Infra-red,
Visible and UV region. In all there are five sets of discrete lines.
• The set of lines in the Visible region are known as Balmer Series, those in Ultra-Violet as
Lyman series and there are three sets of lines in Infra-red region : Paschen, Brackett and
Pfund series.
• Balmer and Rydberg gave an empirical relation to define the wavelength of the lines in
each series in terms of a parameter called as Wave Number
F 061, Rev 01, dtd 10th March 2020
4 Structure of atom
• These observations led Bohr to conclude that electrons in an atom are not randomly
distributed, but were arranged in definite energy states. The energy of each state (or level)
was fixed or quantised (from characteristic nature of H-atom spectra). The complete theory
developed by him is organised in his postulates.
BOHR’S MODEL FOR HYDROGEN ATOM
The important postulates of Bohr's Model are :
• The electron in the hydrogen atom can move around the nucleus in a circular path of fixed
radius and energy. These paths are called orbits, stationary states or allowed energy
states.
• The energy of an electron in the orbit does not change with time. However, the electron
will move from a lower stationary state to a higher stationary state when required amount
of energy is absorbed by the electron or energy is emitted when electron moves from higher
stationary state to lower stationary state.
• The frequency of radiation absorbed or emitted when transition occurs between two
stationary states that differ in energy by ∆E, is given by Where E1
and E2 are the energies of the lower and higher allowed energy states respectively. This
expression is commonly known as Bohr’s frequency rule.
F 061, Rev 01, dtd 10th March 2020
5 Structure of atom
• The angular momentum of an electron is quantised. In a given stationary state it can be
expressed as in equation Where me is the mass of
electron, v is the velocity and r is the radius of the orbit in which electron is moving.
NOTE :
1. Energy of the orbit where RH is called Rydberg constant
and its value is 2.18×10–18 J.
2. For Hydrogen like species (He +, Li2+, Be 3+)
where Z is the atomic number
3. Energy change for transistion
4. Frequency
5. Wave number
LIMITATIONS OF BOHR’S MODEL OF ATOM
• Unable to explain the spectrum of multi-electron atoms (For example – helium atom which
contains two electrons)
• Unable to explain splitting of spectral lines in electric field (Stark effect) or in magnetic
field (Zeeman effect)
• Unable to explain the fine spectral lines in H atom.
•
F 061, Rev 01, dtd 10th March 2020
6 Structure of atom
THE DUAL NATURE OF MATTER (THE WAVE NATURE OF ELECTRON)
• Like light matter also exhibit dual character -ie both particle like and wave like character.
• De Broglie proposed dual behavior to matter.
• According to de-brogile every form of matter (electron or proton or any other particle)
have wave and particle nature .
• Hence a particle of mass m moving with a velocity v should have a wavelength
Where p= momentum This is called de-brogile relation.
Significance of de-brogile relation :
• Wavelengths of objects having large masses are so short that their wave properties
cannot be detected.
• Wavelengths of particles having very small masses (electron and other subatomic
particles) can be detected experimentally.
Q. Why don’t we observe the wave properties of large objects like cricket ball?
Because of their large mass, wavelength is small, hence cannot be detected.
HEISENBERG’S UNCERTAINTY PRINCIPLE:
• Heisenberg uncertainty principle states that “it is impossible to determine
simultaneously, the exact position and exact momentum (or velocity) of an electron”.
• Mathematically, it can be given as
• Significance of Uncertainty Principle:
Heisenberg’s uncertainty principle rejects the existence of definite paths or
trajectories of electrons and other similar particles.(orbit is replaced by orbital)
Reasons for Failure of Bohr’s model:
• It ignores the dual behavior of matter.
• It contradicts Heisenberg’s uncertainty principle.
F 061, Rev 01, dtd 10th March 2020
7 Structure of atom
QUANTUM MECHANICAL MODEL OF ATOM:
The branch of science that takes into account the dual behaviour of matter is called
quantum mechanics.
QUANTUM NUMBERS:
• These are a set of numbers with the help of which we can get a complete information about
the electron in an atom. The main quantum numbers are
1. Principal Quantum Number (n)
2. Azimuthal Quantum Number or Orbital Angular Momentum or Subsidiary
Quantum Number ( l)
3. Magnetic orbital quantum number. (ml)
4. Spin Quantum Number ( ms)
• An orbital is designated by first three set of quantum numbers (principle, azimuthal,
magnetic).
