Professional Documents
Culture Documents
Malmo 1981
Uploaded by
Víctor FuentesCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Malmo 1981
Uploaded by
Víctor FuentesCopyright:
Available Formats
THE BEHAVIORAL AND BRAIN SCIENCES (1981) 4, 459-514
Printed in the United States ot America
Reticulo-cortical activity and behavior: A
critique of the arousal theory and a new
synthesis
C.H. Vanderwolf
Department of Psychology, University of Western Ontario, London,
Ontario, Canada N6A 5C2
T.E. Robinson
Neuroscience Laboratory, University of Michigan, Ann Arbor, Mich. 48109
Abstract: It is traditionally believed that cerebral activation (the presence of low voltage fast electrical activity in the neocortex
and rhythmical slow activity in the hippocampus) is correlated with arousal, while deactivation (the presence of large amplitude
irregular slow waves or spindles in both the neocortex and the hippocampus) is correlated with sleep or coma. However, since
there are many exceptions, these generalizations have only limited validity. Activated patterns occur in normal sleep (active or
paradoxical sleep) and during states of anesthesia and coma. Deactivated patterns occur, at times, during normal waking, or
during behavior in awake animals treated with atropinic drugs. Also, the fact that patterns characteristic of sleep, arousal, and
waking behavior continue in decorticate animals indicates that reticulo-cortical mechanisms are not essential for these aspects of
behavior.
These puzzles have been largely resolved by recent research indicating that there are two different kinds of input from the
reticular activating system to the hippocampus and neocortex. One input is probably cholinergic; it may play a role in stimulus
control of behavior. The second input is noncholinergic and appears to be related to motor activity; movement-related input to
the neocortex may be dependent on a trace amine.
Reticulo-cortical systems are not related to arousal in the traditional sense, but may play a role in the control of adaptive
behavior by influencing the activity of the cerebral cortex, which in turn exerts control over subcortical circuits that co-ordinate
muscle activity to produce behavior.
Keywords: arousal; cerebral cortex; EEG; hippocampus; motor activity; reticular system; sleep
A considerable portion of any comprehensive current consciousness has been reproduced in many textbooks
textbook of neuroscience is usually devoted to the (Fig. 1). According to some authors, the relation
physiological basis of consciousness, attention, sleep, between internal states of arousal and overt behavior is
and arousal. A basic source of data for such discussions complex (Hebb 1955; Malmo 1959). Thus, total behav-
is the presumed relation between brain wave activity ioral immobility may occur during low arousal (as in
(electroencephalogram, EEG) and behavior. It is boredom, drowsiness, or stupor) or during high arousal
usually stated that the presence of low voltage fast (as in freezing from terror).
activity (LVFA) in the neocortex is a correlate of The above ideas originated in investigations carried
arousal, alertness, attention, or consciousness, while out mainly in the years between 1930 and 1960 (see
spindles and large amplitude slow activity are corre- reviews by Lindsley 1960 and Moruzzi 1972). During
lates of drowsiness, sleep, or coma. The definition of this period it was established that large amplitude slow
these various states in experimental animals is usually wave activity is very prominent in the neocortex
based on common sense criteria such as waking or during sleep while LVFA is generally present during
sleeping posture, spontaneous movement, and respon- the waking state. The differing characteristics of the
siveness to sensory stimuli. According to Rossi and human electroencephalogram (EEG) at various times
Zanchetti (1957, p. 386), "The close relationship that during normal sleep led to the recognition of a number
exists between EEG patterns and behavioral manifesta- of different stages of sleep (Hess 1964). The observa-
tions of wakefulness and sleep appears to justify the tion that dreaming is frequently associated with LVFA
assumption that electrocortical records may be (see below) led many investigators to assume that three
regarded as a reliable test of behavior, even in those distinct states of consciousness occur normally. 1)
preparations in which it is impossible to analyze waking consciousness, 2) dreaming consciousness, both
directly the behavior itself." A figure from Penfield associated with LVFA, and 3) loss of consciousness,
and Jasper (1954) which illustrates the widely accepted associated with large slow waves.
view that cortical wave activity is related to the level of The physiological basis of natural sleep-waking
Q1981 Cambridge University Press 0140-525X/81/030459-56/$04.00/0 459
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
EXCITED r occurs upon awakening, regardless of whether awaken-
ing happens naturally or as a result of stimulation of the
reticular formation. Although these observations
RELAXED
suggest that there is a relation between neocortical
I activity and stages in a sleep-waking continuum, other
facts indicate that the correlation is not a strong one.
Previous discussions (Mirsky & Pragay 1967; Moruzzi
DROWSY
1972) have not brought out the full importance of these
facts for the conventional theory of arousal.
There are two main classes of phenomena which
ASLEEP
indicate that the state of neocortical slow wave activity
(i.e., presence of LVFA, spindles, or large slow waves)
does not correlate well with arousal or consciousness as
DEEP SLEEP conventionally understood. Since many of these
phenomena are well known, they can be described
briefly.
1. Examples of the first class of phenomena consist
of instances in which neocortical LVFA occurs during
natural sleep or other states of low arousal or uncon-
sciousness.
a. Active sleep is a condition in which LVFA is
SOjiV
prominent in animals lying in a sleeping posture and
1 SEC.
relatively unreactive to stimuli. The musculature is
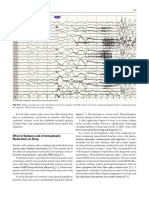
Figure. 1. Characteristic electroencephalograms during atonic but, despite this, twitches of a variety of muscle
variations in states of conciousness. (From Penfield & Jasper groups occur periodically (Dement & Kleitman 1957;
1954, p. 188; reprinted with permission of Little, Brown & Dement 1958; Jouvet 1967). The term "paradoxical
Co.) sleep" is sometimes applied to this stage, presumably
because its occurrence was puzzling in terms of the
original theory of arousal.
phenomena was clarified by a long series of investiga- b. During chemically induced anesthesia, the usual
tions which indicated that high levels of activity in the overt signs of arousal (waking posture, reactivity to
ascending reticular activating system (ARAS) produce stimuli) are largely abolished. Consequently, the ortho-
LVFA in the neocortex while low levels of activity in dox view of arousal would suggest that large slow
this system result in slow waves or spindles in the waves should be present. In fact, slow waves are not
neocortex. Slow waves or spindles may also occur as a always present when an animal is anesthetized. It is
result of increased activity in an ascending reticulocor- common for a form of LVFA to occur during the
tical slow wave inducing system which appears to act as surgical anesthesia produced by volatile anesthetics
an antagonist to the ARAS (Moruzzi 1972). A widely such as diethyl ether or choloroform (Beecher &
accepted interpretation of these findings is that natu- McDonough 1939; Bremer 1936; Rossi & Zirondoli
rally occuring sleep and waking states are dependent 1955; Vanderwolf, Kramis, Gillespie & Bland 1975). If
on ascending reticulo-cortical activity. LVFA is absent during surgical anesthesia, stimula-
Although the foregoing ideas originated many years tion of the reticular formation will usually produce it,
ago, they remain influential today. In the words of one but waking behavior will not necessarily appear
recent reviewer (Kandel 1979, p. 540) "Even a rapid (Moruzzi & Magoun 1949).
review of the literature of the last 10 years makes it c. Human patients in deep coma following brain-
clear that our understanding of the relation of the stem injury sometimes display normal or near-normal
reticular formation to behavioral arousal has not been LVFA in the electroencephalogram (Loeb, Rosadini &
significantly advanced" (since the late 1950s). We Poggio 1959). A similar phenomenon has been
believe that this assessment is inaccurate. There is a observed in cats following large lesions of the basal
wealth of data which shows that the textbook view of diencephalon (Feldman & Waller 1962) or following
reticulo-cortical activity and behavior described above transection of the pons at a midpontine pretrigeminal
(the arousal theory) is wrong in several basic respects. level (Batini, Moruzzi, Palestini, Rossi & Zanchetti
Further, we shall present an alternative point of view 1959).
which appears to provide a satisfactory interpretation 2. The second class of phenomena consists of
of most of the data in this field. instances in which large slow waves or spindles occur in
the neocortex during waking behavior.
a. A large number of investigators have shown that
How well does neocortical slow wave activity slow waves and spindles frequently occur in neocortex
correlate with the level of arousal? in normal rats and cats during such waking behaviors
as standing motionless with the head held up against
Even a casual examination of neocortical activity in a gravity and the eyes open, as well as during shivering
normal laboratory animal (usually a rat or cat) indi- (rats), grooming (rats and cats), and lapping milk (cats)
cates that long trains of slow wave activity are very (Buchwald, Horvath, Wyers & Wakefield 1964,
common during behavioral sleep but LVFA invariably Grandstaff 1969; Hackett & Marczynski 1969; Marc-
460 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
zynski & Hackett 1969; Marczynski, Rosen & Hackett anatomical differentiation is probably associated with
1968; Roth, Sterman & Clemente 1967; Rougeul 1958; functional differentiation as well. This suggests that the
Rougeul, Letalle & Crovisier 1972; Vanderwolf 1975; ascending reticular formation is concerned with a vari-
Whishaw & Vanderwolf 1971). It might be supposed ety of functions, some of which may be only indirectly
that normal waking animals displaying slow wave related to "arousal level" and sleep.
activity are less "aroused" or less "attentive" than they Finally, ablation experiments indicate that cortical
are when displaying LVFA. However, there is no activity is irrelevant to the main phenomena of sleep.
evidence of this. The animals remain fully reactive to Totally decorticate cats retain both the postures and
sensory stimuli when the slow waves are present, show- ocular activity characteristic of quiet sleep and the
ing, for example, good responses to conditioned stimuli atonia and muscular twitches characteristic of active
in learning tasks (Chase & Harper 1971; Rougeul et al. sleep (Villablanca & Marcus 1972). In rats, sleep
1972). Possible human analogies to this situation are postures and a circadian rhythm of motor activity
described by Larsson (1960) and Mulholland (1969), persist following total decortication (Vanderwolf,
who showed that the alpha rhythm, not LVFA, may Kolb & Cooley 1978).
predominate in humans engaged in tasks requiring We conclude that neocortical slow wave activity is
concentrated attention. not well correlated with arousal level or consciousness,
If one follows the logic underlying the use of the and further, that the states of sleep and waking are not
term "paradoxical sleep," then it would be reasonable dependent on the integrity of the cerebral cortex or of
to use the terms "paradoxical waking" or "paradoxical ascending reticulo-cortical projections.
alertness" to refer to situations in which slow waves are
present in the neocortex of alert waking animals. (We
do not seriously recommend the use of these terms!) A behavioral approach to brain function
b. Antimuscarinic drugs, such as atropine, produce
an abundance of large delta waves in the neocortex It is commonly assumed that a major goal of brain-
with no evidence of sleep or coma (Longo 1966; Wikler behavior research is the discovery of physiological
1952). Conversely, an anticholinesterase such as correlates of psychological processes, including "arous-
eserine produces LVFA without producing behavioral al," "activation level," "vigilance," and the related
arousal (Bradley & Elkes 1957; Bradley & Hance 1957; concepts of motivation, emotion, and drive. In this
Longo & Silvestrini 1957). A variety of other drugs also connection it is important to distinguish clearly
produce effects that do not conform to the orthodox between: 1) behavior, which can be defined approxi-
view of reticulo-cortical activity in relation to behavior. mately as the totality of the postures and movements
For example, small doses of reserpine produce behav- which an animal displays, and 2) psychological
ioral sedation together with continuous LVFA (Jouvet processes, which cannot be known directly, and whose
1967). existence is inferred on the basis of data obtained in
The foregoing phenomena are often accounted for some other domain. For many authors, "arousal" seems
by the hypothesis that neocortical activity and arousal to be such an inferred process, one whose status can be
are correlated under normal conditions but that some estimated on the basis of measuring such variables as
surgical and pharmacological manipulations of the brain waves, muscle tension, heart rate, or skin resis-
brain destroy this correlation, producing a "dissocia- tance (Lindsley 1951; Malmo & Belanger 1967). Other
tion" of cortical electrical activity from behavior. This authors who use the terms "aroused," "alert," "atten-
attempt to salvage the original arousal theory is unreal- tive," etc., mean by them nothing more than short-
istic for several reasons. First, reticulo-cortical activity hand designations for the fact that an animal is not
in normal animals is not related to behavior as would comatose or asleep and is engaged in ordinary behavior
be expected on the basis of the arousal theory. Sleeping such as standing up and walking about. We approve
animals may display either LVFA (active sleep) or wholeheartedly of the intent to use descriptive termi-
spindles and large amplitude slow waves (quiet sleep). nology. However, the terms to which we have referred
Similarly, waking animals may also display either do not describe what animals actually do in sufficient
LVFA (during most kinds of waking behavior) or detail to permit valid correlations with reticulo-cortical
spindles and large amplitude slow waves ("paradoxical activity. As we shall see, it is important to know
waking," point #2a above). Consequently, there is no whether animals are totally motionless or else moving
clear relation between the presence or absence of their head, walking about, chewing a piece of food,
LVFA and the occurrence of behavioral wakefulness or etc.
sleep. Second, the statement that a drug or surgical The distinction between behavior and inferred
lesion dissociates brain activity and behavior (if it were psychological processes is basic to a fundamental
true) contributes little to an understanding of how such difference of opinion on the best way to conduct
agents affect the brain and behavior. A comprehensive research in the brain-mind-behavior field. Many
theory is required to account for the two classes of psychologists regard behavior (in the sense intended
phenomena listed above as well as many others to be here) as a topic of interest chiefly insofar at it provides
discussed below. Third, advances in anatomical tech- a means of investigating the nature of the mind.
niques in the past 20 years have revealed that the According to one eminent authority (Hebb 1980, p. 1)
"reticular formation" of the 1950s comprises a variety "Mind is the central psychological problem. . . . It is
of ascending or descending cholinergic and aminergic inaccurate - worse, it is misleading - to call psychology
pathways (Azmitia 1978; Lewis & Shute 1978; Linde- the study of behavior." Hebb suggests that the mind
vall & Bjorklund 1978). It is widely recognized that this consists of the brain processes that control behavior.
THE BEHAVIORAL AND BRAIN SCIENCES (1981). 4 461
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
However, the complexity of these processes is such that Table 1. Abbreviations used in text
studies involving direct intervention in the nervous
system can make only limited headway. The mind
must be studied indirectly, by inference from behavior. ACh acetylcholine
As a guide for research, this approach suggests that one ARAS ascending reticular activating system
should: 1) study behavior in order to identify the CA catecholamine
presence of a specific psychological process, such as CAl cornu Ammonis 1
arousal, attention, motivation, or learning, then 2) DA dopamine
search in the brain for an anatomical, physiological, or EEG electroencephalogram
biochemical basis for the psychological process. It is LC locus coeruleus
probable that a large part of current brain-behavior LIA large amplitude irregular activity (hippo-
research is guided by this point of view. campus)
Students of behavior such as Skinner (1938, 1969, LSD-25 D-lysergic acid diethylamide
1974) or the European ethologists (Tinbergen 1951, LVFA low voltage fast activity (neocortex)
1972, 1973), believe that behavior should be investi- NA noradrenalin
gated as a phenomenon in its own right and not merely 6-OHDA 6-hydroxydopamine
as an index of processes occurring in the mind or the PGO ponto-geniculo-occipital (spike)
brain. As pointed out by Skinner (1950) in another RSA rhythmical slow activity (hippocampus)
context, an investigator who is preoccupied with the
problems posed by inferred processes is likely to regard
behavior as a topic of secondary interest only and is
unlikely to describe or analyze it effectively. although the specific waveforms produced in the
A behavioristic or ethological approach is compatible neocortex are different from those in the hippocampus
with the view that direct measures of brain activity (archicortex). Stimulation produces LVFA in the
(electrophysiological, biochemical) can be correlated neocortex but rhythmic slow activity in the hippocam-
with behavior itself without the necessity of hypothe- pus (RSA; theta). Since neocortical LVFA was thought
sizing a psychological concept. According to this point to be correlated with arousal, and since the occurrence
of view behavior should first be accurately described in of hippocampal RSA initially appeared to be correlated
terms of posture and movement without reference to with the occurrence of LVFA, the RSA waveform
inferred psychological processes. A level of detail must came to be known as the hippocampal "arousal
be chosen which is appropriate to the problem being rhythm."
investigated. For example, the study of spinal reflex
In studying the relations of hippocampal electrical
physiology typically requires describing muscle activ-
ity in more detail than does the study of the forebrain activity to behavior, the technique initially employed
control of behavior. This initial step can be followed by consisted simply of recording hippocampal slow wave
an analysis of: 1) environmental or internal physiolog- activity during spontaneous behavior in a small record-
ical factors (hormones, interoceptors) that determine ing cage or other apparatus (using manually operated
the occurrence of particular behaviors, and 2) the signal markers and a movement sensor). The behaviors
relations among measures of brain activity, the occur- examined included locomotion, head movement, sniff-
rence of particular behaviors, and the presence of ing, components of feeding, mating, grooming, swim-
behavior-controlling factors such as environmental ming, digging, sleep, response to various sensory stim-
stimuli. uli, and performance in simple learning tasks. As far as
possible, behavior was described in terms of movement
If results with broad applicability are to be obtained, and posture. Inferential terms such as perception,
it is important to study a wide range of behaviors, attention, arousal, motivation, memory, and so on,
especially in the early phases of investigation (Lorenz were deliberately avoided in describing the behavior of
1973; O'Keefe & Nadel 1978). Behaviors do not all the animals.
depend on the same control mechanisms and do not all
As work progressed it became evident that clear
operate according to the same rules. As problems
become more clearly defined, research can be focused correlations between slow wave activity and behavior
on a few key areas but it is a mistake to restrict could be obtained only if recording electrodes were
investigation at the outset to a small number of arbi- placed correctly in the generator zones for the hippo-
trarily chosen tests. campal slow waves. The critical importance of this
factor has been emphasized in a recent review (Robin-
son 1980). Since the slow wave potentials in stratum
oriens of area CAl and in stratum granulosum-molecu-
Hippocampal slow wave activity and behavior lare of the dentate gyrus are of opposite phase (Bland,
Andersen & Ganes 1975; Bland & Whishaw 1976;
This general approach wasfirstapplied to an investiga- Winson 1974, 1976a, 1976b), a difference recording
tion of the behavioral correlates of hippocampal slow from electrodes placed in these areas will sum their
wave activity (Vanderwolf 1969; Vanderwolf et al. potentials, producing a signal with an amplitude of up
1975). The hippocampus, like the neocortex, is strongly to 3 mV. Electrical activity generated by other nearby
influenced by the ascending reticular formation brain structures tends to yield in-phase signals with
(Green & Arduini 1954). Sensory stimulation, or elec- strongly attenuated amplitudes. By this means, it has
trical stimulation of the reticular formation, produce been possible to identify consistent relations between
changes in the electrical activity throughout the cortex, behavior and hippocampal slow wave activity without
462 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
resorting to averaging or spectral analytical proce- ing, the startle response, vocalization, shivering,
dures. Moreover, when computational methods have tremor, face-washing, scratching the fur, pelvic thrust-
been applied, in conjunction with adequate records of ing, ejaculation, defecation, urination, and piloerec-
behavior, they have confirmed the conclusions drawn tion. Many of the behaviors in this class have a
from visual inspection of the analogue records relatively simple reflexive or consummatory character
(Arnolds, Lopez Da Silva, Aitink & Kamp 1979a, and have been referred to as automatic or Type 2
1979b, 1979c; Black 1975; Coenen 1975; Morris & behavior (defined as behavior with no consistent rela-
Black 1978). tion to RSA). If behaviors of the two groups occur
When adequate recordings are taken from the simultaneously, RSA also occurs. For example, a short
hippocampus of a freely moving rat, the wave pattern burst of RSA appears against a background of LIA if a
observed at a fixed electrode changes rapidly from rat changes posture while licking water continuously
moment to moment in relation to concurrent behavior. from a dish.
Thus, if a rat is standing motionless, head held up and A major finding was that RSA is correlated with
eyes fully open (alert immobility), large amplitude overt behavior in itself rather than with the environ-
irregular activity (LIA) is generally present, but RSA mental circumstances or the psychological processes
appears if the rat takes a step forward. RSA is contin- which might be assumed to cause the behavior. Thus,
ually present when a piece of food is picked up and locomotion is always accompanied by RSA regardless
carried about but it gives way to LIA if the rat pauses of whether it occurs in a novel environment ("explora-
for a moment and stands motionless. When eating a tion"); as a result of food deprivation, treatment with
large piece of solid food, rats usually hold it motionless amphetamine, or pinching of the tail; or following
between their paws, squirrel-fashion, when chewing a training to obtain food or avoid shock in tests of
mouthful, but they manipulate it actively with mouth operant behavior. RSA also persists if rats are forced to
and forepaws whenever a fresh bite is taken. Such walk for hours in a motor driven treadmill or trained to
manipulation is accompanied by RSA, but LIA occurs jump out of a box several thousand times in quick
during chewing, provided that the rat is truly motion- succession (Vanderwolf & Cooley 1974; Whishaw &
less (apart from respiration and the rhythmic jaw Vanderwolf 1973). If a drowsy rat changes posture, for
movements). If a rat sits up to wash its face, RSA example, changing from a position of lying on the left
appears briefly during the change in posture but LIA side to lying on the right side, RSA accompanies the
appears during the rhythmic movements of face- movement although the eyes remain closed. On the
washing. If the rat then changes from face-washing to other hand, behavioral immobility is accompanied by
licking the genitalia, RSA again appears briefly during LIA in most conditions reagardless of the level of
the change in posture but LIA appears during the "arousal" or "excitement" a human observer might
licking movements. attribute to the rat. Thus, LIA is observed when a rat is
A large number of observations of this type have motionless, resting quietly on the floor, but it is also
suggested that rat behavior in general can be divided observed when the rat has just received a strong electric
into two broad classes (Fig. 2). One class - including shock to the feet which (after an initial violent scram-
walking, running, swimming, rearing, jumping, ble accompanied by RSA) causes it to stand motionless
digging, and manipulation of objects with the fore- with its hair erect, its eyes protruding slightly, and its
limbs, isolated movements of the head or one limb, and teeth chattering loudly. Further, during fighting, LIA
changes of posture - is always accompanied by RSA in is present in a rat while it is being bitten by another rat,
the hippocampus. This class has been referred to as provided that it remains motionless (Frederickson,
voluntary or Type 1 behavior. The second class of Miczek, Zurawin & Frederickson 1977).
behaviors, whose performance is ordinarily not accom- A further point is that RSA does not seem to be a
panied by RSA, include immobility (in any posture) as result of proprioceptive or cutaneous feedback from
well as licking, chewing, chattering of teeth, sneez- the accompanying movements since it is not abolished
by curarization and is not produced by a purely passive
movement imposed by the experimenter. Thus, RSA
NORMAL RELATION OF HIPPOCAMPAl ACTIVITY TO BEHAVIOR IN THE RAT
may play some role in the control of motor activity.