• In order to designate the electron an additional quantum number called as SPIN
QUANTUM NUMBER is also needed to specify spin of the electron.
Principle quantum number (n) :
• It describes the size of the electron (distance from the nucleus) and the total energy of the
electron.
• It has integral values 1,2,3,4......., etc, and is denoted by K,L,M,N..........,etc.
• The maximum number of electrons present in a principal energy shell, is equal to 2n2.
Azimuthal quantum number (l) :
• It denotes the sub shell and shape of the orbital.
• The number of sub shells in a shell is equal to the value of n.
• It can have values from 0 to (n – 1).
Magnetic quantum number (ml):
• It describes the orientations of the sub shells and number of orbitals.
• It can have values from –l to +l including zero.
• It can have a total of (2l + 1) values for each subshell.
• Each value corresponds to an orbital.
Spin quantum number (s) :
• It describes the spin of the electron.
• It has values +1/2 and –1/2.
• (+) signifies clockwise spinning and (–) signifies anticlockwise spinning.
• It indicates each orbital can have two electrons
F 061, Rev 01, dtd 10th March 2020
8 Structure of atom
CONCLUSION FROM QUANTUM NUMBERS:
Value of n 1 2 3 4
Shell K L M N
Value of l 0 1 2 3
Subshell s p d f
Main shell (n) Subshell ( l) No.of Orbitals No.of electrons
l=0 to (n-1) (ml) (2l+1) (Also 2n2)
n=1 1s 1 1*2=2
n=2 2s, 2p 1+3=4 4*2=8
n=3 3s, 3p,3d 1+3+5=9 9*2=18
n=4 4s,4p,4d,4f 1+3+5+7=16 16*2=32
Subhell No.of No.of electrons
Orbitals(2l+1)
s 1 1*2=2
p 3 3*2=6
d 5 5*2=10
f 7 7*2=14
Maximum number of electrons for a main shell (n)= 2n2
Number of orbitals for a shell (n) = n2
Number of subshells= Value of n
SHAPES OF ATOMIC ORBITALS & BOUNDARY SURFACE DIAGRAMS:
S Orbital :
• S orbital is non-directional and sperically symmetrical.
• As the value of n increases, size of S orbital increases. 4s> 3s>2s>1s
• As the value of n inrcreases, the electron is located farther away from the nucleus.
P Orbital :
• P orbital is dumb bell shaped.
• There are three p orbitals- Px , Py , Pz
• The size, shape and energy of the three orbitals are identical. They differ however, in the
way the lobes are oriented. Hence they are called degenerate orbitals.
F 061, Rev 01, dtd 10th March 2020
9 Structure of atom
d-Orbital :
• The five d-orbitals are designated as dxy, dyz, dxz, dx2–y2 and dz.
• The shapes of the first four d orbitals are similar to each other, where as that
of the fifth one, dz is different from others. But all five 3d orbitals are equivalent in
energy and are said to be degenerate orbitals.
• The three d orbitals 3dxy, 3dyz, 3dxz are similar and its lobes are oriented in between the
axis .
• In 3dx2–y2 the lobes are oriented along the axis x and y.
• The 3dz2 consists of lobes along the z axis and a ring of high density in the xy plane.
NODES AND NODAL PLANE:
• The region where the probability of finding the electron is zero is called nodal surface or
node.
• Radial nodes are nodes present along the axis.
• Angular nodes are nodes at the plane bisecting the lobes passing through the nucleus.
• Total node = n-1 Radial node = n-l-1 Angular node =l
Qn). Claculate the radial node, angular node and total number of nodes of 2p orbital.
For 2P (n=2 l =1)
Total nodes = n-1, 2-1=1 Angular nodes = l =1 Radial nodes = n-l-1 = 2-1-1=0
ENERGIES OF ORBITALS:
• In a multi-electron atom, there are two types of interactions
1. attraction between the electron and nucleus.
2. repulsion between every electron and other electrons present in the atom.
• Thus the stability of an electron in a multi-electron atom is because total attractive
interactions are more than the repulsive interactions.
• On the other hand, the attractive interactions of an electron increases with increase of
positive charge (Ze) on the nucleus.
F 061, Rev 01, dtd 10th March 2020
10 Structure of atom
• The net positive charge experienced by the outer electron from the nucleus in a
multielectron atom is called effective nuclear charge.