HIPPOCAMPUS BEHAVIOR
Since RSA can be generated by stimulation of the
Type I. walking, running, swimming, rearing,
reticular formation and can be totally abolished by
jumping, digging, manipulation of objects interruption of reticulo-septo-hippocampal pathways,
with the (orelimbs. isolated movements of
the head or one limb, shifts ol posture. its occurrence under physiological conditions is proba-
Related terms: voluntary, appetitive.
bly due to increased activity in the ascending reticular
Instrumental, purposive, operant, or "theta"
behavior.
formation (Green & Arduini 1954; Brugge 1965).
Therefore, the behavioral data suggest that there is a
reticulo-hypothalamo-septo-hippocampal pathway
Type 2. a) aleri immobility in any posture.
t>) licking, chewing, chattering the teeth,
which becomes more active if, and only if, Type 1
sneezing, startle response, vocalization,
shivering, tremor, face-washing, scratching
behavior is being performed.
the fur, pelvic thrusting, ejaculation, defecation,
urination, piloerection.
Related terms; automatic, reflexive, consummatory.
respondent, or "non-theta" behavior.
Two types of RSA
Figure. 2. Normal relation of hippocampal activity to
behavior in the rat. Upper record, RSA; lower record LIA. A major complication with the simple picture outlined
(From Vanderwolf et al. 1975, with permission.) thus far is the fact that RSA is very prominent in some
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 463
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
circumstances when animals are largely or entirely However, the RSA accompanying Type 1 behavior is
motionless. Rats anesthetized with urethane, ethyl alco- resistant to this drug.
hol, or volatile anesthetics (ether, chloroform, ethylene Comparable effects occur in rats. It has been known
chloride) show long trains of RSA spontaneously or in for several years that the RSA accompanying Type 1
response to sensory stimuli. Movement, of course, is behavior in rats is not abolished, even by extremely
absent in this condition. In some species, RSA is promi- large doses of atropine (up to 150 mg/kg, i.p.) or
nent in the absence of movement even in the normal scopolamine (10 mg/kg, i.p.) (Vanderwolf, Bland &
waking state (Harper 1971; Kramis, Vanderwolf & Whishaw 1973; Vanderwolf 1975). In contrast, the RSA
Bland 1975; Robinson, 1980; Winson 1972). For exam- occuring during anesthesia (induced by volatile anes-
ple, if a rat is startled by the sound of a sudden thetics, alcohol, or urethane) is abolished by atropinic
handclap when it is lying down quietly, it is likely to drugs. If atropine is administered first, followed by a
leap to its feet, then stand motionless with its eyes volatile anesthetic, RSA ceases to appear at approxi-
opened widely. A hippocampal recording made during mately the same time that struggling movements cease.
this behavior shows either continuous LIA or a transi- Therefore, it appears that the RSA component which is
tion from LIA (accompanying the behavior of lying resistant to atropine is sensitive to anesthetics (Kramis
motionless) to a pattern of small amplitude irregular et al. 1975; Vanderwolf et al. 1975; Vanderwolf et al.
activity (SIA). RSA is not observed unless the rat makes 1978; Whishaw 1976). This suggests that there are not
additional head movements or runs away. In contrast, one, but two distinct reticulo-hippocampal pathways,
with a rabbit, a sudden handclap will produce a long each capable of producing RSA. One pathway, resis-
train of RSA even though the animal remains perfectly tant to atropine but sensitive to anesthetics, is active in
motionless, apart from a brief startle response. Such waking rats and rabbits only in relation to the occur-
observations, plus similar ones made in cats, have led to rence of Type 1 behavior. The second pathway, sensi-
a good deal of controversy concerning the behavioral tive to atropine but resistant to volatile anesthetics, can
correlates of hippocampal RSA (Bennett 1975; Cole- be active during waking immobility, especially in
man & Lindsley 1975; Kemp & Kaada 1975; Klemm rabbits. The existence of two such pathways has
1976). recently been confirmed by a study of hippocampal
A solution to these problems is provided by evidence rhythms evoked by rhythmic bursts of electrical stimu-
that RSA is not a unitary phenomenon (Fig. 3). If a lation of the medial septum (Kramis & Vanderwolf
waking rabbit is given atropine SO4 (5 mg/kg, i.v.) all 1980).
traces of RSA during behavioral immobility or face- The atropine-sensitive and anesthetic-sensitive com-
washing quickly vanish but the RSA accompanying
ponents of RSA differ in mean frequency although
head movements, postural changes, and hopping
persists almost unchanged. Thus the relations of RSA to there is some overlap in the two frequency distribu-
behavior in an atropine-treated rabbit are very similar tions. In both rats and rabbits the RSA accompanying
to those seen in an undrugged rat; RSA occurs if, and Type 1 behavior ranges in frequency from about 7-12
only if, certain motor patterns occur (Kramis et al. Hz while the RSA which sometimes accompanies
1975). Like rats, rabbits display RSA when they are waking immobility or other Type 2 behavior usually
anesthetized with urethane, ethyl alcohol, or volatile has a frequency of about 6 Hz. In the anesthetized state
anesthetics. Such RSA is abolished readily by an injec- RSA frequency may be as low as 4 Hz.
tion of atropine SO4 (Kramis et al. 1975; Vanderwolf, Another line of evidence which suggests that RSA
Kramis & Robinson 1978; Whishaw 1976). Thus, RSA does not constitute a unitary phenomenon comes from
occuring in rabbits during behavioral immobility in the studies of the ontogeny of hippocampal electrical activ-
anesthetized as well as during the unanesthetized state ity (Creery & Bland 1980). By 8 days of age rabbits
is sensitive to atropine. The RSA which accompanies show the correlation between Type 1 behavior and
face-washing in rabbits is also sensitive to atropine. RSA characteristic of the adult. However, the lower
frequency RSA induced (in the absence of Type 1
behavior) by sensory stimulation or an anticholinester-
NO ATROPINE ATROPINE ase drug does not develop until the rabbit is 14 days of
age. The differences in the rate of development of the
NO
ETHEf
I^VM' 1 ' 1 !!'!!!!,'' 1 1 ! ''''iii1')1"'1''!!)
Av* fl|il|'*|f|!l( 1.0
two types of RSA may be due to differences in the
mV
immobile walking im mobile * walking ontogenesis of two different neurotransmitter systems
involved in the generation of RSA.
Atropine-sensitive RSA may result from activity in a
retieulo-septo-hippocampal pathway which contains
muscarinic cholinergic synapses at one or more points.
Evidence favoring this hypothesis is based mainly on
studies using systemic administration of drugs, a
method that raises many problems of interpretation.
Figure. 3. Hippocampal slow wave activity under four However, it has been shown that the RSA occurring
conditions. (1) Undrugged state; (2) after atropine SO4 (50
mg/kg, i.p.); (3) during ether anaesthesia, (4) during ether during Type 2 behavior or anesthesia, which is sensi-
anaesthesia after atropine SO4 (50 mg/kg, i.p.). Note: low tive to atropine or scopolamine, is not abolished by a
frequency RSA during anaesthesia, higher frequency RSA variety of other drugs, including reserpine, nicotinic
during walking, and total absence of RSA if atropine is blockers such as mecamylamine, various antipsychotic
combined with anaesthesia. (From Vanderwolf et al. 1978, drugs, enzyme blockers such as a-methyl-p-tyrosine
reprinted with permission of Excerpta Medica.) and p-chlorophenylalanine as well as various anes-
464 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
thetics (Assaf & Miller 1977; Vanderwolf et al. 1978). doses of 25-50 mg/kg to produce an extensive occupa-
Further, the injection of cholinergic agonists (acetyl- tion of muscarinic cholinergic receptor binding sites in
choline, eserine, diisopropylfuorophosphate) by a vari- rat brain. Such doses are considerably larger than those
ety of routes (including intracarotid injections) required to block peripheral muscarinic synapses. The
produces long trains of RSA without producing behav- high doses necessary for effects on the brain are at least
ioral activation, i.e., without producing Type 1 move- partly due to the fact that atropine or atropine SO4 do
ment (Monnier & Romanowski 1961; Sailer & Stumpf not penetrate the brain nearly as well as they penetrate
1957; Bradley & Nicholson 1962; Torii & Wikler 1966; other tissues (Albanus, Hammarstrom, Sundwall,
Vanderwolf 1975). Such RSA can be abolished by a Ulberg & Vangbo 1968; Albanus, Sundwall, Vango &
subsequent injection of atropine. Windbladh 1968; Gosselin, Gabourel, Kaiser & Wills
An additional piece of evidence which supports the 1955; T^nnesen 1948). Further, the data of Whishaw,
concept of a cholinergic pathway producing RSA Bland & Bayer (1978) on the effects of X-irradiation of
during Type 2 behavior and anesthesia is the recent neonatal rats on subsequent development of hippocam-
finding of Robinson & Green (1980) that intraventricu- pal RSA suggest that normal rhythms may persist even
lar injections of hemicholinium, which deplete brain when only a fraction of the normal cell population is
acetylcholine (ACh), abolish RSA in urethane-anesthe- capable of activity. Thus, atropine may abolish immo-
tized rats. The RSA accompanying Type 1 behavior in bility-accompanying RSA only when it is present in
freely moving rats is unaffected, supporting the concentrations large enough to block virtually all react-
hypothesis that a noncholinergic pathway is active in ing cells.
producing RSA in association with such behavior. If atropine-sensitive RSA is dependent on activity in
A probably anatomical and biochemical basis for the cholinergic synapses, an obvious question is raised.
production of "cholinergic" RSA has been described in What neurotransmitter system(s) is necessary to
a series of studies of cholinergic reticulo-septo-hippo- produce atropine-resistant RSA? It has been suggested
campal pathways (Kuhar 1975). The evidence for such that catecholaminergic neurons ascending from the
pathways includes the demonstration of reticulo- locus coeruleus may be involved in the production of
septo-hippocampal fibres containing acetylcholinester- RSA, since stimulation of the locus coeruleus has been
ase (Lewis & Shute 1967). Recent studies using immu- reported to produce RSA in cats (Macadar et al. 1974).
nohistochemical methods have shown that cells in the This suggestion is supported by studies on the effects of
medial septum and diagonal band contain not only adrenergic drugs on hippocampal electrical activity.
acetylcholinesterase, but choline acetyltransferase as Intracarotid injections of adrenaline or noradrenaline
well (Kimura, McGeer, Peng & McGeer 1980). Septal (NA) (Kawamura and Oshima 1962), or systemic injec-
lesions result in a loss of choline acetyltransferase tions of amphetamine produce hippocampal RSA
activity in the hippocampus, suggesting that some (Bradley & Nicholson 1962; Longo 1962; Stumpf, 1965;
septo-hippocampal fibres contain this enzyme (Lewis, Vanderwolf 1975).
Shute & Silver 1967). High-affinity uptake of choline The neuroanatomical basis for a role of the locus
and the acetylcholine content of the hippocampus are coeruleus in the modulation of hippocampal activity
also reduced by septal lesions (Kuhar, Sethy, Roth & has been well documented with a variety of techniques
Aghajanian 1973). Thus, there appear to be septo- (see Moore 1975 for review). Fluorescence histochemi-
hippocampal fibres which take up choline and which cal and immunohistochemical studies have revealed
possess the apparatus for the manufacture, storage, and that noradrenaline-containing (or dopamine /3-hy-
destruction of acetylcholine. Stimulation of the septal droxylase-containing) cells in the locus coeruleus give
nuclei or the reticular formation (Smith 1972; Dudar rise to fibers which ascend via the median forebrain
1975) results in a release of acetylcholine from the bundle to innervate the hippocampus. Locus coeruleus
hippocampus. Since muscarinic receptors appear to be or medial forebrain bundle lesions greatly reduce the
present in the hippocampus (Biscoe & Straughan 1966; NA histofluorescence (Ungerstedt 1971) and assayed
Yamamura, Kuhar & Snyder 1974), such released NA levels in the hippocampus (Kobayashi et al. 1974).
acetylcholine might be expected to have postsynaptic In addition, hippocampal pyramidal cells usually
effects, including the generation of RSA. Ott, Malish & respond to micro-iontophoretically applied NA with a
Krug (1977) have shown that RSA can be produced by long latency, long duration inhibitory response (Biscoe
the injection of a cholinergic agonist (oxotremorine) & Straughan 1966; see Straughan 1975 for review). The
directly into the hippocampus. However, since hemi- inhibitory effect of NA is blocked by a /3-adrenergic
cholinium may block hippocampal RSA when it is
antagonist and facilitated by desmethylimipramine,
injected into the hypothalamus there may be choliner-
gic neurons involved in RSA production at this site as which prevents NA re-uptake (Segal & Bloom 1974).
well (Friedman & Wikler 1970). Lastly, the hippocampus contains /3-adrenergic recep-
tors (Melamed et al. 1977).
It is curious that the RSA occurring during anesthesia However, further studies strongly suggest that NA is
or Type II behavior in the waking state can be not important for the production of hippocampal RSA.
abolished by atropine (base), atropine SO4, or scopol- In rats, electrical stimulation of the locus coeruleus is
amine HBr only with relatively large doses. In neither ineffective in producing hippocampal RSA, except at
rats nor rabbits are intravenous doses of atropine SO4 of very high current intensities (Robinson et al. 1977;
less than about 5 mg/kg consistently effective. With Robinson & Vanderwolf 1978; M. Segal, personal
intraperitoneal injection in rats, a dose of about 25 communication). Earlier reports (Macadar et al. 1974
mg/kg is required. Similarly, Yamamura, Kuhar & that stimulation of the locus coeruleus does produce
Snyder (1974) showed at atropine must be given in RSA may have been due to current spread to the
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 465
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
underlying reticular formation. Stimulation of the tex (Krnjevic 1974; Phillis 1976). Strong evidence for
reticular substance just ventral to the locus coeruleus is an ascending cholinergic activating system is provided
very effective in producing RSA (Robinson et al. 1977). by studies which show that electrical stimulation of the
In addition, atropine-resistant RSA, which is correlated midbrain reticular formation increases the rate of ACh
with Type 1 behavior, fails to be abolished by a variety release from the neocortical surface and produces
of treatments that deplete brain NA, including: 1) LVFA (Kanai & Szerb 1965; Szerb 1967). Many
systemic neonatal injections of 6-hydroxydopamine (6- neocortical cells have been shown to be cholinoceptive
OHDA; Robinson et al. 1977); 2) systemic injections of (for reviews see Phillis 1974, 1976). Post-synaptic
a-methyl-p-tyrosine or FLA-63 (Robinson et al. 1977); effects of ACh on these cells may be mediated by
or 3) intraventricular injections of 6-OHDA in adult muscarinic receptors, which have been shown to be
rats (Whishaw et al. 1978). Electrolytic lesions of the located on cortical neurons (Krnjevic & Phillis 1963a,
locus coeruleus also fail to abolish atropine-resistant 1963b; Yamamura, Kuhar, Greenburg & Snyder 1974;
RSA (Kolb and Whishaw 1977). Since some of these Yamamura et al. 1974).
treatments also severely deplete brain dopamine (e.g., Taken together all these data provide strong
a-methyl-p-tyrosine or intraventricular 6-OHDA) it is evidence that the "cholinergic reticular formation"
unlikely that either catecholamine is necessary for the plays an important role in controlling neocortical acti-
production of atropine-resistant RSA. vation. Therefore, in the context of the traditional
Thus, the neurotransmitter(s) involved in a noncho- arousal theory, it is surprising that antimuscarinic
linergic reticulo-hippocampal pathway responsible for drugs such as atropine result in the appearance of
the production of RSA in association with Type 1 extensive large amplitude slow wave activity in an
behavior, remain unknown. [According to C. Destrade apparently alert active animal.
and T. Ott, (personal communication) glutaminergic Consequently, it appeared to us, worthwhile to rein-
synapses may be involved.] vestigate the effects of atropine in rats, using the same
behavioral methods which had proved successful in
work on the hippocampus. During the course of the
Neocortical activation and behavior: atropine studies it was found that the location of the
Cholinergic influences recording electrodes plays a key role in the type of data
obtained (Fig. 4). Conventional bipolar screw elec-
A good deal of evidence from early studies suggests trodes do not reveal clearly some of the effects of
that acetylocholine (ACh) is also involved in the atropine on neocortical activity. The reason for this is
production of neocortical LVFA. The injection of ACh that the large slow waves produced by atropine are
or other cholinergic agonists by a variety of routes,
including local application to the cortical surface, abol-
ishes large amplitude slow waves or produces neocorti-
cal LVFA (Bonnet & Bremer 1937; Bremer & Chaton-
net 1949; Miller, Stavraky & Woonton 1940; Monnier
& Romanowski 1961). Low rates of ACh release from
the neocortical surface have been shown to be asso-
ciated with large slow waves during slow wave sleep,
following brainstem transection, and during chloralose
or barbiturate anaesthesia, whereas high rates of ACh
release and LVFA tend to occur together in the waking
state and in active sleep (Celesia & Jasper 1966; Jasper
& Tessier 1971; Kanai & Szerb 1965; Sie, Jasper &
Wolfe 1965; Szerb 1967). It is interesting that volatile
anaesthetics, which permit the occurrence of a good
deal of LVFA, also permit higher rates of ACh release
than such anaesthetics as chloralose and barbiturates, Figure. 4. Slow wave activity in the parietal neocortex of a
which modify or abolish LVFA (Phillis 1968). Lastly, rat following atropine SO4 (50 mg/kg) and trifluoperazine (10
there is an extensive literature claiming that choliner- mg/kg) injection. LSD, left surface-to-depth bipolar lead;
RSD, right surfact-to-depth bipolar lead; RD, monopolar
gic antagonists, such as atropine and scopolamine, record from right cortex 1 mm below surface; RS, monopolar
abolish LVFA, resulting in an animal that emits contin- record from right cortical surface. In surface-to-depth elec-
uous slow waves (Bradley & Elkes 1953, 1957; Funder- trode pairs an upward deflection indicates relative negativity
burk & Case 1951; Montplaisir 1975; Rinaldi & at deep electrodes. In monopolar leads an upward deflection
Himwich 1955; Torii & Wikler 1966; Wikler 1952). indicates negativity at the active lead (with respect to an
The effects of ACh on neocortical electrical activity indifferent referent in the skull over the cerebellum). Left
may very well be mediated by the "ascending choliner- panel. Note large slow waves during immobility and low
voltage fast waves during struggling induced by handling.
gic reticular formation" described by Shute & Lewis Changes are especially clear in SD derivations. Right panel.
(1967). This system of ascending reticulo-cortical fibers Note that: (a) activity in right and left cortices is closely in
contains acetylcholinesterase (Shute & Lewis 1967; phase, and (b) activity at surface and deep sites in the right
Jacobowitz & Palkovits 1974), may contain choline cortex tends to be of opposite phase. Time marks indicate 1
acetyltransferase (Hebb, Krnjevic & Silver 1963; Hoov- and 5 sec intervals. (From Vanderwolf & Pappas, 1980;
er, Muth & Jacobowitz 1978), and is, therefore, thought reprinted with permission of Elsevier/North-Holland Bio-
to contribute a diffuse cholinergic input to the neocor- medical Press.)