• Effective nuclear charge experienced by the orbital decreases with increase of Principle
quantum number and if same principle quantum number it will decreases with increase in
azimuthal quantum number (l). s>p>d>f
• The shielding of the outer shell electrons from the nucleus by the inner shell electrons is
called shielding or screening effect.
• The order of sreening effect is s>p>d>f.
(n + l ) OR BOHR- BURY RULE:
1. Lower the value of (n + l) for an orbital, lower is its energy and it is filled first.
Example: 4s has lower (n+l )value than 3d. So 4s is filled first
2. If two orbitals have the same value of (n + l), then the orbital with lower value of n
will have lower energy and can be filled first.
Example: Both 2p and 3s have same value of (n+l ) then 2p with lower value of n is
filled first.
RULES FOR FILLING OF ORBITALS:
Aufbau Principle :
• Aufbau is a German word and its meaning 'Building up'.
• Aufbau Principle states that In the ground state of the atoms, the orbitals are filled
in order of their increasing energies.
• The order of energies of the orbitals is
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 4f, 5d, 6p, 7s…
Pauli Exclusion Principle:
• According to this principle : No twoelectrons in an atom can have the same set of
four quantum numbers.
• Pauli exclusion principle can also be stated as : “Only two electrons may exist in the
same orbital and these electrons must have opposite spin.”
Hund’s Rule of Maximum Multiplicity:
It states that pairing of electrons in the orbitals belonging to thesame subshell
(p, d or f) does not take place until each orbital belonging to that subshell is singly occupied.
ELECTRONIC CONFIGURATION OF ATOMS:
• The distribution of electrons into orbitals of an atom is called its electronic configuration.
It is based on above rules.
• The electronic configuration of different atoms can be represented in two ways.
F 061, Rev 01, dtd 10th March 2020
11 Structure of atom
Qn ). Write the electronic configuration of elements from atomic number from 1 to 30 .
Qn). Write the expected and actual electronic configuration of chromium and copper.
Account
Chromium ( Cr) has half filled (d5) and copper (Cu) has completely filled ( d10) orbitals.
Hence Cr and Cu have extra stability due to symmetrical distribution of electrons and high
exchange energy.
EXERCISE QUESTIONS:
1. What are the frequency and wavelength of a photon emitted during a transition from n = 5
state to the n = 2 state in the hydrogen atom?
2. Calculate the energy associated with the first orbit of He+. What is the radius of this orbit?
3. What will be the wavelength of a ball of mass 0.1 kg moving with a velocity of 10 ms–1 ?
4. A microscope using suitable photons is employed to locate an electron in an atom within a
distance of 0.1 Ao. What is the uncertainty involved in the measurement of its velocity?
F 061, Rev 01, dtd 10th March 2020
12 Structure of atom
5. A golf ball has a mass of 40g, and a speed of 45 m/s. If the speed can be measured within
accuracy of 2%, calculate the uncertainty in the position.
6. What is the total number of orbitals associated with the principal quantum number n = 3 ?
7. Using s, p, d, f notations, describe the orbital with the following quantum numbers
(a) n = 2, l = 1, (b) n = 4, l = 0, (c) n = 5, l = 3, (d) n = 3, l = 2
8. Using s, p, d notations, describe the orbital with the following quantum numbers.
(a) n=1, l=0; (b) n = 3; l=1 (c) n = 4; l =2; (d) n=4; l=3.
9. An electron is in one of the 3d orbitals. Give the values of n, l &ml for this electron.
10. (i) An atomic orbital has n = 3. What are the possible values of l and ml ?
(ii) List the quantum numbers of electrons for 3d orbital.
(iii) Which of the following orbitals are possible? 1p, 2s, 2p and 3f.
11. How many electrons in an atom may have the following quantum numbers?
(a) n = 4, ms = – ½ (b) n = 3, l = 0
12. Among the following pairs of orbitals which orbital will experience the larger effective
nuclear charge? (i) 2s and 3s, (ii) 4d and 4f, (iii) 3d and 3p.
13. The unpaired electrons in Al and Si are present in 3p orbital. Which electrons will
experience more effective nuclear charge from the nucleus ?
14. Indicate the number of unpaired electrons in : (a) P, (b) Si, (c) Cr, (d) Fe and (e) Kr.
15. (a) How many subshells are associated with n = 4 ? (b) How many electrons will be
present in the subshells having ms value of –1/2 for n = 4
16. Among the following pairs of orbitals which orbital will experience the larger effective
nuclear charge? (i) 2s and 3s, (ii) 4d and 4f, (iii) 3d and 3p.
17. Write the electronic configurations of the following ions: (a) H – (b) Na+ (c) O2– (d) F–
18. What are the atomic numbers of elements whose outermost electrons are represented by
(a) 3s1 (b) 2p3 and (c) 3p5
19. Differentiate between an orbit and an orbital.
20. Show that the circumference for the Bohr orbit for Hydrogen atom is an integral multiple of
the de brogile wavelength associated with the electron revolving around the orbit,.
F 061, Rev 01, dtd 10th March 2020
13 Structure of atom
You might also like
- Section:BOYS' &GIRLS' Date:7-04-20 Class & Div.:XI (All Divisions) Subject:CHEMISTRY Lesson / Topic: ATOMIC STRUCTURE - 1Document8 pagesSection:BOYS' &GIRLS' Date:7-04-20 Class & Div.:XI (All Divisions) Subject:CHEMISTRY Lesson / Topic: ATOMIC STRUCTURE - 1Mohammed IliasNo ratings yet
- Structure and Properties of AtomsDocument10 pagesStructure and Properties of AtomsRishabh Garg33% (3)
- 11 Chemistry Notes Ch02 Structure of AtomDocument18 pages11 Chemistry Notes Ch02 Structure of AtomSayantanBanerjee0% (1)
- 02-Structure of AtomDocument100 pages02-Structure of Atomtorodoki15No ratings yet
- Structure of Atom Class 11 Chemistry CBSE BoardDocument5 pagesStructure of Atom Class 11 Chemistry CBSE BoardminimataNo ratings yet
- Atomic Structure 11 DM 11Document102 pagesAtomic Structure 11 DM 11Harsh YadavNo ratings yet
- Chapter 2_ Structure of AtomDocument18 pagesChapter 2_ Structure of AtomstudyforiittomeetbtsNo ratings yet
- Structure - of - Atom - Class 11 CbseDocument10 pagesStructure - of - Atom - Class 11 CbseAnshuman SinghNo ratings yet
- Discovery of fundamental particles and electromagnetic radiationDocument38 pagesDiscovery of fundamental particles and electromagnetic radiationAdil KhanNo ratings yet
- Atomic Structure Short Notes 7 PageDocument7 pagesAtomic Structure Short Notes 7 PageSubhajit GoraiNo ratings yet
- Atomic Structure and Models ExplainedDocument17 pagesAtomic Structure and Models ExplainedprashanthNo ratings yet
- JEE Main Atomic Structure - pdf-98Document12 pagesJEE Main Atomic Structure - pdf-98Zameer AnsariNo ratings yet
- 2nd MeetDocument48 pages2nd MeetIntan CahyaningrumNo ratings yet
- 2 Atomic StructureDocument114 pages2 Atomic StructurekhushiNo ratings yet
- NCERT Solutions Structure of AtomDocument18 pagesNCERT Solutions Structure of Atomjazi_4uNo ratings yet
- Structure of Atom: Rutherford's Nuclear ModelDocument12 pagesStructure of Atom: Rutherford's Nuclear ModelGautham GrimaceNo ratings yet
- Atom and Subatomic Particles ExplainedDocument16 pagesAtom and Subatomic Particles ExplainedMahin ChandwaniNo ratings yet
- 11th Structure of Atom Notes - 1908516557Document12 pages11th Structure of Atom Notes - 1908516557Sachin SinghNo ratings yet
- Atomic StructureDocument11 pagesAtomic StructureAmiya Kumar PandaNo ratings yet
- CBSE Class 11 Chemistry Notes: Atomic StructureDocument13 pagesCBSE Class 11 Chemistry Notes: Atomic StructureSonaakshi Jagadeesh BabuNo ratings yet
- Atomic Structure Theory - EDocument33 pagesAtomic Structure Theory - EthinkiitNo ratings yet
- CHM131 - CH 3 - The Electronic Structure of Atoms and Periodic Table PDFDocument102 pagesCHM131 - CH 3 - The Electronic Structure of Atoms and Periodic Table PDFRabiatul AdawiyyahNo ratings yet
- Atom and Its Structure Class 11 Notes NEET Chemistry (PDF)Document11 pagesAtom and Its Structure Class 11 Notes NEET Chemistry (PDF)Ankit KumarNo ratings yet
- Atomic StructureDocument49 pagesAtomic StructureFatimaNo ratings yet