466 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
closely in phase over wide areas of neocortex within a such as alert immobility, face washing, licking or biting
hemisphere, and waves in one hemisphere are in phase the fur, gnashing the teeth, chewing food, or drinking
with those in the other. Consequently, bipolar screw water. However, at times, especially during prolonged
electrodes will be exposed to in-phase signals which periods of alert immobility with the head held up and
will largely be rejected by the differential amplifiers the eyes open ("freezing"), LVFA may suddenly give
generally used in laboratories. Monopolar records way to 6-9 Hz rhythmical spindle activity, which often
taken from the pial surface (used, for example by assumes a spike-and-wave pattern with an amplitude
Bradley & Elkes 1953) also do not reveal the relations up to 2 mV (Klingberg 1971; Semba et al. 1980;
to behavior very clearly, although they are obvious in Vanderwolf 1975). These waves are frequently accom-
monopolar records taken from a point about 1 mm panied by a rapid tremor of the vibrissae and head but
below the pial surface. Slow waves recorded monopo- they never occur during even slight head movements
larly from the pial surface tend to be phase-reversed of the type displayed during exploratory scanning,
with respect to the large waves recorded monopolarly searching for food, etc., or during any other Type 1
about 1 mm below the surface (Schaul, Gloor, Ball & behavior. Mild sensory stimuli or stimulation of the
Gotman 1978). Consequently, a bipolar surface-to- reticular formation also readily abolish these waves.
depth recording yields slow waves of especially large Spontaneous 6-9 Hz spindle activity can be recorded
amplitude (often 2 mV) while rejecting the largely in a wide area of neocortex in the rat. However, it is
in-phase signals from other sources, such as the hippo- morphologically similar to the rhythmic afterdischarge
campus. Using such electrodes it is possible to demon- localized to striate cortex following a light flash
strate clearly phenomena which are revealed only (Kimura 1962). The flash-triggered afterdischarge, like
imperfectly (or not at all) by electrodes placed on the the spontaneous spindle activity, has a relation to
mopial surface (Figs. 4 & 5). behavior which is reminiscent of the relation of hippo-
When the relation of neocortical activity to behavior campal RSA to behavior in a waking normal rat. Thus,
was studied in normal undrugged rats it was observed Pickenhain & Klingberg (1965) observed that rhythmic
that LVFA was invariably present during head move- afterdischarges can be evoked in waking rats during
ment, locomotion, and other behavior of the type immobility or grooming behavior but not during loco-
normally associated with hippocampal RSA. LVFA is motion.
also usually present during the occurrence of many Small doses of atropine SO4 (0.1-1.0 mg/kg, i.p.)
waking behaviors not normally associated with RSA, produce no clear change in the normal patterns of
neocortical activity but a larger dose (5.0 mg/kg)
results in occasional bursts of large amplitude slow
CONTROL wave activity; these occur only during behavioral
A CORTEX immobility. Such large slow waves occur in a pattern
similar to the one observed during natural slow wave
BEHAVIOR /
sleep, as originally noted by Funderburk & Case (1951;
cf. Montplaisir 1975). Doses larger than 5 mg/kg
increase the occurrence of these large slow waves. At
h h > an atropine dose of about 25 mg/kg, LVFA virtually
ceases to occur during periods of immobility but it
ATROPINE persists during head movement, walking, rearing,
struggling when held, and other Type 1 movements
f'l^^^^^^-^ljt (Vanderwolf 1975). Such persisting LVFA can be
referred to as atropine-resistant LVFA since it is unaf-
fected by doses of atropine as large as 150 mg/kg. The
large slow waves produced by atropine occur during
such behaviors as tremor or chattering of the teeth, just
as they do during total immobility. During face-
washing LVFA accompanies the postural changes
involved in sitting up on the hind legs and the initial
part of the face-washing sequence. However, if face-
washing persists for several seconds without noticeable
changes in posture, large slow waves appear. Thus, the
occurrence of LVFA in an atropinized rat is associated
with the same general type of behavior as that asso-
ciated with RSA in an undrugged rat. A further parallel
with RSA is observed in the finding that a purely
Figure 5. Neocortical slow wave activity in relation to passive movement (imposed by the experimenter) is
behavior in a rat. A: control record after trifluoperazine H Cl not associated with atropine-resistant LVFA. This
(5 nig/kg, i.p.); B: after addition of atropine SO4 (50 mg/kg, suggests that atropine-resistant LVFA is not due to
i.p.); C: low speed record after trifluoperazine and atropine. proprioceptive feedback from moving body parts, a
h, head movement; S, stepping. Note relation between slow conclusion which is supported by the fact that the wave
waves and motor activity in B and C. Trifluoperazine coun- pattern continues to occur spontaneously in rats that
teracts the excessive motor activity produced by atropine. are curarized (Whishaw, Bland, Robinson & Vander-
(From Vanderwolf et al. 1978, reprinted with permission of wolf 1976). Presumably, atropine-resistant LVFA
Excerpta Medica.)
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 467
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
would remain correlated with action potentials in the Type 1 behavior in a normal undrugged rat. It may
motor nerves of curarized animals, but this point has also be responsible for the suppression of flash-
never been examined. triggered afterdischarges during Type 1 behavior, as
The presence of large amplitude slow waves in the described by Pickenhain & Klingberg (1965).
neocortex of a motionless rat might be regarded as an
indication of inattention or even of sleep. However,
immobility may sometimes be a sign of arousal and Neocortical activation and behavior:
attention. If an active atropinized rat is startled by a Monoaminergic influences
handclap or another sudden stimulus it will sometimes
stand absolutely motionless for several seconds (freez- Numerous investigations have indicated that brain
ing behavior), usually facing the source of the stimulus. monoaminergic systems may play a role in the activa-
On other occasions the sudden stimulus may be tion of the neocortex. Some experiments have
followed by an increase in head movements, stepping, suggested that adrenalin or noradrenalin may be a
and sniffing. Both types of reaction might equally be transmitter in the ascending reticular formation (Cor-
regarded as indicative of arousal or attention but, deau, Champlain & Jacks 1971; Dell 1958; Rothballer
nonetheless, they are correlated with different patterns 1956). Other studies have not favored this hypothesis
of slow waves. Freezing behavior in this situation is (Baust, Niemczyk & Vieth 1971; Mantegazzini, Poeck
invariably associated with large slow waves while head & Santibanez 1959). However, the idea that ascending
movements and stepping are associated with LVFA. norandrenergic fibers may be involved in neocortical
Thus neocortical slow wave activity in an atropinized activation was revived with the discovery of diffuse
rat is more closely correlated with overt behavior than noradrenergic projections from the locus coeruleus to
with inferred processes such as attention or arousal. the hippocampus and neocortex (Anden, Dahlstrom,
Further, the relations of slow wave activity to behavior Fuxe, Larsson, Olson & Ungerstedt 1966).
are identical in a situation involving shock avoidance Jouvet has been the most vigorous proponent of the
performance and one involving spontaneous behavior idea that the ascending NA projections from the locus
on a small open platform (Vanderwolf 1975). It there- coeruleus are important for the electrocortical activa-
fore appears that the neocortical activation accom- tion occurring during waking (Jouvet 1972, 1974,
panying a given behavior is similar regardless of 1977). The evidence to support this idea is derived
whether the behavior occurs spontaneously or as a primarily from studies involving pharmacological
result of training. manipulations or lesions of the locus coeruleus; it
If an atropinized rat is anesthetized with a volatile includes the following: 1) Increasing the brain levels of
anesthetic such as diethyl ether or trichloroethylene, catecholamines (CA) by administrating L-dopa causes
the atropine-resistant LVFA disappears at approxi- behavioral and EEG arousal; 2) depletion of brain CA
mately the same level of anesthesia that struggling causes a decrease in the behavioral and EEG arousal
movements cease. It cannot then be elicited at all, even which usually follows amphetamine administration; 3)
by painful peripheral stimulation or by strong stimula- destruction of the ascending NA fibers from the locus
tion of the reticular formation. If the anesthetic agent is coeruleus (and surrounding area) depresses "electro-
given first, the LVFA which normally occurs during cortical waking," and this depression of "electrocorti-
anesthesia produced by volatile anesthetics can be cal waking" is correlated with the loss of forebrain NA
abolished by a subsequent injection of atropine (Van- (Jones, Bobillier, Pin & Jouvet 1973). On the basis of
derwolf, Kramis, Gillespie & Bland 1975). Similar these studies, Jouvet and his collaborators have
effects can be demonstrated using large doses of ethyl concluded that brainstem NA neurons are important in
alcohol as an anaesthetic agent (Whishaw 1976). mediating "tonic cortical activation" during waking
These results suggest that neocortical LVFA, like behaviors. This hypothesis has received support from a
hippocampal RSA, is controlled by two independent number of other researchers using similar procedures
inputs from the reticular formation. A cholinergic (Bolme, Fuxe & Lidbrink 1972; Fuxe, Hokfelt &
reticulo-cortical pathway is probably responsible for an Ungerstedt 1970; Fuxe, Lidbrink, Hokfelt, Bolme &
atropine-sensitive form of LVFA which occurs during Goldstein 1974; Lidbrink 1974). For example, Lidbrink
immobility and other Type 2 behavior in waking (1974) reported that injections of 6-OHDA into the
animals and can also occur during the surgical anes- locus coeruleus depleted forebrain NA and produced a
thesia produced by volatile anesthetics. As would be correlated decrease in waking time (determined elec-
expected, it is possible to activate this pathway by an troencephalographically). It may be necessary to
injection of eserine without producing concomitant repeat at this point that the occurrence of LVFA should
motor activity (Bradley & Elkes 1957; Bradley & not be equated with "waking" in a behavioral sense in
Hance 1957; Longo & Silverstrini 1957). In addition to such experiments.
this presumed cholinergic pathway, there is a second The hypothesis that a monoamine (but not a cate-
type of reticular formation input to the neocortex cholamine; see below) is directly involved in neocorti-
which is resistant to atropine but sensitive to the action cal activation is supported by a recent series of experi-
of volatile anesthetics and alcohol. This input appears ments which have made use of reserpine and other
to be active during, and only during, such behaviors as drugs as a means of altering brain monoamine function
walking, spontaneous changes in posture, head turning, (Vanderwolf & Pappas 1980; Vanderwolf, Robinson &
or struggling when held (Type 1 behavior). Presum- Pappas 1980). Large doses (10 mg/kg) of reserpine
ably, it is this atropine-resistant input which is responsi- produce a behavioral state of catalepsy and akinesia in
ble for the fact that LVFA is invariably present during rats, as is well known (Bein 1956). Spontaneous neocor-
468 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
tical activity accompanying this state consists of peri- compounds in the nervous system, including histamine
ods of rather irregular large slow waves resembling (Adam & Hye 1966) and various amino acids (Santini
those of natural slow wave sleep, or of rhythmic spin- & Berl 1972). Consequently, the observation that atro-
dles resembling the 6-9 Hz spindles which occasionally pine-resistant LVFA is sensitive to reserpine need not
occur in normal rats. LVFA of normal appearance indicate that a monoamine is involved in its produc-
occurs spontaneously at times or can be elicited by tion.
natural stimuli, such as stroking the fur, or by stimulat- More definitive evidence that a monoamine plays a
ing the midbrain reticular formation. LVFA can occur key role in the production of atropine-resistant LVFA
during total behavioral immobility as well as during is provided by the observation that the reserpine-
episodes of head movement or attempts to walk. If induced blockade of atropine-resistant LVFA can be
atropine (25-50 mg/kg, i.p.) is administered 12-24 hr prevented by prior treatment with a monoamine
after the reserpine, all LVFA usually disappears. Even oxidase inhibitor (nialamide, 50 mg/kg). This shows
painful peripheral stimuli or strong stimulation of the that the reserpine-induced blockage can occur only in
midbrain reticular formation or hypothalamus - which the presence of uninhibited monoamine oxidase.
all produce vigorous motor activity - fail to alter the Therefore, it appears that some compound which is
continuous pattern of large irregular slow waves, which essential for the appearance of atropine-resistant
resemble those that occur in a motionless nonreserpi- LVFA is a substrate of monoamine oxidase. Known
nized rat following treatment with atropine. Thus, it substrates of monoamine oxidase which are normally
appears that reserpine abolishes the atropine-resistant present in the brain include noradrenalin, dopamine,
LVFA which normally accompanies Type 1 motor serotonin, and various trace amines such as /3-phenyl-
activity (Fig. 6). ethylamine, tyramine, and octopamine.
It is well known that large doses of reserpine produce The possible role of a number of these amines in
a severe depletion of eateeholamines and 5-hydroxy- producing atropine-resistant LVFA has been assessed
tryptamine in the brain and other tissues. This deple- by several different procedures. It is quite unlikely that
tion occurs as a result of inhibition of uptake of the the unknown amine essential for the occurrence of
amines by intracellular storage granules, which thereby atropine-resistant LVFA is either dopamine or nora-
increases exposure to mitochondrial monoamine drenalin. Atropine-resistant LVFA in rats is not abol-
oxidase (Kirschner 1962; Kopin 1972). However, reser- ished by: (a) depletion of neocortical noradrenalin by
pine also affects a variety of nonmonoaminergic neonatal systemic injection of 6-OH dopamine (Robin-
son, et al. 1977); (b) depletion of noradrenaline and
dopamine together by intraventricular injection of 6-
PIMOZIDE + ATROPINE OH dopamine in adults (Whishaw, Robinson, Schallert,
DeRyck & Ramirez 1978); (c) blocking the synthesis of
dopamine and noradrenalin by means of «-methyl-
p-tyrosine or blocking the synthesis of noradrenalin
alone by means of FLA-63 (Robinson, et al. 1977); (d)
MOVEMENT SENSOR
treatment with catecholamine receptor blockers such
as chlorpromazine, trifluoperazine, haloperidol, phen-
oxybenzamine, pimozide, and propranolol (Vander-
wolf et al. 1978; Vanderwolf & Pappas 1980).
The conclusion that NA is not important for the
RESERPINE + ATROPINE
production of neocortical LVFA is further supported
by recent studies in which the locus coeruleus was
destroyed (see Jacobs & Jones 1978, for review). Jones
et al. (1977) reported that lesions of the locus coeruleus,
severely depleting forebrain NA, do not significantly
disrupt EEG activation in cats. Kolb & Whishaw (1977)
reported that after locus coeruleus lesions in rats, both
atropine-sensitive and atropine-resistant LVFA were
present, and the latter showed its normal relations to
Figure 6. Neocortical slow wave activity in a rat following ongoing motor behavior.
the administration of reserpine (10 mg/kg) plus atropine (50 The results reviewed above provide strong evidence
mgAg) o r pimozide (5 mg/kg) plus atropine (50 mg/kg). that the eateeholamines are not essential for the gener-
Upper traces: One hr after pimozide injection and 30 min ation of either atropine-sensitive or atropine-resistant
after atropine, rat is severely cataleptic but makes occasional LVFA in the neocortex. Serotonin is probably likewise
spontaneous head movements which are associated with low of little importance in this function. Blocking the
voltage fast activity (LVFA). Walking and struggling are also synthesis of serotonin with p-chlorophenylalanine does
associated with LVFA but large slow waves occur during not abolish atropine-resistant LVFA. Suppressing the
immobility. Lower traces: Same rat 24 days later. Forty-eight activity of central serotonergic neurons with LSD-25 is
hr after reserpine injection and 30 min after atropine, rat
displays moderate spontaneous activity, moving the head, also ineffective, as is treatment with presumed seroton-
stepping, and walking. LVFA is absent. Time marks indicate ergic receptor blocking drugs such as methysergide
1 and 5 sec intervals. (From Vanderwolf & Pappas 1980; bimaleate.
reprinted with permission of Elsevier/North-Holland Bio- A different approach to the problem has been
medical Press.) suggested by the possibility of restoring amines selec-
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 469
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
tively, after their depletion by reserpine. Using this Quite different results were obtained when
technique, Carlsson, Lindqvist & Magnussen (1957) (8-phenylethylamine was administered following pre-
showed that restoring catecholamines by 1-dopa treat- treatment with reserpine and atropine (Fig. 8). Within
ment reversed the catalepsy and akinesia produced by 2-5 min after injection of this compound good LVFA
reserpine. 5-Hydroxytryptophan was ineffective. This appeared, together with a behavioral syndrome of head
provides strong evidence that reserpine-induced cata- movements and stepping. These behavioral effects are
lepsy is due to depletion of catecholamines. Applying probably due to a release of residual dopamine (Fuxe,
this method to the identification of the amine involved Grobecker & Jonsson 1967), which is only partially
in atropine-resistant LVFA showed that 1-dopa does not eliminated by the reserpine pretreatment. The appear-
restore atropine-resistant LVFA (Fig. 7). Similar results ance of the atropine-resistant LVFA does not appear to
were obtained with apormorphine (a dopamine depend on the presence of dopamine. Rats treated with
agonist), clonidine (a NA agonist), and amphetamine a combination of reserpine and a-methyl-p-tyrosine
(an indirect agonist of both dopamine and NA). retain virtually no dopamine. Consequently they
Administering these compounds (alone or in various display little or no motor activity when /3-phenyleth-
combinations) to rats following pretreatment with ylamine is injected, but atropine-resistant LVFA is
reserpine and atropine results in pronounced behav- undiminished. Therefore, it appears that /3-phenyl-
ioral hyperactivity accompanied by continuous large ethylamine is capable of restoring atropine-resistant
amplitude slow wave activity in the neocortex. This LVFA and that this restoration does not depend on the
finding, together with the negative results of selective presence of catecholamines.
interference with catecholamines (summarized in the It is unlikely that restoration of atropine-resistant
preceding paragraph) indicates that the catechol- LVFA in reserpinized rats by /3-phenylethylamine is
amines are neither necessary nor sufficient for the due to a direct effect on catecholaminergic receptors.
production of atropine-resistant LVFA in the neocor-
tex. RESERPINE + ATROPINE
RESERPINE
CTX + RO4-4602 + 1-D0PA
5.0 sec
t fl-PHENYLETHYLAMINE
• ATROPINE
. ^ ^ ^
Figure 7. Effects of reserpine, 1-dopa, and atropine on
neocortical electrical activity and behavior. CTX: neocortex;
MVMNT: movement sensor output. Top traces: After treat-
f
ment with reserpine (10 mg/kg/day x 2). Note: 1, low Figure 8. Effect of /3-phenylethylamine (80 mg/kg) on
voltage fast activity (LVFA) during immobility; 2, LVFA neocortical electrical activity in a rat pretreated with reser-
during spontaneous movement; 3, irregular slow waves pine (10 mg/kg) and atropine (50 mg/kg). Abbreviations as in
during immobility; 4, rhythmic spindle activity during Fig. 4 except that L and R refer to left and right parietal
immobility. Middle traces: 60 min after injection of Ro- cortex. Top traces: 16 hr after reserpine injection and 0.5 hr
4-4602 (50 mg/kg) and 30 min after 1-dopa (300 mg/kg), after atropine. At times marked "p," rat is induced to struggle
LVFA is nearly continuous. The rat moves its head, rears, and by being picked up. LVFA is absent. Lower traces: Nine min
walks. Bottom traces: 10 min after injection of atropine (50 after injection of phenylethylamine (PEA) nearly continuous
mg/kg), all LVFA is abolished although rat is very active, LVFA is present together with spontaneous stepping move-
walking and gnawing at the apparatus. (From Vanderwolf et ments of the forelimbs. (From Vanderwolf et al. 1980;
al. 1980; reprinted with permission of Elsevier/North- reprinted with permission of Elsevier/North-Holland Bio-
Holland Biomedical Press.) medical Press.)
470 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
First, the effects of /3-phenylethylamine are distinctly also result from dopaminergic activation of cholinergic
different from the effects of direct-acting catechol- corticopetal neurons.
aminergic agonists such as dopamine (introduced via 3. In normal rats, unlike those pretreated with reser-
1-dopa), apomorphine, or clonidine. Second, the effec- pine, the LVFA produced by amphetamine is resistant
tiveness of /3-phenylethylamine was not abolished by to atropine (Vanderwolf 1975; Schallert, De Ryck &
blocking dopamine and norepinephrine receptors with Teitelbaum 1980). This might be due to trace-aminer-
chlorpromazine, propranolol, or trifluoperazine. gic neurons, excited by catecholamine release, produc-
Therefore, it is likely that /3-phenylethylamine ing LVFA in the neocortex. Alternatively, trace amines
produces atropine-resistant LVFA in reserpinized rats may be released by amphetamine directly, without the
through an interaction with an unknown type of mediation of dopamine.
monoamine receptor which is distinct from the norepi- 4. It has been reported that dopaminergic blocking
nephrine and dopamine receptors. The nature of this drugs, such as phenothiazines, potentiate the slow-
receptor and the identity of its ligand under normal wave-producing effects of atropine (Bradley & Hance
conditions remain problems for future research. For 1957). Such potentiation can probably be understood in
various reasons, it need not be assumed that /3-phenyl- terms of the behavioral effects of these drugs (Vander-
ethylamine is normally a transmitter or modulator in wolf 1975). Atropine produces a behavioral syndrome
an aminergic reticulo-cortical pathway, although this is of hyperactivity in rats, consisting of head movements,
a possibility. /3-Phenylethylamine is not depleted by stepping, and forward locomotion. Such behaviors are
reserpine, at least not in the hypothalamus (Boulton, accompanied by atropine-resistant LVFA. Doparnine-
Juorio, Philips, & Wu 1977) while the compound blocking drugs, such as phenothiazines, reduce both the
essential to atropine-resistant LVFA is depleted (or hyperactivity and the occurrence of atropine-resistant
otherwise inactivated) by reserpine. Furthermore, /3- LVFA, but the remaining spontaneous motor activity
phenylethylamine, unlike acetylcholine or norepineph- remains strongly correlated with the occurrence of the
rine, is not readily released from rat neocortex by LVFA. d-Amphetamine intensifies atropine-induced
electrical stimulation (Saldate & Orrego 1978). There- hyperactivity, resulting in continuous movement
fore, /3-phenylethylamine may be a direct or indirect accompanied by continuous LVFA (Vanderwolf 1975;
agonist of an unknown trace amine which normally Schallert, De Ryck & Teitelbaum 1980). These effects
plays an essential role in the production of atropine- of amphetamine and dopamine-blocking drugs suggest
resistant LVFA. that dopaminergic mechanisms control both the occur-
Although catecholamines do not play an essential rence of spontaneous motor activity and the correlated
role in cortical activation, they may act indirectly by occurrence of atropine-resistant LVFA. It is even possi-
somehow exerting an excitatory influence on: (a) cho- ble that dopaminergic mechanisms play a role in the
linergic neurons presumably responsible for atropine- normal correlation between atropine-resistant LVFA
sensitive LVFA, and (b) trace-aminergic neurons and Type 1 motor activity. This is suggested by the
which may be responsible for atropine-resistant LVFA. observation that rats treated with a combination of
Such influences are suggested by a number of different a-methyl-p-tyrosine and reserpine (virtually eliminat-
phenomena. ing brain dopamine) display good atropine-resistant
1. Motionless rats treated with large doses of reser- LVFA in the absence of significant motor activity
pine or dopamine receptor blockers such as phenothi- following the administration of /3-phenylethylamine.
azines tend to display less LVFA than undrugged Presumably, /3-phenylethylamine stimulates the atro-
motionless waking rats. It may be hypothesized that pine-resistant pathway directly, but in the absence of
cholinergic corticopetal neurons receive a reduced dopamine, Type 1 motor activity cannot occur.
excitatory input under these circumstances, a sugges-
tion consistent with the decreased release of acetylcho- Reticulo-cortical activity during sleep
line from the neocortex following treatment with
chlorpromazine (Phillis & Jhamandas 1971) and with Thus far we have discussed primarily the relations of
the increased acetylcholine content of the brain in electrocortical activity to behavior during the waking
reserpinized animals (Hrdina & Ling 1973; Malhotra & state. However, slow wave recording techniques are
Pundlik 1959). Increased acetylcholine content is also used extensively as an index of reticulo-cortical
usually correlated with decreased release (Szerb, Malik activity during sleep. In the light of the behavioral and
& Hunter 1970). neuropharmacological correlates of reticulo-cortical
2. The finding that dopamine agonists (1-dopa, activity during waking which we have presented
amphetamine, apomorphine, and bromocriptine) all above, an obvious question is whether or "not these
increase the release of acetylcholine from the neocortex correlations generalize to reticulo-cortical activity
may be due to increased excitation of corticopetal during sleep. In the following paragraphs we attempt
cholinergic neurons by a dopaminergic mechanism to answer this question.
(Pepeu & Bartolini 1968; Pepeu, Mantovani & Pedata
1978). This explanation could also account for the fact a) Quiet (slow wave; synchronized) sleep. Prior to
that the LVFA produced by dopamine agonists (1- sleeping most animals seek out a quiet protected loca-
dopa, amphetamine, apomorphine, LSD-25) in reser- tion, assume a characteristic "sleep posture," and,
pinized rats is sensitive to atropine (Vanderwolf, having adopted this posture, remain relatively immo-
Robinson & Pappas 1980). The increased LVFA bile. During this period of immobility large slow waves
following 1-dopa treatment in cats (Jouvet 1967) may gradually appear in the neocortical EEG record. The
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 471
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
hippocampal record is characterized by LIA. It must (1977) suggest that the spikes occur in active sleep as
be emphasized that the occurence of hippocampal LIA responses to spontaneously generated "sensory" stim-
and neocortical large amplitude slow waves during the uli. Phasic phenomena also occur in hippocampal and
immobile phase of sleep is not unique to sleep since neocortical slow wave activity, presumably in response
similar wave patterns occur during immobility in the to ascending reticular volleys. During the intervals
waking state as well (see above). A waking atropinized between phasic episodes, hippocampal RSA has a
rat, like the rat in quiet sleep, displays continuous large frequency of about 6 Hz but the frequency rises to 8
slow waves during immobility. The latter observation Hz or more during bursts of muscular activity (Robin-
suggests that perhaps an atropine-sensitive (presum- son, Kramis & Vanderwolf 1977; Soulairac, Gottesman
ably cholinergic) reticulo-cortical input, which is capa- & Thangapregassam 1965; Whishaw & Vanderwolf
ble of producing LVFA during immobility, is inactive 1973). Hippocampal "theta cells" which selectively
during quiet sleep. This suggestion is supported by increase their firing rate during Type 1 behaviors in
reports that ACh release from the neocortical surface is the waking state also increase their firing rate during
reduced during quiet sleep, relative to the release seen active sleep (Rank 1973; 1975).
during waking or active sleep accompanied by LVFA It appears possible to obtain active sleep in heavily
(Celesia & Jasper 1966; Jasper & Tessier 1971). atropinized rats provided that they have been deprived
Periodically animals move (i.e., shift posture) during of active sleep for several days. In such rats the 6 Hz
quiet sleep. Such postural shifts are invariably accom- RSA, which is normally present in the intervals
panied by the appearance of neocortical LVFA and between the phasic episodes of active sleep, is abol-
hippocampal RSA (Glotzbach 1975). This occurs even ished. However, the higher frequency RSA which
though the animal may not open its eyes, lift its head, normally accompanies the phasic muscular twitches of
or show any other signs of behavioral "arousal." The active sleep is well preserved (Robinson et al. 1977;
research on waking animals reviewed above would Usui & Iwahara 1977). Furthermore, if active sleep-
suggest that this movement-related LVFA and RSA deprived rats are anesthetized with urethane they
accompanying postural shifts during quiet sleep is due
display long trains of 5-6 Hz RSA but do not display
to activation of noncholinergic reticulo-cortical path-
ways. At present there is no direct evidence to support phasic muscular twitches or the higher frequency RSA
this contention. normally associated with these twitches. Therefore, it
appears that the RSA occurring during active sleep is
b) Active (paradoxical, desynchronized, rapid eye
comprise of a 5-6 Hz atropine-sensitive and urethane-
movement) sleep. At a behavioral level, active sleep is resistant component which can occur during immobil-
characterized by a tonic reduction in muscle tonus ity as well as of a higher frequency component which is
interrupted periodically by bursts of phasic activity in atropine-resistant, urethane-sensitive, and occurs in
virtually all the somatic muscles, including the extra- relation to phasic muscular activity. These data suggest
ocular muscles (thus producing rapid eye movements, that: (1) a (presumed) cholinergic input which
REM). Work by Pompeiano and others (Chase 1974; produces RSA is tonically active during active sleep,
Pompeiano 1967) has revealed that the reduction in and (2) the presumed noncholinergic input which
muscle tonus results from a postsynaptic inhibition of produces higher-frequency RSA correlated with Type
alpha and gamma spinal motor neurones as well as a 1 behavior in the waking state is phasically active in
phasic presynaptic inhibition of la spinal sensory affer- association with the muscular twitches of active sleep.
ents. The phasic bursts of muscular twitches appear to During active sleep in atropinized rats neocortical
result from activity in descending systems including activity consists of: (a) short periods of LVFA asso-
the pyramidal tract, rubrospinal tract, and reticulospi- ciated with the phasic bursts of muscular activity, and
nal fibres (Pompeiano 1967; Siegel 1979a; 1979b; (b) periods of large amplitude irregular slow waves
Vertes 1979). Since the spinal motor neurones and during the quiescent intervals. Undrugged rats tend to
reflex afferents are strongly inhibited, the muscular display continuous LVFA throughout an entire episode
activity which results from these descending discharges of active sleep (although 6-9 Hz rhythmic spindle
is slight in comparison with that occurring during activity occasionally occurs during immobile periods
waking behavior. However, the pattern of impulses between the bursts of phasic muscle activity, just as in
descending from the brain during the phasic episodes the waking motionless rat). Therefore, during active
of active sleep is probably similar to the pattern sleep the neocortex, like the hippocampus, may receive
occuring during some active waking behaviors. Siegel both a tonic cholinergic input and a phasic noncholin-
(1979a; 1979b) has summarized data showing that the ergic input correlated with phasic motor activity (Rob-
firing patterns of many reticular formation neurones is inson et al. 1977).
similar during waking movement and the phasic events The foregoing data suggest that the mechanisms
of active sleep (see also below). which produce hippocampal RSA and neocortical
LVFA in the waking state also produce these wave
Ascending brainstem influences fluctuate in relation patterns during active sleep. However, Monmaur,
to the phasic descending influences or peripheral Houcine & Delacour (1979) have recently reported
phenomena of active sleep. Increases in the frequency that posterior septal lesions abolish the RSA which
of ponto-geniculo-occipital (PGO) spikes, for example, usually accompanies waking behaviors without sup-
are correlated with other phasic events in active sleep. pressing the RSA associated with active sleep. Lesions
Since similar PGO spikes occur in response to a variety located more anteriorly in the septum, and in the
of novel stimuli in waking cats, Bowker & Morrison region of the diagonal band of Broca, abolish the RSA
472 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
associated with active sleep, but not that associated A new synthesis
witli waking behaviors. While these experiments are
not incompatible with the idea that the same neuro- In the past 30 years there has been almost unanimous
chemical systems produce RSA during waking and agreement among neuroscientists that the projections
active sleep, they do suggest that different neuroana- from the brainstem reticular formation to the cerebral
tomical systems may underlie hippocampal activation cortex play an essential role in sleep and waking
in these two different states. behavior and in the psychological phenomena of
In general, it is probably that brain activity and consciousness, vigilance, awareness, alertness, atten-
patterns of motor output during active sleep are quite tion, and arousal. We have reviewed evidence that this
similar to the patterns that occur during waking (with view is fundamentally unsound. The ascending reticu-
the exception that environmental stimulus control of lo-cortical pathways whose activity results in neocorti-
these patterns is largely absent during active sleep). cal LVFA and hippocampal RSA appear to consist of at
This is suggested by studies of active sleep in cats least two distinct subsystems. The normal activities of
following chronic lesions of the pons, which appear to these subsystems are related to behavior in different
abolish the muscular atonia characteristic of normal ways but neither of them appears to correlate with
active sleep. In such cats, active sleep episodes take the behavior in the way suggested by the classical concepts
form of dramatic displays of motor activity which of arousal, vigilance, or consciousness. What theoretical
resemble such waking behaviors as exploration, attack, approach can we now offer to replace the venerable
or the capture of prey (Jouvet & Delorme 1965; Henley reticulo-cortical arousal theory?
& Morrison 1974; llendricks, Morrison & Mann 1980; A good place to begin is to consider the behavior of
Sastre & Jouvet 1977). Despite this vigorous motor totally decorticate animals in which all reticulo-cortical
activity, the cats appear to be "asleep," since they are effects are necessarily abolished. A striking feature of
unresponsive to visual stimuli, their nictitating decorticate or even decerebrate rats (Berntson & Micco
membranes are relaxed and their pupils are miotic. 1976; Grill & Norgren 1977; Vanderwolf, Kolb &
Furthermore, the motor displays are easily interrupted Cooley 1978; Woods 1964) and of decorticate cats
by auditory stimuli which awaken the cats. (Bard & Rioch 1937) is the quality of preservation of
Since the phasic motor components of active sleep the many distinctive motor patterns normally observed
are generally accompanied by atropine-resistant hippo- in these species. Totally decorticate rats sniff or nibble
campal RSA in normal rats, one might expect that the at proffered objects, walk, jump, and sit up to wash
motor activities released following locus coeruleus their faces in a normal or near-normal manner. They
lesions would consist largely or entirely of Type 1 also sleep, often while lying in a curled-up posture, as
behavior. This question has not yet been examined in normal rats frequently do. Auditory or tactile stimuli
rats, but llendricks, Bowker & Morrison (1977) report applied during sleep elicit an arousal or awakening
that in cats with locus coeruleus area lesions, the motor reaction consisting of raising the head, opening the
displays of active sleep resemble "orienting, searching, eyes, and the assumption of a standing position. Simi-
stalking, rage, and attack." Behaviors resembling "eat- larly, decorticate and destriate (thalamic) or decere-
ing, drinking, grooming, urinating, defecating, or brate cats display at various times the postures and
reproductive behavior" were not observed. However, ocular activity characteristic of quiet sleep, active
Sastre & Jouvet (1977) did observe grooming behavior sleep, and the waking state, although there are quanti-
during active sleep in cats with locus coeruleus lesions. tative changes in the proportion of the day spent in
Although more detailed descriptions would be desir- these different states (Villablanca 1966; Villablanca &
able, it does appear that Type 2 behavior is relatively Marcus 1972). Finally, decorticate rats display a circa-
rare in these preparations during active sleep, while dian rhythm of spontaneous head movements, walking,
Type 1 behavior is common. and rearing (Fig. 9). Lesions of the hypothalamus, not
A question which arises from the foregoing experi- the cerebral cortex, disrupt circadian rhythms (Richter
ments is: Which transmitter system(s) is (are) responsi- 1967; Rusak & Zucker 1975; Stephan & Zucker 1972).
ble for producing neocortical LVFA and hippocampal These results indicate unequivocally that the dience-
RSA during the periods of phasic motor activity of phalon, brainstem, cerebellum, and spinal cord contain
active sleep? Jouvet (1972; 1974) has suggested that NA the neural networks necessary for the principal behav-
has an important role in the generation of LVFA in ioral phenomena of quiet sleep, active sleep, awaken-
both active sleep and the waking state. The experi- ing from sleep, and the postures and phasic motor
ments discussed above (in the section called "Neocorti- patterns characteristic of the waking state. Ascending
cal activation and behavior: Monoaminergic in- reticulo-cortical systems do not play an essential role in
fluences") have led us to conclude that NA is not the production of these behavioral states. However,
critical for the production of LVFA during waking. On descending reticular projections appear to be of great
the basis of other evidence, other authors have importance. The behavior of decorticate or decere-
concluded that NA is not critical for the production of brate preparations is presumably dependent on reticu-
LVFA during active sleep either (Jacobs & Jones 1978; lospinal projections, which, together with vestibulospi-
Jones ft al. 1977; Ramm 1979). It is possible, however, nal, rubrospinal, and other descending pathways exert
that a trace amine is essential for the generation of a powerful control over the activity of spinal motoneu-
LVFA during active sleep; the experiments necessary rones (Shapovalov 1975). This conclusion is supported
to examine this hypothesis have not yet been carried by the fact that the firing of many neurons in the
out, however. reticular formation in freely moving animals is strongly
THE BEHAVIORAL AND BRAIN SCIENCES (1981). 4 473
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
% TIME ACTIVE % TIME ACTIVE % TIME ACTIVE
o o o o o o o 5888883888
Figure 9. Percentage of time per hour occupied by head movement, walking, and rearing in three totally decorticate rats
during a 3-day period. (From Vanderwolf, Kolb & Cooley 1978).
correlated with motor activity (Siegel 1979a). Destruc- involved in the stimulus control of behavior. Behavioral
tion of reticulospinal pathways of the medial brainstem investigations have revealed a number of different
results in severe behavioral incapacitation (Kuypers mechanisms, such as elicitation (release), guidance, and
1964). reinforcement, by which environmental stimuli control
If the ascending reticulo-cortical pathways are not animal behavior (Eibl-Eibesfeldt 1970; Hinde 1970;
directly involved in the basic phenomena of sleep, Skinner 1938; 1969; 1974). Interoceptive and proprio-
arousal, and waking, then what is their function? The ceptive stimuli also affect behavior. Animals or humans
data discussed above indicate that the ascending reticu- who are demented, psychotic, or intoxicated may be
lar activating system comprises at least two functional said to display impaired stimulus control of behavior
components with quite different relations to behavior. when they react inappropriately to environmental
One component, which is probably dependent on cho- signals. It may be that brain cholinergic systems,
linergic transmission at one or more points, is responsi- including the atropine-sensitive ascending reticular
ble for any hippocampal RSA or neocortical LVFA activating system, play an essential role in normal
sh%$Uwhich occurs during behavioral immobility or responses to environmental situations. These hypoth-
simple reflexive or consummatory behavior (Type 2 eses are supported by recent evidence that brain cho-
behavior). This component is also responsible for the linergic systems are selectively impaired in senile
RSA and LVFA occurring during the quiescent inter- dementia. Choline acetyl transferase and acetylcholin-
vals of an active sleep episode or during the anesthesia esterase activity is very low in the neocortex, hippo-
produced by volatile anesthetics. We refer to this campus, and striatum of senile demented patients (Alz-
component as "atropine-sensitive," since atropine has heimer's disease) but other brain enzymes appear to be
provided the chief method for investigating it. The well preserved (Davies & Maloney 1976; Perry, Perry,
occurrence of atropine-sensitive RSA and LVFA Blessed & Tomlinson 1977; Perry, Perry, Gibson,
during the anesthetic state and the inter-twitch inter- Blessed & Tomlinson 1977; Perry, Tomlinson, Blessed,
vals of active sleep emphasizes the fact that activity in Bergman, Gibson & Perry 1978; Reisine, Yamamura,
the reticulo-cortical pathways producing these wave- Bird, Spokes & Enna 1978). In normal humans and
forms is not dependent on the maintenance of muscle aged experimental animals, treatment with choline or
tone or the occurrence of phasic motor activity. cholinergic agonists has been reported to improve
In all species studied so far, atropine-sensitive performance in learning tasks (Bartus, Dean, Goas &
neocortical LVFA can be readily elicited by sensory Lippa 1980; Davis, Mohs, Tinklenberg, Pfefferbaum,
stimulation, without concurrent elicitation of phasic Hollister & Kopell 1978; Sitaram, Weingartner & Gillin
motor activity. Atropine-sensitive hippocampal RSA 1978). Interference with central cholinergic function
can be elicited by sensory stimuli in this way in some due to a choline deficient diet (Bartus et al. 1980) or
species (rabbit, guinea pig) but not in others (rat). The central muscarinic blocking drugs, disrupts both
significance of these species differences, and of the fact learned and spontaneous (untrained) behavior (see
that atropine-sensitive LVFA occurs spontaneously reviews by Brimblecombe 1974; Hunter, Zornetzer,
much more frequently than atropine-sensitive RSA in Jarvik & McGaugh 1977; Longo 1966). These data are
all species, is unknown at present. consistent with the hypothesis that cholinergic reticulo-
The fact that atropine-sensitive LVFA and RSA can cortical fibres play a role in normal stimulus control of
often be elicited by sensory stimuli may suggest that behavior. At present, however, it is not possible to
these waveforms reflect the activity of mechanisms exclude a role for other central cholinergic systems as
474 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Vanderwolf & Robinson: Reticulo-cortical activity and behavior
well. ized only by being normally subject to control by
An important conclusion of this review is that there complex cerebral systems which are not directly
is a second component of the ascending reticular acti- involved in the control of simpler reflexive and
vating system which is noncholinergic and is resistant consummatory behavior. Analysis of these cerebral
to large doses of atropine. This component produces control systems is a problem for future research. The
hippocampal RSA and neocortical LVFA if, and only possible physiological role of a diffuse trace amine-
if, voluntary behaviors such as locomotion or head dependent input to the neocortex, for example,
turning (Type 1 behavior) are being performed or if remains unknown.
muscular twitches are occurring during an episode of In this connection, is is of interest that trace amine
active sleep. The effect of this input is not obvious in activity appears to be abnormal in paranoid schizo-
the spontaneous electrical activity of the neocortex in a phrenia and depression (Potkin, Karoum, Chuang,
normal animal but it becomes very clear following Cannon-Spoor, Phillips & Wyatt 1979; Sandier, Ruth-
treatment with atropine. Presumably, atropine un- ven, Goodwin & Coppen 1979). Thus, it is conceivable
masks the effect of a noncholinergic input by eliminat- that malfunction of an ascending trace amine-depen-
ing concurrent cholinergic inputs. Atropine-resistant dent reticulo-cortical system is involved in some
hippocampal RSA and neocortical LVFA are abolished psychiatric disorders.
by volatile anesthetics and morphine and the produc- A neglected approach to reticulo-cortical function in
tion of atropine-resistant LVFA appears to be depen- relation to behavior is provided by the study of decorti-
dent on the presence of a trace amine. cate animals. Decortication produces a profound disor-
There has not yet been an intensive study of the ganization of behavior, affecting both specially trained
brainstem origin of reticulohippocampal and reticulo- ("learned") behavior and behaviors which are
neocortical pathways whose activity is correlated with frequently regarded as "innate" or "unlearned,"
Type I behavior. Stimulation experiments suggest that including feeding, food hoarding, nest building,
this origin is rather diffuse (Robinson & Vanderwolf mating, grooming, and general social behavior (Lash-
1978). Vertes (1979) has made an important finding in ley 1929; 1935; Vanderwolf et al. 1978). Decorticate
this field by demonstrating the existence of pontine animals appear to retain most of the motor components
neurons which fire in relation to Type 1 behavior and of normal adaptive behavior but fail to display these
the phasic events of active sleep. These neurons may components at the correct times and places. For exam-
activate the hippocampus, as Vertes suggests, but may ple, a normal rat will pick up and carry home (hoard)
also activate the neocortex. food pellets that are lying about in the open. A decorti-
Considering the nature of their relation to behavior, cate rat never does this, even though it is perfectly
it seems possible that atropine-resistant reticulo-cortical capable of picking up food in its mouth and runs about
inputs may play a role in the control of Type 1 very actively.
behavior, but have no direct role in Type 2 behavior. We suggest that the cerebral cortex is involved in
Unfortunately, there are, as yet, no clear behavioral behavior control systems which, in normal animals,
criteria by which Type 1 or Type 2 behavior can be determine the occasions on which particular motor
classified. In general, Type 2 behavior includes alert patterns are to be displayed. The behavior of decorti-
immobility and various reflexive or consummatory cate animals suggests that subcortical mechanisms are
behaviors, while Type 1 behavior includes walking, largely capable of generating the "motor score" (Weiss
manipulation, and other actions of a type which might 1941) characteristic of a given behavior pattern such as
be called "voluntary" or "purposive" (Fig. 2). It is walking or rearing, but that the cortex plays a major
interesting that most Type 1 behaviors are readily role in determining when walking or rearing should be
shaped by operant conditioning procedures while a performed. The activity of the cortex, in turn, is
number of Type 2 behaviors are resistant to such influenced by specific sensory input and by reticulo-
shaping (Amiable & Weardon 1979; Shettlevvorth cortical inputs. Output from the cortex may exert a
1975). However, no detailed analysis of possible rela- descending control over posture and movement
tions between ease of operant conditioning and the (behavior) largely by way of reticulospinal, rubrospi-
electrocortical correlates of behavior has yet been nal, and other descending pathways which originate in
attempted. the brainstem. Numerous corticosubcortical anatomi-
Another possible distinction between Type 1 and cal connections suggest the possibility of this type of
Type 2 behavior is that Type 1 behaviors usually control (see Knook 1965 for extensive references).
involve movement from one place to another while The effects of cortical damage on behavior are
Type 2 behaviors are performed in a single place frequently interpreted in terms of a loss of the projec-
(O'Keefe & Nadel 1978). [See also BBS multiple book tion areas of specific sensory systems. While such loss is
review of O'Keefe & Nadel 1978 in BBS 2(4) 1979.] undoubtedly a major factor, a part of the behavioral
While this distinction often does apply, there seem to deficit may result from interruption of diffusely
be some Type 1 movements such as head scans (Schal- projecting reticulo-cortical pathways. Many years ago,
lert et al. 1980) which are accompanied by atropine- Lashley (1929) suggested that the cerebral cortex had
resistant RSA and LVFA even though they do not some general function in behavior distinct from the
involve movement from one place to place in the sense analysis of specific sensory input or the organization of
intended by O'Keefe & Nadel. It may be that no single specific motor output, but he did not specify the
denning behavioral characteristic of Type 1 behavior anatomical basis of this general function. Perhaps the
will ever be found. Type 1 behaviors may be character- nonspecific pathways from the brain stem to the
THE BEHAVIORAL AND BRAIN SCIENCES (1981). 4 475
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
neocortex are the basis of the general function hypo- longer in their infancy, and cognitive processes such as
thesized by Lashley. The diffuse nature of these path- attention, perception, and consciousness are today considered
ways might account for Lashley's finding that large to be just as legitimate for study as were overt movements for
lesions at any location in the cerebrum produce a the behaviorists many years ago.