- Atoms:: Particle Electron Proton Neutron Discovery Nature of Charge Negative Amount of Charge MassDocument6 pagesAtoms:: Particle Electron Proton Neutron Discovery Nature of Charge Negative Amount of Charge MassNasser SsennogaNo ratings yet
- Final Junoon-e-Jee - Atomic Structures - 21 DecDocument184 pagesFinal Junoon-e-Jee - Atomic Structures - 21 DecAnshu BhawsarNo ratings yet
- Inorganic Chemistry 1Document70 pagesInorganic Chemistry 1Korir BiwottNo ratings yet
- Class 11 Chemistry Chapter 2 Structure of AtomDocument15 pagesClass 11 Chemistry Chapter 2 Structure of AtomgokulNo ratings yet
- Unit-1 Learning Objective Radiation Units Work EnergyDocument31 pagesUnit-1 Learning Objective Radiation Units Work EnergyManisha khanNo ratings yet
- Jee Advanced Atomic Structure and Chemical Bonding Revision NotesDocument12 pagesJee Advanced Atomic Structure and Chemical Bonding Revision Notesluubkhan037No ratings yet
- Chemistry I: Emtinan DiabDocument35 pagesChemistry I: Emtinan DiabMohamed GariballaNo ratings yet
- Unit 11 Modern PhysicsDocument90 pagesUnit 11 Modern PhysicsPeril LousNo ratings yet
- Chemistry Notes Chap 2 Structure of An AtomDocument15 pagesChemistry Notes Chap 2 Structure of An AtomJo ParkerNo ratings yet
- Fundamental particles and isotopes explainedDocument16 pagesFundamental particles and isotopes explainedMuhammad ShakeelNo ratings yet
- Atomic Structure - One Shot by Sakshi Mam #BounceBackDocument231 pagesAtomic Structure - One Shot by Sakshi Mam #BounceBackchansiray7870No ratings yet
- Chemistry Mastery Test ReviewDocument26 pagesChemistry Mastery Test ReviewaihpendoyNo ratings yet
- Structure of Atom - 404Document72 pagesStructure of Atom - 404vipulvidhya2020No ratings yet
- XI 02 Atomic Structure ModifiedDocument53 pagesXI 02 Atomic Structure Modifiedkaushik2470% (1)
- 2.structure of Atom-Smart Booklet-1Document44 pages2.structure of Atom-Smart Booklet-1nadeemnagthan008No ratings yet
- Atomic Structure PDFDocument28 pagesAtomic Structure PDFArun Kumar100% (2)
- AQA - Physics - Unit 1Document3 pagesAQA - Physics - Unit 1sashabelleNo ratings yet
- ATOMIC STRUCTUREDocument33 pagesATOMIC STRUCTUREWaheba TanveerNo ratings yet
- Chapter-2 Structure of Atom-NotesDocument24 pagesChapter-2 Structure of Atom-NotesKiran KiruNo ratings yet
- Atomic Structure: Chapter - 1Document14 pagesAtomic Structure: Chapter - 1Cube WorldNo ratings yet
- Structure of AtomDocument72 pagesStructure of AtomAditi YadavNo ratings yet
- Notes On Atomic Structure-1Document9 pagesNotes On Atomic Structure-1Manish AgrawalNo ratings yet
- Structure of Atom Class 11Document74 pagesStructure of Atom Class 11Komal VermaNo ratings yet
- Class XI Atomic Structure Notes.Document11 pagesClass XI Atomic Structure Notes.easaNo ratings yet
- (2087) Lecture Notes 1 Atomic Structure eDocument53 pages(2087) Lecture Notes 1 Atomic Structure eRamJiPandeyNo ratings yet
- Chapter 11Document44 pagesChapter 11swezinomg2002No ratings yet
- Modern Physics Hints: Atomic Structure, Spectra, Radiation, and MoreDocument21 pagesModern Physics Hints: Atomic Structure, Spectra, Radiation, and MoreOlajide HeritageNo ratings yet
- Structure of AtomDocument18 pagesStructure of Atomchetnasri01No ratings yet
- Atomic Structure ExplainedDocument8 pagesAtomic Structure ExplainedJames AdibNo ratings yet
- Atomic Structure TheoryDocument14 pagesAtomic Structure TheoryPrithviraj Netke100% (1)
- Structure of Atom ExplainedDocument27 pagesStructure of Atom ExplainedEINSTEINNo ratings yet
- Elementary Particles: The Commonwealth and International LibraryFrom EverandElementary Particles: The Commonwealth and International LibraryNo ratings yet