V&R apparently do not share this view. In studying EEG
general deterioration in behavior.
correlates of behavior they state that "behavior was described
The specific roles of Ach-dependent and trace in terms of movement and posture, and inferential terms such
amine-dependent reticulo-cortical pathways in the as perception, attention, arousal, motivation, memory, and so
overall organization of brain activity and behavior is a on, were deliberately avoided in describing the behavior of
topic for future research. We believe that this review the animals." I take issue with this approach because I believe
has shown that careful descriptive studies of behavior this behavioral analysis to be unnecessarily restrictive at the
are likely to play an important role in such research. stage we have now reached in the development of our
discipline. In addition, such an approach leads to an investiga-
ACKNOWLEDGMENT tor's possibly missing a wealth of information.
Preparation of this paper was supported by a grant (AO-118) In the case of hippocampal EEG correlates of behavior, the
from the Natural Sciences and Engineering Research Council behaviorist approach resulted in Vanderwolf and his
of Canada. colleagues' concluding that hippocampal slow-wave activity
is a correlate of so-called "voluntary " movements. (But is
making a distinction between voluntary versus involuntary or
automatic behaviors not inferential?) Many neuroscientists
believe that Vanderwolf and his colleagues, in rejecting
attention, perception, and memory processes, came to errone-
ous conclusions regarding the significance of hippocampal
Open Peer Commentary slow-wave activity. Instead, these investigators suggest that
the appearance of this biorythm is specifically related to the
cognitive processes which Vanderwolf rejects (see, for exam-
Commentaries submitted by the qualified professional readership ple, Adey 1966; Bennett 1975, 1979; Grastyan, Karmos,
of this journal will be considered for publication in a later issue as Vereczkey & Kellenyi 1966; Isaacson 1974; Kemp & Kaada
Continuing Commentary on this article. 1975). Interestingly, Grastyan originally thought that hippo-
campal slow-wave activity was a correlate of voluntary move-
ment; but further observations, in which he had not made the
a priori decision to reject inferred behavioral processes from
Is a behaviorist's approach sufficient for his analysis (as Vanderwolf and his colleagues have), led
Grastyan to reject his speculation as premature and incorrect
understanding the brain? (personal communication). Those original observations were
never published, and we instead attribute to him the view
Thomas L. Bennett that hippocampal slow-wave activity is a correlate of an alert
Department of Psychology, Colorado State University, Fort Collins, Co. animal; attending to and processing information about a
80523 potentially significant environmental stimulus - a hypothesis
Vanderwolf & Robinson's (V&R) presentation is essentially which a number of us have heartily endorsed.
composed of two articles. The first describes the neurophysi- Actually, it now appears that there are two bandwidths of
ology of reticular, neocortical, and hippocampal electrical hippocampal slow-wave activity, with different behavioral
activity while the second attempts to relate patterns of correlates: a slower, 4-7 Hz, pattern (theta activity) that is a
electrical activity to behavior. I have no major quarrels with correlate of cognitive processes and is blocked by anticholin-
the first, but I am uncomfortable with the second, largely ergic agents such as atropine and scopolamine, and a faster,
because I believe that the authors have been unnecessarily 8-12 Hz, rhythmic activity which is more closely related to
restrictive in their behavioral analysis. the execution of voluntary movement and is not blocked by
The classical arousal theory of reticulo-cortical activity has anticholinergic agents. Please note that this faster activity is
its problems, as V&R point out. However, a feature of this not theta (it falls in the range of alpha), despite the popularity
view is that it is couched in cognitive terms such as attention, of denoting it as such (for example, see V&R's Figure 2).
arousal level, alertness, and consciousness. How can one assess Nevertheless, the point to be made is that rejecting inferred
the validity of this theory for describing the electrophysiologi- behavioral processes from an analysis of the functions
cal correlates of these processes when one rejects such performed by the brain in regulating behavior needlessly
concepts (as the authors do) and refuses to include them in a restricts the wealth of information that can be gleaned from
behavioral analysis of neurophysiological events? such inquiries; indeed, such an approach may produce erro-
I think it would be more accurate to view this paper as a neous conclusions. At the very least, such narrow investiga-
behaviorist's view of the behavioral correlates of reticulo- tions should be prefaced by a statement that they are thus
cortical activity than as a resynthesis of the theory. V&R's limited and followed by the conclusion that the obtained
approach is to look only at body movements when they results have no relevance to describing the functions of the
investigate behavior, following the tradition of behaviorism in brain in higher behavioral processes; rather, such inquiries
the footsteps of Watson and Skinner. The behaviorist view of only describe the brain's functions in overt motor operations.
psychological processes, in which the legitimate subject of Unfortunately, the behaviorist philosophy leads to rather
behavioral analysis is restricted to overt movements, was an narrow conclusions when these constraints are in effect.
important stage in the history of experimental psychology Consider V&R's major conclusion that "the cerebral cortex is
(and especially the psychology of learning), enabling it to involved in behavior control systems which, in normal
"get its feet on firm ground ' during its infancy. Applied to animals, determine the occasions in which particular motor
physiological psychology, this approach proved invaluable for patterns are to be displayed." This is an extremely narrow
the early development of that discipline as well. But experi- view of the contributions to behavior made by the cortex,
mental psychology and physiological psychology are no even in the case of the rat whose cortical mantle is very poorly
476 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
developed. Even the lowly rat has been a rich source of believe that valuable information could be obtained by relat-
information for experiments conducted in the field of cogni- ing physiological indexes of internal conditions to electrical
tive psychology. Physiological psychology no longer needs to rhythms of the brain and using them as another basis for
confine itself to simple descriptive analyses of overt behavior. making inferences about psychological processes. Further-
As a discipline, it is reaching maturity and can make many more, we suggest that a commitment to an extreme operant
well-substantiated statements about how the brain regulates approach to finding electrical correlates of behavior will be
complex behavioral processes. self-defeating in connection with atropine-sensitive activity.
These are thought to occur without movement or behavior,
and consequently there will be nothing with which to corre-
late the electrical activities.
Behavioral problems related to the In our opinion, a more powerful approach is to test animals
in situations in which things are required of them. Situations
interpretation of brain rhythms can be arranged in which ethologically significant behaviors
are obtained and can be analyzed for relationships with the
Gydrgy Buzsaki,*' Robert L. Isaacson," and John H.
electrical rhythms of the brain. Through the use of such
Hannigan, Jr.b paradigms, electrical patterns which occur before meaningful
'Physiology Institute, Medical School. University of Pecs, Pecs, Hungary and specific patterns of behavior can be investigated. Buzsaki,
and "Department of Psychology and Center of Neurobehavioral Sciences, Grastyan, Tveritskaya & Czopf (1979), and Buzsaki, Hauben-
State University of New York at Binghamton, Binghamton, N, Y. 13901 reiser, Grastyan, Czopf & Kellanyi (1981) have found that
The basis of the approach which has been used so extensively signal-elicited behaviors are accompanied by greater amounts
by Vanderwolf, Robinson, and their associates is the associa- of rhythmic slow activity in the theta range than is found in
tion of certain behaviors with electrical activities recorded US (unconditional stimulus)-directed "voluntary" behaviors
from the hippocampus and neocortex. Essential to this under- in both the rat and the cat. The conditioned theta activity
taking is the identification of the behavioral units with which occurring in the absence of locomotion was found to have
correlations are to be made. The authors of the target article high modal frequency peaks (9 Hz in rat, 5 Hz in cat)
say that their aim is to study behaviors in terms of postures indicating it to be of the atropine-resistant type. US-directed
and movements which they believe would be in accord with "voluntary" movement was accompanied by lower frequency
both behavioristic (i.e., Skinnerian) and ethological rhythmic activities in both species. It was of interest that CS
approaches. Frankly, we doubt that the behavioral approach (conditional stimulus)-elicited head movements were accom-
used by Vanderwolf & Robinson (V&R) are appropriate to panied by the higher frequency rhythmic slow activities;
either. We do not think V&R's behavioral units are Skinner- spontaneous head movements were not.
ian, since some of the more-or-less explicit categorizations are We also believe that serious problems exist for a reticular
made on bases other than purely behavioral ones ("manipula- formation-hippocampal theory as it relates to "arousal" as
tion" of objects), and because of the use of the notion that indicated by the rhythmic activities in the hippocampus. The
some behaviors are "voluntary." More important, however, is anatomical question of the actual existence of reticular
our view that V&R's approach is not ethological. Ethologists formation-hippocampal fibers has not been conclusively
have rejected extreme behavioristic approaches and look for resolved. Neurophysiologically, the almost complete domi-
units of behavior which are meaningful patterns in the lives nance of hippocampal electrical activity by slow-wave activ-
of animals. Many of these units consist of complex patterns of ity has been found after prepontine section in the unanesthe-
movements and postures that cannot be isolated from the tized rabbit (Woodruff, Gage & Isaacson 1973), and after
settings which make them meaningful. Examined from an high mesencephalic transections in the cat (Olmstead &
ethological perspective, there is, for example, a great differ- Villablanca 1977; Radii-Weiss, Zernicki & Michalski 1976),
ence between a foreleg movement in stepping toward a and the rat (C. Gottesmann, personal communication). The
receptive female and a similar movement made in getting question of the location of the atropine-sensitive rhythmic
away from a predator. [See also Johnston: "Contrasting slow-wave generating region still seems obscure.
Approaches to a Theory of Learning" BBS 4(1) 1981.] Finally, we have severe reservations about the pharmaco-
Part of the problem is probably due to the methods logic evidence marshalled to argue for the involvement of a
commonly used, namely observation of animals in an open "trace amine" (j3-phenylethylamine) in atropine-resistant
environment. This could account for the relative paucity of neural pathways. It seems unreasonable to postulate a signifi-
behaviors categorized as type I or type II (e.g., V&R's Figure cant role for this agent - which can be considered a parent
2). The small number of behaviors categorized by the authors' compound for a host of catecholamine-related compounds -
procedures is apparent. The method of simple observation in on the basis of incomplete pharmacologic investigations.
a free environment may also account for the fact that hardly
any changes are observed in fundamental movements and NOTE
postures after extensive or total neocortical destruction. A * During 1980-1981 at: Division of Neurosurgery, Department of
more refined analysis could reveal such changes. For exam- Surgery, University of Texas, Health Sciences Center, San Antonio,
ple, we have found increases in the frequency and average Texas 78284.
duration of grooming bouts, as well as altered rates of
habituation of grooming after even small amounts of neocor-
tical damage in rats. These changes are subtle but consistent
(Reinstein, Hannigan, & Isaacson 1981).
Can the decomposition of attention
In addition, V&R's arbitrary categorization of behaviors
restricts measurable correlates of both movement and clarify some clinical issues?
nonmovement. Careful analysis of the rhythmic electrical
Enoch Callaway
activity in the brain associated with physiological changes
remains an appropriate procedure. Considering such vari- Department of Psychiatry, University of California, San Francisco, School
ables as only a "domain" of inference about "psychological of Medicine, San Francisco, Calif. 94143
processes," an opportunity to meaningfully distinguish simi- As William James accurately observed, attention has for each
lar overt behaviors in terms of EEG may be missed. We of us a self-evident and unitary quality. That, it seems, is
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 477
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
Table 1 (Callaway). Dimensions of attention/arousal
Family A Family B References
1. Serial Parallel Posner (1978)
2. Memory driven Data driven Rabbitt (1979)
3. Controlled Automatic Shiffrin & Schneider (1977)
4. Response selection Stimulus evaluation Donchin (1979)
5. Sustained arousal Phasic arousal Pribram (1977)
and effort
6. Attention Pre-attentive Broadbent (1977)
7. Cognitive memory Trace memory Sutton, Spring & Teuting
(1978)
Type I (Aminergic) Type II (Cholingeric)
Motor - Operant Sensory - Consumatory
Purposive - Learned Responsive - Reflex
illusion, since attention is being decomposed nowadays into mine-related toxic effects such as schizophrenic-like psychosis
separable processes. The results of this decomposition seem and response stereotopy. Type I also exerts a direct drive on
confusing, although full of promise. By decomposing electro- Type II since its aminergic activity seems to have an excitory
graphic arousal into Type I and Type II, Vanderwolf & effect on the cholinergic control of Type II. This suggests the
Robinson (V&R) provide an important new addition to the effects of conscious choice on automatic parallel systems and
growing list of dimensions on which arousal/attention can be the general modulation of automatic motor responses by
pulled apart. Furthermore, this newcomer may help to settle controlled attention.
some of the confusion that exists among the earlier arrivals. As V&R correctly point out, schizophrenia appears to be a
Table 1 gives a partial list of arousal/attention processes. It disease of the Type I systems. A considerable literature
is arranged as a sort of a matrix, so that items in each column supports the notion that not only is the defect in schizophrenia
share a family resemblance, while each row represents a confined almost exclusively to the Type I systems, but the
dimension that has some empirical justifications. The refer- Type II systems remain normal, or even supernormal. This
ences on the right provide a guide to the details of these concept of schizophrenia resolves a number of apparent
empirical justifications, although the text by Klatzky (1980) is paradoxes in the literature on schizophrenic performance.
probably a better practical reference. For example, it is noted that while schizophrenics have
No two of these dimensions are the same, yet neither are generally slower reaction times, they have faster than normal
they orthogonal. As in family-type classes, each member of a alpha-blocking. This would seem to be an example of how, in
family has something in common with every other member, schizophrenia, defective functioning of the Type I systems
yet is also has something that makes it different from every slows reaction time, while the Type II systems function more
other member. For example, parallel and data-driven rapidly than normal.
processes both seem to operate rapidly, automatically, and The characteristics of Type II systems remind me of the
generally outside of consciousness. Nevertheless, some paral- sensory distortions and deliria produced by anticholinergic
lel processes can be memory-driven. Consider some of the drugs, and of the general relationship between the cholinergic
classic experiments carried out by Posner (1978) and his system and both mood and pain perception. I am particularly
students (McLean & Schulman 1978). First, a prime stimulus intrigued by the light this new work sheds on some data that
is given as a warning. The prime may be a letter or a nonletter we collected many years ago (Callaway & Dembo 1958;
symbol. Then a pair of target letters is presented and the Callaway & Band 1958). Based on a wide variety of behav-
subject responds "same" or "different." The response time is ioral experiments it seemed that a sort of narrowed attention
measured. If a subject is taught to evoke an image of a prime could be induced by a variety of procedures. These proce-
in response to some arbitrarily designated cue, and if a match dures included a stimulant (amphetamine), sensory stimula-
between the imagined prime and the targets is unlikely, then tion (cold-presser test), and a strong cholinergic (anticholines-
reaction time will be speeded when an actual match occurs, terase nerve gas). To produce the opposite sort of effect and
but will not be slowed if the target is a mismatch. This gain broaden attention, the most effective substance seemed to be
(speeding with the match) without cost (slowing with the atropine. We assumed we were observing classic arousal and
mismatch) is a classic indication of a parallel system in finally stopped pursuing this line of work when subsequent
operation. Yet the fact that the prime was only imagined studies failed to consistently confirm the narrowing effect of
makes it memory-driven. amphetamines. In retrospect, apparently we were inadver-
Before the arrival of Type I and Type II it was difficult to tantly studying Type II arousal and were thrown off by the
think of names for the two families that I have labeled A and weak tendency of Type I arousal to stimulate Type II arou-
B. Family B is generally faster and Id-ish, while A is slow and sal.
Ego-ish. But these are hardly satisfactory definitions. At the In conclusion then, I find that the Type I and Type II
bottom of the Table, I have placed Type I and Type II distinctions provided by V&R suggest a framework for orga-
electrographic arousals. I believe the situation now becomes nizing some of the various ways that attention/arousal can be
less confusing. Type I is concerned with voluntary behavior broken into separable processes. I am sure that this synthesis is
and is response-oriented. It suggests amphetamine-related too simple, but the Type I/Type II distinctions suggest that
benefits such as increased sustained attention and ampheta- we may be on the verge of making a more reasonable
478 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
organization out of some rather confusing observations, both ology of these parts of the brain, to describe the animal's
from the laboratory and from the clinic. behavior in terms of inferred complex processes rather than
the specific movements that are being observed. However,
V&R demonstrate that cortical and hippocampal rhythms
correlate much better with particular classes of behavior than
Is the distinction between Type I and with any internal state that one might reasonably hypothe-
Type II behaviors related to the size. In fact, the data they summarize suggest that the concept
effects of septal lesions? of "general arousal," at least as defined electrophysiological-
ly, has little to recommend it.
Neil R. Carlson V&R do an excellent job describing the data that suggest
the existence of two neurochemically distinct reticulo-fugal
Department of Psychology, University of Massachusetts. Amherst, Mass.
01003
systems that are active during Type I ("voluntary") or Type
II ("automatic") behaviors, independent of the animal's puta-
At the risk of simplifying matters too much, it might be said tive state of alertness. I find their analysis especially interest-
that strategies for research on the neural mechanisms of ing in light of the results of some studies that my students and
behavior can be grouped into two categories: (1) One can 1 have undertaken to determine the functions of the septum.
facilitate or interfere with some part or parts of the nervous Until recently, I would have said that most of the behavioral
system by chemical, surgical, or electrical means and observe effects of lesions of the septum could be attributed to an
the behavioral phenomena that are altered, or (2) one can enhancement of the consequences of reinforcement, caused
watch the behavior of an animal ("spontaneous" behavior or by disinhibition of neural mechanisms of reinforcement. This
behavior elicited by the experimental situation) and see what enhancement would explain many of the phenomena that
chemical or electrical changes may be found in the nervous constitute the "septal syndrome," such as increased response
system. rate during performance of an operant behavior, impaired
An investigator who pursues research that falls under either acquisition of a schedule of reinforcement that requires a low
of these categories must ask the following question: What is rate of responding (DRL, or differential reinforcement for
the best way to categorize the behaviors that are observed? low rate), and more rapid acquisition of alternation tasks,
The level of behavioral analysis has a profound effect on the which may be regarded as discrimination tasks that are cued
kinds of conclusions that can be reached; the conclusions are by the presence or absence of the reinforcer. (See Fried 1972;
constrained, regardless of the results that are finally obtained. Grossman 1974, 1978; Isaacson 1974 for reviews of the "septal
To take a striking example, Delgado (1969) claimed to have syndrome.") However, recent (unpublished)*evidence that we
"pacified" a charging bull by telestimulation, while the have gathered suggests another interpretation, one which
apparent truth of the matter was that he simply stopped might be related to the distinction between Type 1 and Type
attack by stimulating motor systems that caused the animal to 2 behaviors.
turn, thus aborting its charge (Valenstein 1973). In this case a Some classes of behaviors, many of which might be
behavioral description is clearly superior to one that makes regarded as species-typical, are impaired or even abolished by
reference to an inferred process. septal lesions. For example, mice with septal lesions do not
The level of behavioral analysis is determined in large part hoard, build nests (during pregnancy or lactation, or in
by the level of the nervous system that one studies. If one response to cold stress), retrieve their pups, gather string,
studies the spinal cord, behavioral observations will probably climb on the lids of their cages, or run in an activity wheel.
be made in terms of specific muscle movements. Conversely, They do kill cockroaches, mate, eat, and drink. They very
if one is interested in cortical function one generally observes readily perform a variety of arbitrary responses (Type 1
learning, or attention, or arousal, or other "higher" processes. behaviors?) when these are reinforced (e.g., they will press a
The problem is, of course, that these inferred behavioral lever, run through an alley, or poke their head into a hole),
phenomena are poorly and inconsistently defined. Vander- and will sustain a rate of responding far in excess of that of a
wolf & Robinson (V&R) make the case for an objective normal animal. However, they perform very poorly when a
behavioral analysis of reticulo-hippocampal and reticulo- "species-typical behavior" such as string-pulling or-wheel-
cortical "arousal." running serves as the operant response. It is as if a class of
Most of the time, hippocampal synchrony (theta) and behaviors is removed from the animals' repertoire. Even the
cortical desynchrony (beta) indicate "arousal," in the sense behavior of remaining immobile is imparted; mice with septal
that the animal is awake and potentially reactive to stimuli. lesions do not learn to hold their head still in the poke hole,
Of course, these forms of electrical activity are also recorded although they very quickly learn to poke it in and out,
during RKM ("paradoxical") sleep, during which the animal repeatedly.
is presumably reacting to internally generated stimuli, The hypothesis that I previously favored - disinhibition of
('diversely, cortical synchrony and hippocampal desynch- reinforcement mechanisms - might be replaced by a more
rony indicate (most of the time) that the animal is not parsimonious one: The unavailability of a class of behaviors
"aroused," and is either asleep or not "attending to" sensory means that the animal spends more time performing the
stimuli. The relation between electrophysiological activity operant response because it spends less time performing the
and the presumed level of arousal has led many investigators various adjunctive behaviors that occur during almost every
to use the EEC as a measure of activation in an animal that is schedule of intermitent reinforcement.
unable to perform normal behaviors. The behaviors that survive septal lesions appear to conform
V&R point out that there are many examples of noncorres- to V&R's description of Type 1 behavior; the animals readily
pondence between probable level of "arousal" and the elec- perform rapid changes in movement or posture. Unfortunate-
trical activity of the hippocampus and cortex. Instead of ly, the behaviors that are lost do not correspond very well
dismissing these observations as exceptions to a general rule, with Type 2 behaviors, although both might be loosely
the authors assert that one should record specific behaviors characterized as species-typical. The fact that hippocampal
and not attempt to infer the presence of physiological/ rhythms are controlled by neural systems that travel from the
behavioral "states." Since the functions of the hippocampus reticular formation through the septum suggests that V&R's
and neocortex undoubtedly comprise many complex analysis might provide useful guidance for future research in
processes, one is tempted, when studying the electrophysi- this area.
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 479
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
A behaviorist in the neurophysiology lab sniffing of relevant olfactory stimuli. Others have found that
RSA phase is related to the onset of relevant auditory stimuli
Howard Eichenbaum (e.g., Thompson 1980). Each of these experimental paradigms
Department of Biological Sciences, Wellesley College, Wellesley, Mass. was characterized by great detail in timing, replication of
02181 behavioral minutiae, and statistical qualification. Moreover,
the necessary selectivity of behavioral observation was guided
Vanderwolf & Robinson (V&R) and others have added a in each case by a theory of RSA involvement in stimulus
great detail to the description of slow wave correlates of overt analysis. It is impossible to observe all ongoing behavior at this
behavior. But the new synthesis by V&R is not sufficient to level of detail; the selectivity of observation requires a focus
replace a sophisticated arousal theory of cortical-reticular on particular behaviors and on their psychological relevance.
relationships. Investigators 30 years ago knew that decortica- Furthermore, contrary to V&R's suggestion, behavioral
tion does not produce coma, and they knew that neocortical scientists do have quantitative tools for indirect observation of
LVFA occurs during "paradoxical sleep." That is, we have psychological processes. For example, through clever manip-
known for some time that cortico-reticular (as opposed to ulation of reward contingency, Ranck (1974) and O'Keefe
more local brainstem) mechanisms in arousal are complex (1976) discovered that some hippocampal neurons fire prefer-
and subtle. Their understanding will require analyses more entially where and when the subject does not find expected
sophisticated than those provided to date. reward. The key aspect of expectancy was not observable in
V&R suggest that such analyses are better served by the gross behavior of the subject. The procedures suggested
detailed behavioral description than by inference to the here (selective detailed behavioral observation and indirect
underlying psychological phenomena. But their "behavioral observation of psychological process) might best be used in
analysis," as such, is unsuccessful, because the described combination to clarify behavioral correlates-of neurophysio-
EEG-behavioral correlates (a) are dissociable and (b) do not logical phenomena.
avoid inference to psychological process. Unfortunately, V&R's new synthesis of cortico-reticular
The evidence V&R provide against "traditional" slow- involvement in determining "when" behaviors happen is no
wave correlates of behavior consists of (1) the exceptions to more sophisticated than the arousal theory it is meant to
their concurrence in subcomponents of normal aroused replace. Perhaps by the tedious detail and trickery which are
behavior and sleep, and (2) the exceptions to disruption of trade to psychologists we will gain some insight into the subtle
arousal-sleep patterns following brain damage. Yet V&R's questions about the EEG, such as, "why a slow rhythm (RSA)
elegant correlation of behaviors with neocortical LVFA and during active, attentive behavior?"
hippocampal RSA are also dissociable, in this case by pharma-
cological manipulations. The finding that LVFA can occur in
the brain-damaged, comatose animal convinces V&R that
LVFA is not the proper EEG correlate of arousal. By similar
logic, the finding that atropine eliminates slow-RSA (4-8 Hz) Is hippocampal theta an artifact?
but not "automatic" behaviors, suggest that "automatic"
Glynne Hirschman
behavior is not the proper behavioral correlate of slow-RSA.
Fast-RSA (7-12 Hz) and voluntary movement are more Department of Psychology, University of Cape Town, Rondebosch 7700,
Republic of South Africa
difficult to dissociate with anesthetics. This may be because
voluntary movement and fast-RSA are causally linked. Following the interpretation of Green and Arduini (1954),
(Although anesthetics which affect fast-RSA eliminate both investigators commonly assume, as do Vanderwolf & Robin-
voluntary and automatic movements.) Curare eliminates son (V&R), that hippocampal theta (RSA) indicates an acti-
movement and leaves fast-RSA intact. The relevant effect of vated hippocampus. This is by no means a universal senti-
curare may in this case be entirely peripheral, but the clear ment, however. Several authors have suggested that theta in
dissociation of EEG from overt behavior makes obvious the fact reflects an inactive hippocampus (Bennett 1973; Black et
need for considering internal mechanisms. al. 1970; Douglas 1967; Grastyan et al. 1959) and, indeed,
Vanderwolf's contrasting of "voluntary" vs. "automatic" recent evidence seems to support this contention.
behavior is insightful and appealing, but such terms cannot, 1. Unit activity during paradoxical sleep (PS): PS presents
as V&R admit, be described as specific movement patterns, an ideal opportunity for assessing the activity of hippocampal
and they definitely infer the underlying psychological neurons with regard to theta, since virtually uninterrupted
purposes of behavior. The terms are no less clear than those theta is present throughout this state. Most studies have
they are meant to replace (e.g., "attention"), although they do reported a decrease in the rate of firing during PS (Delacour
introduce a potentially valuable, ethologically relevant 1980; Mink et al. 1967; Ranck 1973), and discrepant findings
dimension. Nevertheless, it seems unavoidable to use psycho- (e.g., Noda et al. 1969) have been clarified by Ranck's
logical intermediaries when discussing the hippocampus and distinction between projection (output) cells and interneurons
neocortex. The elimination of such terms will come when we (Fox and Ranck 1975; Ranck 1973). With this classification it
can substitute sufficient mechanistic detail to obviate their has been convincingly shown that hippocampal output cells
need in explanation. are strongly inhibited during PS, while interneurons (which
V&R suggest that we would do better to compare the are generally thought to inhibit output cells) fire rapidly.
details of behavior to EEG, but the success of this strategy Since it is only via its output neurons that the hippocampus
requires a good choice of what to observe, and in how much can exert any influence, the conclusion seems inescapable
detail. For example, Vanderwolf et al. (1975) found "no that, in this case, theta indicates an inactive hippocampus.
consistent phase relation" between RSA and sniffing in corre- 2. Unit activity and theta during the waking state: In
lating EEG and inhalation cycle for long periods during general, findings have been equivocal: Some units increase
which other details of exploratory behavior were not consid- their rate of firing during theta, while others decrease it
ered. But exploratory sniffing is a complex, epochal behavior (Lidsky et al. 1974; Mays & Best 1975; O'Keefe & Dostrovsky
(Welker 1964), and the appropriate "level of detail" for 1971). Delacour (1980), however, has been able to make a
behavioral description must take into account both its more definite statement. He found that during the elicitation
complexity and intermittency. Macrides (1974) and Eichen- of a classically conditioned neocortical desynchronisation
baum et al. (1979) observed a preferred phase relation (which is usually associated with hippocampal theta) hippo-
between inhalation cycle and RSA in short bouts of intense campal Type I (output) units were clearly inhibited.
480 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary /V anderwolf & Robinson: Reticulo-cortical activity and behavior
Certainly it is the conclusion of Ranck (1973) that different artifact. Specifically, I would suggest brain movement as the
output neurons are concerned with different types of behav- artifact-generator, since it has long been established as a
iour, rather than the presence of theta. The only cells which source of EEG artifact (see, e.g., Li & Jasper 1953; Walsh
show a consistent activation during theta are interneurons. 1956), and can be produced by any agent which causes
While a recent study by Bland et al. (1980) disagrees with this fluctuations in cerebrospinal fluid pressure (e.g., respiration -
finding, it should be noted that they used urethanised especially sniffing - heart beat, head movements, and vigor-
animals - a fact which makes interpretation of their results ous body movements). Support for this contention derives
difficult (Mercer et al. 1978). from a series of experiments designed to investigate this
It is perhaps not surprising that sampling the activity of a phenomenon (Hirschman, in preparation), the main points of
minute percentage of neurons during the waking state (when which can be summarised as follows:
many stimuli occur simultaneously) does not present a clear («) "Theta" could be produced in a dead rat (whose head
picture. To assess the general excitability level of large was fixed in a stereotaxic frame) by rhythmically squeezing
numbers of neurons at any particular point in time it is the thorax, or moving the body relative to the head.
perhaps more appropriate to turn to evoked potentials. Segal (ii) Hippocampal tissue movement and the concomitant
(1978), using unrestrained rats, produced evoked potentials in EEG were recorded in lightly anaesthetised animals. Brain
the hippocampus by stimulating the hippocampal commi- movement was found to bear a one-to-one correspondence to
sural fibres. He found that during theta the primary wave of theta.
the evoked potential was much reduced, while the late (iii) Under conditions of anaesthetisation heartbeat was
component was enhanced. This result is consistent with the found to be ineffective in pulsing the brain. However, when
interpretation that the output neurons (which are the first to halothane anaesthesia was applied to the point of respiratory
receive the commissural activation) are in a more inhibited depression, the consequent increase in strength of cardiac
state during theta, while the interneurons (whose firing is contraction did produce neural tissue movement; and a well-
secondary to that of the output neurons) are in a more excited developed theta, which bore a one-to-one relation to heart-
state. beat, was seen.
3. Amphetamine, which is routinely administered to (iv) Sniffing was found to be an especially effective cause
produce theta, has been shown to inhibit hippocampal unit of brain movement and theta. A bout of sniffing was always
activity (Segal & Bloom 1976). accompanied by theta, and each sniff corresponded to a theta
4. Theta can be recorded during locus coeruleus (LC) wave. Figure 1 illustrates the correspondence between theta,
stimulation (Macadar et al. 1974; Robinson et al. 1977) even hippocampal tissue movement, and respiration. The large
though such stimulation specifically inhibits what appear amplitude deflection resulted from a sharp intake of breath as
from their firing characteristics to be hippocampal output the animal changed from normal respiration to sniffing.
neurons (see Segal & Bloom 1976). [Note: Robinson et al. (v) In the awake curarised animal, theta was found to
(1977) have argued that theta production during LC stimula- follow the respirator setting, provided that the ventilation
tion is due to current spread, but this presumably does not pressure was adequate. This finding supports earlier reports
alter the fact that LC-mediated inhibition is still operative.] to this effect (Li & Jasper 1953) and contrasts with Komisa-
5. Both ablation (see Altman et al. 1973; Douglas 1967; ruk's (1970) failure to find such a relationship. It seems
Kimble 1968) and stimulation studies (e.g., Bland & Vander- possible that the discrepancy is due to Komisaruk's underven-
wolf 1972a; Vanegas & FLynn 1968; Votaw 1960) implicate tilating his animals, since his records show a close correspon-
the hippocampus in some form of behavioural inhibition, and dence between heartbeat and theta.
yet it is not theta, but low voltage fast activity (LVFA) in the (tri) The freely moving animal represents a more complex
hippocampus which is associated with both behavioural and situation in that many other variables can produce brain
spinal reflex inhibition (Yokoto & Fujimora 1964). movement, thus obscuring the simple relationships observed
Taken together, the above evidence implies that, in the during anaesthetisation. Nevertheless, several authors have
hippocampus as elsewhere, it is EEG fast activity which noted a one-to-one correspondence between sniffing and
indicates activation; and since the output cells are large and synchronous EEG activity in the unrestrained animal (Gault
numerous, it is they that seem likely to most influence the & Leaton 1963; Komisaruk 1970; Lippold 1973; Macrides
EEG. Certainly the elegant work of Steriade (1978) supports 1975).
this contention with regard to the neocortex. He has shown (vii) Distortions introduced by the capacitative coupling of
clearly that it is the activity of the large output cells of the the electrode to the electrolyte are especially important when
neocortex that is closely related to neocortical LVFA. The two or more variables combine to produce brain movement.
interneurons show the opposite behaviour, being active Under these conditions brain movement does not always bear
during synchronous EEG activity.
Unfortunately, there is little work on unit activity during
hippocampal LVFA, but the work of Whishaw et al. (1978) is I
suggestive. From their records it can be seen that as an
electrode traverses the hippocampus, fast activity (15-55 Hz)
is closely related to the pyramidal and granule cell layers. In
addition, theta was not markedly affected when approxi-
mately 90% of the granule cells were destroyed by X-
irradiation, but the amount of fast activity was reduced
dramatically.
The fact that theta is present when the output neurons are
inhibited presents somewhat of a puzzle - if theta is not
produced by the output neurons, what then does cause theta?
The possibility that interneurons (which do fire in phase with Figure 1 (Hirschman). Illustrating the correspondence
theta) might be involved is weakened by the fact that they are between theta (HPC), hippocampal tissue movement
few in number (less than 1% according to Fox & Ranck (MOUbr), and respiration (RESP). The large deflection marks
1975). the transition from normal respiration to sniffing. A one-
Thus, in the absence of a suitable neural substrate, I would to-one correspondence between the three measures is present.
like to submit the rather radical proposal that theta may be an Cal: 500 nV, 1 sec.
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 481
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
a simple relation to the induced EEG fluctuations. Moreover, Understanding the physiological correlates
it was found that theta produced by thoracic manipulation of a behavioral state as a constellation of
could be blocked by LVFA. it is suggested that this blocking is
due to high levels of neural activity and thus that theta will be events
seen only in a relatively inactive hippocampus.
Barbara E. Jones
(viii) The fact that theta has been found to be dependent on
the integrity of the septum (Green & Arduini 1954) may seem Laboratory of Neuroanatomy, Montreal Neurological Institute, Department
of Neurology and Neurosurgery, McGill University, Montreal, Quebec,
to provide strong evidence that theta has an exclusive neuro- Canada H3A 2B4
biological origin. However, there are other possible explana-
tions. For example, the medial septal nucleus has been shown Rather than creating a new synthesis of current concepts,
to exert an inhibitory effect on the hippocampus (Miller & Vanderwolf & Robinson (V&R) merely reject an anachronis-
Groves 1975). Thus its destruction will release the hippocam- tic and oversimplified view of the relation of electrocortical
pus, resulting in excess neural activity and therefore a activity to behavior, thus setting up a strawman; moreover,
predominantly desynchronised hippocampal EEG. they do so with less rigor, offering a piecemeal model of the
brain reconstructed by surgical and pharmacological means
to explain pathological behavioral states.
With contemporary computer technology, a monolithic
analysis, involving a single physiological correlate of behavior
EEG, pharmacology, and behavior such as electrocortical activity, has now been superseded
(Friedman & Jones 1981). Through the quantification of the
Herbert H. Jasper amplitude or frequency of multiple physiological parameters
Department of Physiology, University of Montreal, Montreal, Quebec, (including electrocortical activity, neck muscle tonus, eye
Canada H3C 3T8 movements, ponto-geniculo-occipital spikes and yes, V&R's
preferred variable hippocampal activity), it becomes appar-
Although I very much appreciate Vanderwolf & Robinson's ent that all these physiological measures covary with observ-
(V&R's) sincere efforts and their critical review, I believe that able behavior through the sleep-waking cycle. Contrary to
there are too many points of question in their treatment of the V&R's statement, electrocortical activity, like each of these
subject for me to deal with adequately in a brief commentary. variables, is indeed strongly correlated with behavioral state
Furthermore, the complications of arguments based on phar- in the normal animal. However, any one variable, including
macological evidence, of which V&R make extensive use, do behavioral phenomena (as V&R admit) is inadequate for the
not, I believe, provide a very firm basis for conclusions. For reliable identification of the sleep-waking state. Instead, the
example, there is much evidence that the effect of atropine on states of waking, (high amplitude) slow wave sleep, and
behavior and on the electroencephalogram is more compli- paradoxical sleep are defined and reliably identified as a
cated than V&R would imply; if proper testing is carried out, particular constellation of physiological and behavioral
there are very significant alterations in behavior, and espe- events. In fact, through the simultaneous quantification of
cially in learning processes, under the influence of atropine multiple physiological variables (particularly electrocortical
accompanied by EEG slow waves. (Scopolamine, for exam- activity, muscle tonus, and ponto-geniculo-occipital spikes),
ple, is used clinically to produce amnesia while maintaining cluster analysis algorithms have been successfully applied to
reactivity in patients during childbirth or surgery.) The the computerized classification of the sleep-waking states in
assumption that animals are as alert under atropine as under the normal animal.
normal waking conditions certainly does not involve a very In an experimental animal with surgical destruction of the
critical approach, yet it is one upon which much of the cortex or pharmacological interference with the acetylcholine
evidence for V&R's evaluation of the arousal hypothesis is receptors, the physiological and behavioral events of the
based. normal constellations are (and here I disagree with V&R)
On the other hand, I do feel that the suggestion that more truly dissociated. These pathological states should therefore
than one transmitter may be involved, such as, for example, not be used (as they have been by V&R) to evaluate the
acetylcholine and amino acids, is a very good one; we have normal correlation between electrocortical activity and
positive evidence that there is a remarkable change in the behavior. Sleep, which is normally characterized by high
liberation of amino acids from the cortical surface in arousal amplitude electrocortical activity, is no longer normal sleep
as compared to sleep states, as well as marked change in without cortical activity, even though one aspect, the behav-
measures of acetylcholine. This would tend to favor V&R's ioral phenomena, may appear normal. Waking, which is
conclusions. The evaluation of the effects of the catechol- normally characterized by low amplitude electrocortical
amines is somewhat doubtful, however, since they may also activity is no longer normal waking when this activity
interact with either amino acids or acetylcholine; such inter- increases in amplitude with atropine, even though certain
actions have been thoroughly demonstrated. superficial behavioral parameters might appear normal.
The above is just a sample of some of the many complica- Studies would suggest, in fact, that the entire constellation of
tions involved in the evaluation of V&R's approach. The events, including the behavior, has changed after these
neurophysiological mechanisms underlying the various manipulations. Recently, cluster analysis of multiple physio-
behavioral "states of consciousness" are more complex than logical and behavioral variables has revealed a complete
the original EEG arousal hypothesis presumed; however, the alteration of the associations among these parameters after
apparent exceptions or "contradictions" in states designated brainstem lesions in the cat (Jones & Friedman 1981).
as "sleep " or "waking " do not contradict the hypothesis but These pathological states have been more appropriately
serve to indicate the need for more careful and critical used to determine the essential mechanisms for states or for
behavioral analysis of the mechanisms underlying the many particular components of states. V&R have not taken into
different behavioral states designated as "wakefulness" or account the existing suggestion that two different reticulo-
"sleep." V&R have made an important contribution in this telencephalic systems may be important for tonic electrocor-
direction. [For further information see Jasper 1966; Celesia & tical activation and behavioral arousal of the waking state (see
Jasper 1966; Jasper & Koyama 1967; Jasper & Krnjevic 1969; Jones, Bobillier, Pin & Jouvet 1973). By definition, it cannot
Jasper & Tessier 1971; Reader, Ferron, Descarries & Jasper be denied that the reticulo-cortical system (as V&R call it) is
1979.] essential for the tonic cortical activation of waking. The fact
482 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
that other reticulo-telencephalic and reticulo-spinal systems authors emphasize its nonexistence (i.e., Type 2 behavior is
are necessary for behavioral arousal has already been demon- "ordinarily not accompanied by RSA") and other times they
strated. emphasize its blockage (i.e., by atropine). Furthermore, there
As to the specific role of catecholamine neurons in waking, is a question as to which components of the RSA are blocked
(proposed by Jones et al 1973), numerous critical studies by atropine; in Fig. 3, upper right, although the waves do not
involving selective lesion and pharmacological techniques persist in a steady rhythm, 2 or 3-wave segments can
have permitted a full appraisal of their importance in arousal nevertheless be seen. There is also a problem regarding the
and have thus made it necessary to assign a modulatory, criteria for the absence of theta. For example, in Fig. 2 (lower
rather than an essential mediating, arousal function to these trace) in the example shown of hippocampal activity related
neurons (Jacobs & Jones 1978). As V&R suggest, the re- to Type 2 ("nontheta") behavior, evenly spaced wave crests
ticulo-telencephalic systems that underlie the regulation of occurring at the theta frequency are evident. Thus, even in
behavioral state are probably multiple and include as constit- this assumed absence of theta, rhythmic brain activity at the
uents the monoamine neurons. As these authors emphasize, theta frequency is nevertheless present. Thus the difference
pharmacological and biomedical evidence suggests that between theta and nontheta activity may be quantitative
acetylcholine neurons are also a component of re- rather than qualitative.
ticulo-telencephalic activating and arousal systems. However, However, let us assume that criteria can eventually be
without histochemical identification and neuroanatomical identified that reliably distinguish two different RSA mecha-
localization of acetylcholine neurons, they cannot be submit- nisms. A question then remains of whether these two mecha-
ted to the same rigorous physiological tests as have the nisms are operational in the normal awake rat, and if so, what
monoamine neurons (see Jacobs & Jones 1978). Therefore, to their functions are. For example, are the cholinergic and
formulate a model of the essential role of acetylcholine noncholinergic mechanisms both active in concert with each
neurons and their target cells in arousal, especially based other during Type 1 behavior? Do they mediate sensory and
upon the action of one drug (atropine), is quite premature. motor patterns, respectively, in an integrated system?