- The Enigmatic Electron: Electron Behaviour and How It Influences Our LivesFrom EverandThe Enigmatic Electron: Electron Behaviour and How It Influences Our LivesNo ratings yet
- Advances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenFrom EverandAdvances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenR. BrillNo ratings yet
- SocialDocument1 pageSocialSora RoseNo ratings yet
- AdbkbDocument1 pageAdbkbSora RoseNo ratings yet
- Ncert Solutions For Class 10 Maths Chapter 12 Ex 2Document11 pagesNcert Solutions For Class 10 Maths Chapter 12 Ex 2Sora RoseNo ratings yet
- CamScanner 05-22-2021 12587Document2 pagesCamScanner 05-22-2021 12587Sora RoseNo ratings yet
- Landscape of the Soul: Exploring Art and SpiritualityDocument6 pagesLandscape of the Soul: Exploring Art and SpiritualityrajniNo ratings yet
- TwitterDocument1 pageTwitterSora RoseNo ratings yet
- IntroductionDocument1 pageIntroductionSora RoseNo ratings yet
- RegDocument1 pageRegSora RoseNo ratings yet
- English ProjectDocument8 pagesEnglish ProjectSora RoseNo ratings yet
- 2021 Units & MeasurementDocument9 pages2021 Units & MeasurementSora RoseNo ratings yet
- WebserviceDocument1 pageWebserviceSora RoseNo ratings yet
- Chem ProjectDocument17 pagesChem ProjectSora RoseNo ratings yet
- 2021 Yech 1 WorksheetDocument1 page2021 Yech 1 WorksheetSora RoseNo ratings yet
- 2021 y Notes3 - MobileDocument3 pages2021 y Notes3 - MobileSora RoseNo ratings yet
- 2021 y NOTES - 2Document5 pages2021 y NOTES - 2Sora RoseNo ratings yet
- 2021 Worksheets SetsDocument2 pages2021 Worksheets SetsSora RoseNo ratings yet
- Mathematics Project On Applications of Euclid's GeometryDocument20 pagesMathematics Project On Applications of Euclid's GeometrySora RoseNo ratings yet
- CLASS 11 Computer Science Practical AnswersDocument5 pagesCLASS 11 Computer Science Practical AnswersSora Rose100% (1)
- S BlockDocument6 pagesS BlockSora RoseNo ratings yet
- InstagramDocument1 pageInstagramSora RoseNo ratings yet
- FacebookDocument1 pageFacebookSora RoseNo ratings yet
- AispeeDocument1 pageAispeeSora RoseNo ratings yet
- AispeeDocument1 pageAispeeSora RoseNo ratings yet
- AispeeDocument1 pageAispeeSora RoseNo ratings yet
- Human Impact On The EnvironmentDocument39 pagesHuman Impact On The EnvironmentMyrna Ramento AppalNo ratings yet
- Good Morning Everyone.. Today I Sargha Will Be Delivering A Speech On How Malnutrition Affects Children and MothersDocument1 pageGood Morning Everyone.. Today I Sargha Will Be Delivering A Speech On How Malnutrition Affects Children and MothersSora RoseNo ratings yet
- Boq For Ifad Pump Project (Solar System With Drip & Microsprinkler Water Distribution)Document4 pagesBoq For Ifad Pump Project (Solar System With Drip & Microsprinkler Water Distribution)Mr. 420No ratings yet
- Solution Manual For Chemistry For Today General Organic and Biochemistry 8th EditionDocument37 pagesSolution Manual For Chemistry For Today General Organic and Biochemistry 8th Editionrepastgraffitie17pv100% (14)
- End Time ProphecyDocument16 pagesEnd Time ProphecyMarven JabianNo ratings yet
- Pro Boxberg en DownloadDocument6 pagesPro Boxberg en Downloadftzo3439No ratings yet
- Microwave TubesDocument36 pagesMicrowave TubesHINDUSTAN KNOW 1No ratings yet
- Harvia Sauna: Glass, light and beautiful contrast – read more about the new Harvia Claro sauna on page 11Document36 pagesHarvia Sauna: Glass, light and beautiful contrast – read more about the new Harvia Claro sauna on page 11Kadiri Olanrewaju100% (1)