In summary, I would agree with V&R that multiple re- We submit that a behavior pattern which is particularly
ticulo-telencephalic systems, probably including monoamine well suited to such an analysis has not been considered in
and acetylcholine neurons, contribute to the electrographic V&R's present discussion, although they raise questions about
and behavioral manifestation of sleep-waking states. But I the sensory and motor functions of RSA. This behavior
would suggest that these mechanisms should be approached pattern is sniffing-vibrissal-whisking during exploratory
through a more holistic view, involving constellations of behavior. In this behavior pattern, which is almost invariably
physiological and behavioral events that define sleep-waking associated with the Type 1 RSA activity, motor and sensory
states. systems are precisely coordinated with each other (see Komis-
aruk 1970). The motor patterns of inhalational sniffs and
protractions of the vibrissae generate sensory bursts of olfac-
tory and vibrissal tactile afferent activity. We have proposed
Rhythmic modulation of sensorimotor (Komisaruk 1970; 1977; Semba & Komisaruk 1978) that each
individual theta (RSA Type 1) wave can be viewed as a single
activity in phase with EEG waves excitation cycle of systematically varying sensory and motor
neural excitability. These excitability cycles normally occur
Barry R. Komisaruk* and Kazue Sembab one after the other, in rhythmic succession, forming the theta
'Institute of Animal Behavior, Rutgers University, Newark, N.J. 07102 and rhythm. We have suggested that the function of this mecha-
'Department of Anatomy, College of Medicine and Dentistry of New Jersey, nism is to enable the CNS to sample and respond to the
Rutgers Medical School. Piscataway, N.J. 08854 sensory environment in successive "bits." Characteristically,
A major conclusion of Vanderwolf & Robinson's (V&R's) although not invariably, there is one sniff cycle to one theta
review is that hippocampal rhythmical slow activity (RSA) is wave in rats (Komisaruk 1970) and hamsters (Macrides 1975).
controlled by two different neural pathways, each of which is In rats, this one-to-one relationship is maintained over the
active in a different behavioral context. In this commentary, normal range of sniffing and theta frequencies (Komisaruk
we point out certain limitations of the criteria on which V&R 1973).
base their conclusions, and discuss an aspect of RSA that they The sensory "gating" property of the theta rhythm is
have not addressed. V&R present evidence that, under appro- indicated by the finding that the number of unit action
priate conditions, two different pathways control hippocam- potentials generated in the lateral hypothalamus by constant
pal RSA. In anesthetized rats, RSA that becomes activated current single shocks to the olfactory bulb varies systemati-
spontaneously or in response to sensory stimuli is blocked by cally as a function of the phase of the theta rhythm at which
atropine. Atropine also blocks the RSA that occurs during the stimulus was delivered (Komisaruk 1977). Similar effects
immobility when rats are awake (Type 2 behavior), so the in hippocampal afferents have been demonstrated recently
authors contend that this RSA has a neural basis in common by Rudell, Fox & Ranck (1980) and Buzsaki, Grastyan, Czopf,
with that under anesthesia. They propose that this Type Kellenyi & Prohaska (1981). Motor performance is also modu-
2 - anesthesia RSA is under cholinergic control and is lated in phasic relation to the hippocampal theta rhythm.
involved in the "stimulus control of behavior." They contrast Rats were trained to press a lever ad lib for a food pellet
this pathway with the RSA of "Type 1 - voluntary behavior" reward. We found that they were most likely to press the
(e.g., walking) that is abolished by anesthesia but not by lever at the peak of their spontaneous concurrent theta wave,
atropine and "appears to be related to motor activity." and were most likely to withdraw their paw from the lever at
This classification into two different RSA systems is unfor- the opposite polarity peak of their theta wave (Semba &
tunately not so clear-cut as V&R imply, even on the basis of Komisaruk 1978). Integration of sensory, motor, and atten-
their own findings. They claim that Type 2 behavior, which tional processes in relation to the theta wave was demon-
includes immobility is "ordinarily not accompanied by RSA." strated by the study of Eichenbaum & Macrides (1979) in
However, the EEG recording shown in their Fig. 3 (upper which individual sniffing cycles and hippocampal theta
left) shows a pattern during the immobile (Type 2) condition cycles became entrained and phase-correlated with each
other when stimulus salience was reversed in an olfactory
that they indicate is an RSA pattern and is sensitive to
discrimination task. The authors concluded that entrainment
atropine (Fig. 3, upper right). This points up an ambiguity in of sniffing and theta rhythms is involved in the assessment of
their criteria for the occurrence of RSA, for sometimes the
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 483
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
the significance of odors. Since Vanderwolf & Robinson (V&R) emphasize the appar-
Modulation of sensorimotor activity in phasic relation to ent involvement of cholinergic pathways in behavioral arou-
brain rhythms appears to be a general phenomenon, as sal, I would like to comment more specifically on cellular
demonstrated by similar findings in relation to the thalamo- mechanisms of cholinergic activation in the brain and why
neocortical rhythmic EEC This approximately 9 Hz pattern they may be particularly involved in some forms of arousal.
(which V&R call "spindle activity") appears in rats during In the first place, it is now clear that as a muscarinic
alert immobility ("freezing"). We have observed that it is (atropine-antagonized) agent, ACh is not a simple transmitter
characteristically accompanied by a low amplitude tremor of in the sense that ACh (nicotinic) is an all-or-none transmitter
the vibrissae and occasionally of the lower jaw in the absence of excitation in muscle. Its role can be characterized more
of gross bodily movement (Semba, Szechtman & Komisaruk usefully as that of a modulator, because its primary action
1980). Both the vibrissal tremor and the EEG pattern cease appears to be to reduce membrane outward currents:
when the vibrissae are touched lightly. A remarkable syn- Normally, such currents tend to slow down neuronal depolari-
chrony exists between each individual tremor movement of zation, thus impeding the generation of action potentials;
the vibrissae, recorded as EMG (electromyogram), individual moreover, by accelerating repolarization after a Spike, they
neocortical waves, and individual bursts of neuronal activity also prevent repetitive firing. Hence, a depression of outward
in the ventrobasal thalamic nuclear complex. We have identi- currents facilitates excitation and promotes repetitive
fied this EEG pattern as "alpha rhythm" in the rat, on the discharges in response to single inputs. [See also Dismukes:
basis of its frequency, its neuroanatomical and functional "New Concepts of Molecular Communication Among
characteristics, and the behavioral context in which it occurs Neurons" BBS 2(3) 1979.]
(for further discussion, see Semba et al. 1980). It does not Two kinds of outward currents are now well known: One
occur during whole body tremor or tooth-gnashing (chatter- set, the K currents, is intrinsic to the neuronal membrane,
ing). As in the case of the theta rhythm, phasic modulation in
being generated in response to depolarization. Cl" current on
relation to individual waves of the alpha rhythm has been
reported for sensory thresholds and reaction time (see Komis- the other hand, is elicited especially by inhibitory synaptic
aruk 1977, for review). action. There is now strong evidence that in structures such as
the neocortex and the hippocampus (and no doubt various
The observations described above provide specific behav- sites in the brain stem)ACh selectively inactivates these
ioral endpoints that are correlated with specific brain EEG outward currents through two different mechanisms.
paterns and could help provide insight into the sensory, The first is the muscarinic depression of neuronal K
motor, and integrative mechanisms which V&R suggest are conductance originally described in the neocortex (Krnjevic
differentially related to these EEG patterns. The findings of et al. 1971), and even more recently shown to operate also in
phasic relationships between individual EEG waves and indi- the hippocampus (Dodd et al. 1981; Ben-Ari et al. 1981). In
vidual muscular movements raise the basic question that the
both regions, ACh makes pyramidal-type neurons strikingly
authors have not addressed: What is the functional signifi-
cance of the rhythmic nature of the EEG patterns, the Type 1 more responsive to any form of depolarizing input (electrical
RSA: 7-12 Hz; Type 2 RSA: 6 Hz; and neocortical spindle or synaptic). According to Brown & Adams's (1980) study of a
activity during Type 2 behavior: 6-9 Hz. Since V&R have comparable muscarinic facilitation of sympathetic neurons,
raised the question of the behavioral correlates of these brain ACh blocks selectively a special, voltage-dependent K current
rhythms, we suggest that whatever different behavioral ("M-current") which is activated by small depolarizations
systems they may regulate, they may all share the common from the resting level, and is therefore normally particularly
property in which their excitability cycle, i.e., their afferent effective in preventing repetitive discharges.
sensitivity and efferent potency, fluctuates in relation to the The second mechanism first became apparent in very
EEG wave phase. This may serve a variety of functions, such recent studies of the hippocampus (Krnjevic et al. 1980;
as increasing the range of sensitivity to afferent excitation, 1981). It is especially evident when local applications of ACh
maximizing the degree of integration possible by increasing are combined with fimbrial stimulation. In rats under
the number of neurons available for excitation at certain urethane, very low frequency stimulation of the fimbria -
moments (e.g., wave peaks), and maximizing the ability to which activates both excitatory and inhibitory inputs to the
excite postsynaptic neurons, by increasing the likelihood of pyramidal cells of CA1 - does not lead to overt excitation, the
temporospatial summation via a synchronous barrage at the predominant response being strong inhibition (which
wave peaks. presumably masks the excitatory effect of EPSPs). When ACh
is applied, the previously inactive neurons generate large
population spikes, evidently because ACh weakens the inhibi-
tory suppressive action, thus "releasing" excitation. What is
even more significant from the point of view of V&R's target
Cellular mechanisms of cholinergic arousal article is that a similar "release" of population spikes is
provoked by medial septal stimulation (Ropert et al. 1980).
K. Krnjevic Thus, through two quite different mechanisms, one a
Departments of Physiology & Anaesthesia Research. McGill University,
postsynaptic facilitatory influence and the second a presynap-
Montreal. P.O., Canada H3G 1Y6
tic disinhibition, ACh enhances hippocampal responsiveness
to various inputs, presumably carried by more conventional
It is notoriously difficult to interpret "brain waves" (EEG synapses. Although evidence of a disinhibitory action of ACh
activity). When an attempt is made to correlate certain types in the neocortex is less compelling, it is likely that both kinds
of wave activity with specific aspects of behavior - another of cholinergic facilitatory mechanisms also take part in
"phenomenon" that does not readily lend itself to precise, neocortical arousal (Steriade 1978).
objective analysis - the complications are further com- There is increasing evidence that some other agents can
pounded. One can therefore only wonder at the fearlessness increase the responsiveness of certain (more peripheral)
of authors who are willing to expose themselves to the neurons by diminishing K currents: For example, peptides
inevitable accusation of gross oversimplification. And yet this such as substance P (Krnjevic 1977) and luteinizing hormone
kind of approach is important, because even if only tentative releasing hormone (Adams & Brown 1980), as well as mon-
conclusions may be justified at the present stage, they should amines such as noradrenaline and 5HT (VanderMaelen /
point increasingly toward more vigorous tests and better Aghajanian 1980); while another peptide, the opioid enke-
hypotheses. phalin, may also facilitate pyramidal firing in the hippocam-
484 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
pus by causing disinhibition (Zieglgansberger et al. 1979). It is motor activity and degree of change in recorded brain
therefore conceivable that the atropine-resistant phenomena activity, which was studied earlier in Vanderwolf s laboratory
described by the authors are mediated by some of these other (Whishaw & Vanderwolf 1973) with positive results. Other
modulators. studies suggesting such correlations include EEG, MUA, and
unit recording (Arnolds, Lopes da Silva, Aitink & Kamp
1979a, 1979b, 1979c; Klemm 1971; Malmo & Malmo, 1977;
Schwartzbaum, 1975; Siegel, McGinty & Breedlove, 1977).
Needed: More data on the reticular The reliance of V&R on visual inspection of analog records is
information conducive to dichotomizing. Although they cite Arnolds et al.
(1979a, 1979b, 1979c) in defending eye-balling of EEG
Robert B. Malmo and Helen P. Malmo records, Arnolds et al. themselves are extremely critical of
Department of Psychiatry, Neuropsychology Laboratory, McGill University, nonquantitative EEG analyzing and dichotomizing (Arnolds
Montreal, P.O.. Canada H3A 1A 1 et al. 1979a, p. 565). Black (1975) draws the following
We have a strong conflict in commenting on Vanderwolf & conclusion (p. 154): "In general, the operational definitions of
Robinson's (V&R's) paper. We so admire Vanderwolf's 'voluntary' and 'automated' are not clear; it often seems as
pioneering work on moment-to-moment covariations be- though 'voluntary' and 'automated' are defined post-hoc by
tween bodily movements and hippocampal EEGs that we the correlation of hippocampal EEG patterns with a
would like to praise that tremendous contribution and end the response. " V&R themselves say, "Unfortunately, there are, as
commentary there. Behavioral monitoring of brain recording yet, no clear behavioral criteria by which Type 1 or Type 2
is no longer an option, because failure to monitor is failure to behavior can be classified." Yet, throughout the article, they
acquire indispensable data, and possibly results in erroneous write as though these behavioral criteria had in fact been
conclusions. established. For example, in the paragraph preceding the
Data on the reticular formation. There is a curious inconsis- above quotation, V&R stated that Vertes (1979) demonstrated
tency between the choice of reticulo-cortical relations as their "the existence of pontine neurons which fire in relation to
central theme, and the relative neglect of the reticular forma- Type 1 behavior. . ." Actually, Vertes clearly states that the
tion (RF) in the research program of Vanderwolf and his firing of those cells (although concomitant with theta EEG)
co-workers. They mention no RF recording from their labora- was not specific to the kind of movement.
tory, citing only one RF stimulation experiment from it, and EEG recording too limited. Teitelbaum (1976) criticized
they refer to only one RF recording study (Vertes 1979). They the hippocampal EEG electrode placements used by V&R,
hardly mention any studies using multiple-unit (MUA) and it does appear that their program would gain greatly in
recording from the RF in freely moving rats with behavioral flexibility if they were to use some other brain recording
monitoring (Klemm 1971; Malmo & Malmo 1977; Schwartz- techniques, such as unit recording or MUA. This would meet
baum 1975). Figure 1 shows a typical covariation of RF MUA Teitelbaum's objection that the atropine-resistant phenomena
with head movement and body movement in a rat. may be artifacts of their electrode placement.
Dichotomy. It seems to us that in their commitment to the In some unpublished experiments we (Malmo & Mundl)
Type 1-Type 2 dichotomy V&R have paid insufficient atten- have stimulated the RF core serially with a descending
tion to the problem of relations between extent of skeletal- electrode while recording mean arterial pressure, heart rate,
and respiration and found circumscribed facilitatory areas
and depressive areas in the same vertical line of descent.
Therefore, the possibility exists for functionally identifying
two areas with reciprocal autonomic functions and then
recording from these two areas during atropinization, in
order to determine whether only one area was atropine
resistant. It appears that some kind of direct attack on the
problem like this is needed prior to claiming a new synthesis
involving the reticular formation, and one based on a
cholinergic-adrenergic differentiation.
Importance of recording head movement. Even when
sensitive body movement recording drops to baseline, head
movement often continues, and the covariation of head
movement and MUA (which we have found to be highly
significant) cannot be observed satisfactorily without a sepa-
rate recorder for head movement (Mundl & Malmo 1979).
The importance of observing head movements during record-
ing of brain activity has been stressed by Malmo & Malmo
(1977), by Siegel et al. (1977), by Teitelbaum, McFarland, &
Mattson (1977), and by Vertes (1979).
Quantitive neuropsychology. As O'Keefe and Nadel (1979)
say in agreeing with Bures, there comes a time when a
neuroethological approach should be followed by a "quantita-
Figure 1 (Malmo & Malmo). Decline in reticular formation tive neurophyschological approach." Correlation between
multiple unit activity (MUA) when rat stopped walking and brain activity and behavior is higher for midbrain than for
stood still. Top three lines: photographic recording of reticu- forebrain (Malmo & Malmo 1977). Where along this
lar formation MUA (from oscilloscope); fourth line: inte- continuum does the hippocampus fall?
grated MUA; fifth line: head movement; bottom line: body In RF MUA, what are the exceptions to positive covariance
movement. Arrows indicate same moment in time. Horizon- between low RF MUA and quiescence? There are situations
tal line: 2 sec for film and 5 sec for other 3 traces. Vertical in which immobility is associated with a high level of RF
line: 200 jiV calibration for film. (From Malmo and Malmo MUA. One such situation is rising RF MUA preceding move-
1977, with permission of Elsevier/North-Holland Biomedi- ment (see Figure 1 for an example). Moreover, we have
cal-Press). designed appetitive situations that consistently produce high
THE BEHAVIORAL AND BRAIN SCIENCES (1981). 4 485
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
RF MUA in quiescent animals. What is the role of the RF phasic motor activity.
here? What are the species differences in correlations The second arousal system, which is not affected by atro-
between brain activity and behavior? To what extent can one pine, is involved only if voluntary behaviors involving move-
generalize from decorticate rats to decorticate primates? ments such as locomotion and head turning (Type 1 behav-
What are the implications of the findings of Halgren, Rabb & iors) are being performed or if muscular twitches are occur-
Crandall (1978) indicating that hippocampal RSA may be ring during episodes of active (REM) sleep. The effects of this
lacking in humans? We wonder why these questions were not system can be abolished by reserpine and are restored by the
considered by V&R. administration of /3-phenylethylamine but not by L-dopa.
Arousal theory. In human psychophysiology there is a Further, since the effects of /3-phenylethylamine are not
substantial amount of work on relations between quality (or abolished by dopamine or norepinephrine antagonists, it is
efficiency) of performance and accompanying physiological proposed that the /8-phenylethylamine interacts with an
activities (including quantified EEG). Some of this work has unknown type of monoamine receptor. The endogenous
involved the study of human brain-behavior relations with ligand for this receptor is unlikely to be j3-phenylethylamine,
the same kind of close behavioral monitoring as that of which is not depleted by reserpine, and may be another trace
Vanderwolf and his colleagues in their animal studies. The amine.
behavior of the human subject was continuously monitored, Experiments on acetylcholine (ACh) release have yielded
and EMGs (electromyograms) were recorded in addition (see some interesting insights into the nature of the cholinergic
Malmo & Belanger 1967, pp. 309-10). The interest was in pathways to the cerebral cortex. ACh release occurs widely
recording tonic background activities (invisible without from both cerebral hemispheres following unilateral stimula-
instruments) in their relation to quality of performance. It tion and is not restricted to the appropriate primary receiving
was in this experimental context that EMG gradients were area of the cortex for a specific modality of stimulation
discovered (Malmo 1965). We believe that these psychophy- (Phillis 1968). Experiments in which forelimb nerves were
siological experiments went well beyond the point of trying to excited with graded stimute which evoked afferent volleys in
infer a psychological process. In fact, Goodman (personal Group I, Groups I and II, Groups I, II and III, and Groups I,
communication) was following up this line of research when II, III and IV nerve fibers clearly demonstrated that ACh
he recorded directly from the reticular formation of monkeys release is enhanced only by volleys in Group III and IV fibers
during reaction time experiments. In multiple-unit recording (Mullin & Phillis, 1975). Activity in Group III and IV fibers is
experiments, Goodman (1968) demonstrated the role of the also a prerequisite for EEG desynchronization (Pompeiano
reticular core of the brain stem in generating supportive tonic 1973), and like its effects on the EEG, such stimulation elicits
background neural activity for phasic acts. Goodman found a widespread increase in ACh release from both hemispheres.
that there was a level of RF neuronal activity that was ACh release is therefore clearly associated with cortical
optimal for reaction time performance. Above and below this arousal and the pathways involved must have an extensive
optimal level, performance was relatively poor. This work, distribution to most, if not all, cortical regions. Attempts to
which was generated by arousal theory, used the kind of define the cholinergic input with electrophysiological tech-
objective methodology that V&R recommend. Yet it was not niques have revealed the existence of a pathway from the
mentioned. Furthermore, recent reviews of arousal theory globus pallidus/magnocellular nucleus region of the basal
(Fowles 1980; Pribram & McGuinness 1975; and Thayer forebrain to the sensorimotor cortex (Edstrom & Phillis 1980).
1978) were ignored. Therefore, one can hardly regard the Another pathway may project through the septum (Phillis
V&R article as a comprehensive critique of arousal theory. 1974). There is also evidence for local intracortical choliner-
gic circuits (Phillis & York 1968).