- MWRC Policies & Procedures Manual 2020Document10 pagesMWRC Policies & Procedures Manual 2020Emma RyersonNo ratings yet
- Premium detergent market insights and Nirma case studyDocument32 pagesPremium detergent market insights and Nirma case studyBhavya ShahNo ratings yet
- Nurs478 Healthcaredelivery Audrey GohDocument12 pagesNurs478 Healthcaredelivery Audrey Gohapi-316372858No ratings yet
- Thermo Fluids LabDocument23 pagesThermo Fluids LabMuket AgmasNo ratings yet
- Cathodic Protection of Subsea Systems: Lessons LearnedDocument9 pagesCathodic Protection of Subsea Systems: Lessons LearnedNguyen Ninh Binh100% (1)
- PN Junction Formation and Barrier PotentialDocument9 pagesPN Junction Formation and Barrier PotentialchristlllNo ratings yet
- Global Leader: in Glass IonomerDocument2 pagesGlobal Leader: in Glass IonomerAnggini ZakiyahNo ratings yet
- SPE/IADC-189336-MS Pioneering The First Hydraulic Fracturing in Iraq's Complex ReservoirDocument12 pagesSPE/IADC-189336-MS Pioneering The First Hydraulic Fracturing in Iraq's Complex ReservoirKarar AliNo ratings yet
- Audi A6 Allroad Model 2013 Brochure - 2012.08Document58 pagesAudi A6 Allroad Model 2013 Brochure - 2012.08Arkadiusz KNo ratings yet
- D & C G T G N A. R E S P A, NY 12242: Esign Onstruction Roup HE Overnor Elson Ockefeller Mpire Tate Laza LbanyDocument18 pagesD & C G T G N A. R E S P A, NY 12242: Esign Onstruction Roup HE Overnor Elson Ockefeller Mpire Tate Laza LbanyAlexNo ratings yet
- Soalan Pecutan Akhir Fizik SPM 2010 Kertas 2 Set 3 PDFDocument18 pagesSoalan Pecutan Akhir Fizik SPM 2010 Kertas 2 Set 3 PDFAnna Latifah CammryNo ratings yet
- Transformer Protection Techniques for Fault DetectionDocument32 pagesTransformer Protection Techniques for Fault DetectionshashankaumNo ratings yet
- WHO Guidelines For Drinking Water: Parameters Standard Limits As Per WHO Guidelines (MG/L)Document3 pagesWHO Guidelines For Drinking Water: Parameters Standard Limits As Per WHO Guidelines (MG/L)114912No ratings yet
- Klubermatic Lubricant DispensersDocument13 pagesKlubermatic Lubricant Dispenserstatankise100% (1)
- Exe Summ RajatCement Eng PDFDocument12 pagesExe Summ RajatCement Eng PDFflytorahulNo ratings yet
- Cs Varnavrat Land SlideDocument7 pagesCs Varnavrat Land SlideBIJAY KRISHNA DASNo ratings yet
- Airworthiness Directives Record ControlDocument4 pagesAirworthiness Directives Record ControlJuan builesNo ratings yet
- Nigelaycardo 1Document8 pagesNigelaycardo 1ANGELICA AYCARDO FLORESNo ratings yet
- Optimized Die Steel Reference GuideDocument16 pagesOptimized Die Steel Reference GuideKeattikhun ChaichanaNo ratings yet
- Comparative Population Growth and Losses Cause by Beetle Trogoderama Granarium (Everts) To Selected Past and Present Wheat GenotypesDocument12 pagesComparative Population Growth and Losses Cause by Beetle Trogoderama Granarium (Everts) To Selected Past and Present Wheat GenotypesInternational Network For Natural SciencesNo ratings yet
- 08 Ergonomics - 01Document35 pages08 Ergonomics - 01Cholan PillaiNo ratings yet
- Shan - Consumer Evaluations of Processed Meat Products Reformulated To Be HealthierDocument8 pagesShan - Consumer Evaluations of Processed Meat Products Reformulated To Be HealthierMarcos RodriguesNo ratings yet
- Format Bahasa Inggeris UPSR 2016 ENGLISH (013) Section ADocument33 pagesFormat Bahasa Inggeris UPSR 2016 ENGLISH (013) Section AVINOTININo ratings yet
- Hazard Identification 2. Risk AssessmentDocument5 pagesHazard Identification 2. Risk AssessmentNoreen Syakireen Binti NormanNo ratings yet