It has been widely assumed that desynchronized electrocor-
ticogram reflects excitation of the underlying cortical
Acetylcholine, amines, peptides, and neurons. When applied iontophoretically, ACh inhibits many
of the neurons in the more superficial layers of the cerebral
cortical arousal. cortex and excites only those in the deeper layers, including
the output, corticospinal neurons (Phillis 1974). EEG
J.W. Phillis desynchronization may therefore result from inhibition as
Department of Physiology, College of Medicine, University of Saskatche- well as excitation of cortical neurons. This combination of
wan, Saskatoon, Saskatchewan Canada S7N OWO inhibition and excitation may serve to enhance the respon-
In their fascinating exploration of contemporary opinion siveness of the output neurons to incoming thalamocortical
regarding reticulo-cortical activity and behavior, Vander- volleys. Activity in intracortical circuits which normally regu-
wolf & Robinson (V&R) expose a number of deficiencies in late the firing of the output neurons would be suppressed,
the classical interpretation of arousal phenomena and substi- thus facilitating the action of other inputs.
tute an audacious new proposal. Their paper addresses itself Inhibition may be a prominent factor in the EEG desyn-
to some of the paradoxes which have plagued investigators, chronization elicited by stimulation of ascending catechol-
such as how can a decorticate animal exhibit a wide range of aminergic pathways to the cerebral cortex. When applied
relatively normal behaviors, and why does an atropinized iontophoretically bo*h norepinephrine and dopamine inhibit
animal display awake behavior simultaneously with a sleep- the spontaneous firing of cerebral cortical neurons.
type electroencephalogram? To account for these discrepan- /?-phenylethylamine and a number of other trace amines also
cies, the authors propose that there are two pharmacologically have a depressant action on the spontaneous firing of cortical
distinct inputs from the reticular activating system to the neurons (Henwood et al. 1979) and this action may account
cerebral cortex and hippocampus. for the recovery of low voltage fast activity in the electrocorti-
One of these inputs, the traditional cholinergic arousal cogram of reserpinized animals given /3-phenylethylamine.
system, may be responsible for the performance of normal A number of polypeptides present in the cerebral cortex
responses to environmental cues involving both learned and have pronounced depressive or excitatory actions on cerebral
spontaneous behavior. Responses in this category would cortical neurons; these included motilin, substance P, luteiniz-
include behavioral immobility or simple reflexive or consum- ing hormone releasing hormone, vasoactive intestinal peptide,
matory behaviors (classified as Type 2 behavior). The cholin- somatostatin, cholecystokinin, thyrotropin releasing hormone,
ergic cortical arousal response can readily be elicited by and the enkephalins (Phillis & Kirkpatrick 1980). Little is
sensory stimulation in the absence of any accompanying presently known about the putative peptidergic pathways in
486 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary/Vanderwotf & Robinson: Reticulo-cortical activity and behavior
the cerebral cortex, but given their presence and the demon- specific motor relations in the reticular formation. V&R point
strable potency of these peptides, it is likely that they function out many errors in arousal theory. But these more general
as neurotransmitters or neuromodulators in the brain. problems made it unlikely that the theory could be patched
Evidence is emerging that peptides are responsible for some up. It had to be replaced.
of the late slow synaptic potentials in sympathetic ganglia and 2. The Vanderwolf & Robinson theory. V&R's theory recog-
this may be the role that they fulfil in the brain. It seems nizes many behavioral states, but puts them into two major
inevitable that peptidergic neurons will receive more atten- categories in the awake state. This is surely an oversimplifica-
tion in future analyses of the neural pathways involved in tion. However, an important advance is that V&R do not
EEG arousal. [See also Dismukes: "New Concepts of Molecu- introduce a one-dimensional spectrum (arousal) to span across
lar Communication Among Neurons" BBS 2 (3) 1979.] all awake states. Dividing awake states into two categories
naturally invites other categorizations within these states or
cutting across them. Since hippocampal theta rhythm has not
been unequivocally recorded in primates, a major difficulty
with V&R's theory at present is that it is not applicable to
An obituary for old arousal theory primates. In addition, hippocampal theta rhythm has also not
James B. Ranck, Jr.
been clearly seen in nonmammals, so that this limits its
applicability as well. We do not yet know the mechanisms of
Department of Physiology, Downstate Medical Center, State University of
New York, Brooklyn, N. Y. 11203
the hippocampal theta rhythm or of neocortical low voltage
fast or rhythmic waves. Indeed the V&R theory only makes
Over thirty years ago the newly formulated theory of the correlations with slow waves and drug changes and behavior
unitary relation of arousal to neocortical low voltage fast and does not get much involved in mechanisms at all. If the
activity and the midbrain reticular system was a major mechanisms were known, perhaps the theory could be
advance. For the last ten years this theory has been quietly extended to nonmammals and primates.
dying. Its slow death has not been generally noted because the Relatively nonmotor behaviors such as those they call Type
theory does deal with important issues and, however inade- 2 are not well defined by V&R. This is partly because of
quate, there has been nothing to substitute for this old arousal V&R's general approach, as discussed in the next section.
theory. It has survived by default. As in politics, you can't However, these behaviors could be more sharply defined, and
beat something with nothing. Over the last few years, and should be, before the theory can be adequately tested.
especially in this target paper, Vanderwolf & Robinson For rats, which are the only nonhuman animals that I know
(V&R) have given us a substitute theory. Now armed with this well, the V&R theory deals with much of the current data,
substitute, even with its problems, I for one, feel the old and can potentially be expanded to deal with more. Most of
arousal theory has finally died. I will therefore write about it the data V&R use to develop the theory is from rats; I would
in the past tense. I will first drive some nails in the coffin and like to see work on nonrats to test the theory fully in other
discuss what was wrong with the theory, beyond what V&R animals. I think the theory is the best we have now, so it
say. I will then discuss V&R's theory, i.e., as in any obituary I would be worth doing this work on other animals.
will talk about the relatives which survive the deceased. 3. The nature of the V&R approach. Whatever may come of
Thirdly, I will discuss their general approach, which is in their theory, V&R have produced major advance by their
many ways more important than this specific theory. approach. In Vanderwolf's landmark 1969 paper on rat
1. What was wrong with the old arousal theory. It is hippocampal theta rhythm, where the approach is perhaps
certainly the case that there are such things as behavioral first used fully, the rat behavior is described in a variety of
states, i.e., general characteristics of the animal beyond situations. The key is that what is described is what the rat is
specific stimuli or motor acts. An animal is more than just the actually doing in detail, including very small movements.
sum of all stimuli impinging on him and his responses to This is in contrast to some descriptions of animal behavior
them. Arousal, or something like that, is one of the important which only describe a few behavioral facts in a particular task
behavioral states. Thirty years ago in many circles it was not which the describer assumes are sufficient to demonstrate
considered proper to talk about things like behavioral states. that some internal process is occurring. Arousal was usually
However, since arousal seemed to have an electroencephalo- described this way. In most published reports on arousal it
graphic and anatomic correlate it gained a respectability it was difficult to find out much about what the animal was
would not have otherwise had. actually doing or not doing. We were only told that the
In the last thirty years we have changed our standards of animal was at a certain level of arousal. I, for one, often had
how we can properly talk about behavior and we have trouble understanding these descriptions. I did not find that
learned more about behavior and about the nervous system. my estimates of a level of arousal were the same as those of
This theory has not kept up with these changes. I think the other observers. I could never be sure I was using the same
presumed relation of arousal to EEG and the reticular forma- criteria as someone else, because there were only neocortical
tion inhibited clear behavioral definitions of arousal. In any electroencephalographic criteria, no clear behavioral criteria.
case, the arousal was not adequately defined behaviorally. Vanderwolf and his many collegues have shown what can be
There are many behaviors for which I do not know the level done by actually describing behavior.
of arousal (e.g., in eating, REM (Rapid Eye Movement) sleep, There are difficulties with their approach, especially in
running a treadmill) and I do not know how to find out. I dealing with animals that are not very motoric, such as cats
cannot simply rely on EEG criteria. Arousal, or something and rabbits, or with largely immobile states such as attending
like it, deserves a clear behavioral definition independent of (which can in theory be defined behaviorally). In this target
neural correlates, otherwise the correlation cannot be tested. article V&R seem more willing than usual to try to discuss
Our knowledge of the nervous system also outgrew arousal some nonmotoric activities. This will be an important
theory. The reticular formation is now known to be an area advance if they continue to apply their careful descriptions of
mediating many specific functions, rather than being the what an animal really does to less active behaviors.
homogenous "activating center" of 1950. Single neuron stud- V&R also really look at both neocortical and hippocampal
ies of many parts of the midbrain and pontine reticular EEGs and describe what is there in simple terms ("measured
formation do not show a simple relation to arousal (even if with a clear plastic ruler") rather than describing some highly
one assumes it can be defined behaviorally). I think Siegel analyzed property of the EEG. Their approach to describing
(1979a) is close to being right in pointing out the strong the EEG is the same as their approach to describing behavior.
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 487
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
Any analysis throws some data away and makes inferences adequate, the more so since the waking immobility rhythms
about what are important properties to be retained and have nothing in common with spindles or slow waves. Even
examined. Their theory is the result of describing what is human EEG studies, performed with scalp electrodes and
really there in simple terms. I would like to see them do more hence with a very low spatial resolution, have long recognized
analysis along with their descriptions of raw data as they do that the occipital alpha rhythm and the central "mu" rhythm
with behavior. Their simple approach is sometimes down- (the latter probably corresponding to the above described
right primitive. However, a simple, primitive approach is an frontal rhythms, see Jasper & Penfield 1949; Gastaut et al.
essential first step. It has too often been the case with others 1967; Chatrian et al. 1959; Kuhlman 1978; Covello et al.
that the simple first step of observating EEG or behavior was 1975) correlate well with situations that are quite distinct
ignored in order to get on with fancier analysis, and what from sleep. Nobody would seriously identify these alpha or
turned out to be the most important observations were lost. mu "synchronized" activities with sleep patterns!
The V&R approach is a good basis on which to build. More generally, our working hypothesis (based on the
above-mentioned facts, as well as others) is that a number (not
yet completely determined) of thalamocortical sectors may
give rise to synchronized activities of limited extent over the
neocortex (let us call them "modular rhythms"). This devel-
Significance of localized rhythmic activities opment takes place in connection with certain behavioral
occurring during the waking state conditions, especially states of selective attention (to either
visual or somatic stimuli). Due to their spatial restrictions,
A. Rougeul, J.J. Bouyer, and P. Buser these activities may be completely overlooked in a "standard"
Laboratory of Comparative Neurophysiology, University Pierre el Marie
ECoG recording when no special care is taken regarding
Curie, Paris 75005. France localization. Therefore, the development of "slow-activities '
(i.e., non-LVFA in V&R's classification) is by no means
Vanderwolf & Robinson's (V&R's) target article contains paradoxical; it is just that if enough electrodes were placed on
some interesting hypotheses regarding hippocampal activity the neocortex, we could presumably observe a considerable
in relation to behavior. It is possible that this conceptual number of different conditions under which one focal rhythm
model, with two distinct systems for theta activity, one or the other would develop, depending on the organism's
resistant to atropine, the other nonatropine resistant, may one prevailing cognitive or conative state. It is only in the extreme
day contribute to solving the riddle of correlations between states of alertness, with or without movement (emotional
hippocampal activities and behavioral states, a problem that situations, fight or flight) that the whole neocortex would
has generated so many contradictions in the past decades. We eventually display generalized LVFA.
shall not consider this specific aspect of the paper, which goes In sum, it is our feeling that if electrocortical correlates of
beyond our own domain of competence. waking behavior are to be studied, "holistic" electrocortical
On the other hand, we shall briefly comment on points on exploration has to be replaced by more refined and topo-
which we unfortunately disagree. One of V&R's theses is that graphically well-defined investigations. If sleep studies have
neocortical slow wave activity is not well correlated with been so successful, it is in part precisely because in this case a
"arousal levels or conciousness." This assumption is based on limited number of electrodes may suffice, the whole neocor-
what we think is an oversimplistic view that neocortex tex being in more or less the same electrophysiological state.
displays either desynchronized activity (their "LVFA") or Our knowledge of the states of waking, with constant atten-
what they call "slow waves." Their paragraph 2a, where they tional shifts, qualitative (selective) and quantitative (levels of
state that "a large number of investigators have shown that alertness), is far from complete; we are evidently dealing with
slow waves and spindles frequently occur in neocortex during subtle and sometimes very short-term processes. We believe
various kinds of waking behaviors " is erroneous. Semantical- that no clear correlation with ECoG will be established unless
ly, and physiologically, the terminology is unsound. The these focal rhythmic systems are better known, and only if
rhythmic neocortical activities that were described during "recording electrodes [are] placed correctly in the generator
waking by several groups, including our own (Roth et al. zone." (A quotation from V&R, but unfortunately only with
1967; Rougeul-Buser et al. 1975, 1978, Bouyer et al. 1981) are reference to the hippocampus!).
completely distinct from slow sleep activities. The latter With these considerations in mind, we must indicate our
consist, as is well known, of two patterns, delta slow waves disagreement with this part of V&R's "critique of the arousal
(2-3 c/sec) and spindles. On the other hand, at least three sets theory."
of rhythms could be recorded over the anterior cortex during
immobility of the animal (cat or monkey), none of those
displaying the least similarity with sleep patterns. One set of
rhythms (36 c/sec) occurs when the animal is in a high state Neocortical activation and adaptive
of vigilance (e.g., immobile and watching prey). These
rhythms develop over two foci of very restricted size, one behavior: Cholinergic influences
over the pericruciate cortex, the other over the anterior
P. Shiromani and William Fishbein
suprasylvian cortex and only there, the rest of the neocortex
remaining in the LVFA state. Psychobiology Laboratory, Department of Psychology, City College of the
City University of New York, New York, N. Y. 10031
Another set of slightly slower (14 c/sec) rhythms appears
during a state (common in the cat) of "quiet wakefulness," Over the years, we have followed with a great deal of
with similar localization. Finally, when the animal becomes enthusiasm the efforts of Vanderwolf & Robinson (V & R)
drowsy, rhythms of a slower frequency (6 c/sec) occur with a toward understanding the role of the ascending reticular
slightly more extensive distribution. Such rhythms all differ system in behavior. We believe that their work has crucial
from the sleep patterns; moreover, they can only be observed importance because it shows how purposive and reflexive
if the electrodes are placed at the right places on the cortex. If forms of behavior correlate with activity in the hippocampus,
the electrodes are implanted elsewhere, especially middle neocortex, and the reticular system. We have some new data,
suprasylvian cortex, or marginal gyrus, which are the most however, which raise some questions about their conclusion
popular sites for ECoG (electrocorticogram) recording, that the "ascending reticulo-cortical pathways are not
LVFA occurs, at least during the states of attentive behavior. directly involved in the basic phenomena of sleep, arousal,
Therefore, the concept of "paradoxical alertness" is not and waking."
488 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary/VanderwoN & Robinson: Reticulo-cortical activity and behavior
All the amassed evidence suggests that cholinergic mecha- Vanderwolf & Robinson (V & R) present an admirably
nisms are implicated in neocortical activation. However, argued case for abandoning the accepted notion that the
research on the cholinergic system has been handicapped, degree of desynchonization (or LVFA) of the electrocortico-
from the outset, primarily because of the inability to localize graphic activity of the neocortex is a reliable measure of the
and map cholinergic pathways in the CNS. This research may level of alertness. They also succeed in rendering plausible
be gaining momentum as a result of recent advances in their own concept of a duality of the LVFA state, controlled
detection of cholinergic receptor sites. For example, Lewis et by two different systems, and subserving different functions.
al. (1980), using a new autoradiographic technique, have In the light of the report by Adametz (1959) one must,
localized muscarinic receptor sites in the rat brainstem. One however, question whether either or both of the LVFA states
area that was found to contain a high concentration of defined by V & R is dependent on input from the reticular
autoradiographic grains was the pontine reticular formation. formation to the forebrain. The two-stage lesion of the
This finding fits nicely with our own data (Shiromani & midbrain reticular formation, described by Adametz (1959),
Fishbein 1980). In our experiments, with rats, carbachol or presents a gold mine of opportunity for neuropsychological
scopolamine is infused, via an Alzet mini-pump, into two experimentation. Hippocampal and neocortical ECoG (elec-
pontine nuclei: Reticularis Pontis Caudalis and the Giganto- trocorticogram) recording, behavioral experiments, and drug
cellular Tegmental Field (FTG). The results show that carba- studies on such lesioned subjects suggest themselves in such
chol augments active (paradoxical) sleep, i.e., hippocampal variety that it would exceed the limits of this commentary to
RSA and neocortical LVFA, for at least five days while detail them.
scopolamine decreases it. The augmentation is due primarily There is another quite different point of V & R's paper
to an increase in the active sleep cycle frequency and not worth discussing. One must wonder whether there are truly
because of an increase in its duration. Further, the augmenta- sufficient reasons to abandon the concept that synchronized
tion due to carbachol is seen mainly during the night cycle, rhythmic activity in the 0.5-15 cps frequency range repre-
when the animals are normally awake, while scopolamine sents an "idling rhythm" of unengaged neuronal populations.
acts mainly during the day cycle, when the animals are Two considerations are relevant here, one a point of fact, the
normally asleep. other of theory. The slow-wave activity of neocortex observed
Our findings, taken together with other cholinergic stimu- under the influence of atropine in apparently awake animals
lation data (Baxter 1969; George et al. 1964; Hobson & is rather different from the sleep spindles and delta waves
McCarley 1977), as well as electrophysiological results from seen in natural sleep, under general anesthesia, and in the
pontine nuclei (Vertes 1979), would seem to suggest that the coma caused by bilateral midbrain lesion. Furthermore, Elul
eholinergic component of the ascending reticular pathway, (1972) has presented theoretical evidence that, in order to
probably originating in the pontine reticular formation, is the produce electrical waves detectable by surface electrodes, it is
trigger responsible for hippocampal theta and cortical activa- sufficient for a fraction of the available neuronal pool to be
tion during both waking and active sleep. engaged in synchronous activity; the remainder could be
Might the cholinergic pathways be involved in adaptive desynchronized and remain undetected in conventional EEG
behavior? We agree with V & R that the evidence so far or ECoG recordings. This realization may be the key to the
suggests that alterations of central cholinergic function apparent dissociations of behavior and ECoG. Atropinized
improve or impair learned and spontaneous behavior. animals presumably do show neuropsychological deficits. The
However, we must point out that these results come from behaviors that seem relatively intact may be performed by
experiments in which the acetylcholine modulating agents neuron populations which discharge in "desynchronized"
patterns; the deficiencies in performance may be due to the
were administered peripherally. To our knowledge there is as "idling" of those cells which generate the large amplitude
yet no study demonstrating that injection of acetylcholine slow waves.
agonists/antagonists directly into the cholinergic reticular
system alters adaptive behavior, and most specifically
memory. Until this is known, we can only speculate about
how the activity in this pathway determines the rate of firing
of hippocampal theta cells (Ranck 1975) and association
cortical interneurons (Steriade 1978), which in turn may
influence the durability of the memory trace. EEG desynchronization is associated with
cellular events that are prerequisites for
active behavioral states
Reticular formation, brain waves, and coma
M. Steriade
George G. Somjen Laboratory of Neurophysiology, Department of Physiology, Faculty of
Duke University Medical Center, Department of Physiology, Durham, N. C. Medicine, Laval University, Quebec, Canada GIK 7P4
27710
Many concepts have had a rough time before being accepted.
In 1959 Aclametz reported a very basic and important discov- Tribulations have often been due either to the absence of
ery. He found that if the midbrain reticular formation of a cat appropriate tools for testing a hypothesis when it is formu-
is destroyed, not at once, but in two or more stages one to lated or to misinterpretations of the original idea. Both of
three weeks apart, the animal does not go into coma after the these conditions have attended Moruzzi & Magoun's (1949)
operation and its sleep-wake rhythm is not disturbed. The pioneering work. The concept of generalized reticular activa-
finding was confirmed by Chow & Randall (1964), and was tion was taken by a thirsty audience to imply ubiquitous
then peculiarly ignored. Two quite fundamental and so far ascending projections of (unknown) reticular origin, acting in
unanswered questions are raised by this observation. First, a diffuse excitatory way on (all) postsynaptic targets. The
what is the real reason that bilateral acute destruction of the reticular formation was viewed at the time as an undifferen-
midbrain reticular formation invariably causes coma lasting tiated structure. Its neuronal networks were left ill defined
at least three to four weeks? And, equally important, why because they were not thought to be amenable to morphologi-
does the same lesion inflicted in two stages not have the same cal and electrophysiological analyses. This caused temporary
effect? Comparing the condition to spinal shock, or calling it neglect of the concept of a reticular activating system. The
"diaschisis" may amplify the question, but does not answer revived interest in the reticular formation during the last
it. decade is due to the advent of morphological methods which
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 489
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
circumvent the problem of passing fibers and to the develop- to the exclusion of others and to perform the motor patterns
ment of electrophysiological techniques which allow one to of one definite instinct" (Dell 1958, p. 198). If changes in
record the activity of identified neurons in behaving animals. ascending reticular influences are hypothesized to prepare
Another source of confusion has been that, after Moruzzi & conditions for the occurrence of genuine behavioral compo-
Magoun's 1949 paper, the descriptive term "EEG desyn- nents in wake-sleep states, it would be worth studying the
chronization ' and the behavioral notion of arousal were cellular correlates of EEG desynchronization and synchroni-
sometimes used interchangeably by physiologists working on zation processes to determine (a) whether or not neuronal
the thalamus and neocortex of acutely prepared animals. This events in thalamocortical systems adequately account for
was obviously an unjustified leap-one made by many of us. what we expect from an activated or deactivated state, and
Moruzzi & Magoun (1949) reported experiments performed (b) what are the most likely brainstem neuronal candidates
under conditions in which no behavioral observation was endowed with tonic ascending activating influences (reticular
possible; they described the reticularly elicited activation of in the classical sense or the more recently discovered
the EEG, and, aware of T. H. Huxley's dictum that those who monoaminergic systems?) It is clear that point (a) takes for
decide not to go beyond facts rarely get far, they advanced granted that the activity in the thalamus and neocortex is
their theoretical concept. But nowhere in that paper was EEG relevant to complex phenomena of waking and sleep. I
desynchronization equated with waking or EEG synchroniza- understand that decorticate rats may display head move-
tion with sleep. As a rule, the difference in level between ments, walking, and rearing, but I assume that waking
electrical activity and behavior has been cautiously stressed processes transcend this repertoire.
by Moruzzi in subsequent publications: "The main idea is to If the reticular activating concept were valid, one would
make a sharp conceptual distinction between EEG synchroni- expect improved receipt of messages and performance of
zation and sleep, ' and "Clearly EEG synchronization reveals commands either with artificial stimulation of the rostral
. . . a process that must reach a critical level to give origin . . . reticular core or during naturally occurring active behavioral
to genuine manifestations of sleep ' (Moruzzi 1972, p. 136). states. What are the correlates of adaptive behavior at a
The critique of the arousal theory in the target article is cellular level? Basically, synaptic transmission in thalamic
based on some data leading to the claim that the desynchron- nuclei and cortical areas should be facilitated, output
ized and synchronized patterns of EEG rhythms are not well elements (such as pyramidal tract cells) should be in a state of
correlated with "arousal" and "sleep." The first and most adequate depolarization for a quick response, and inhibitory
striking example chosen is the EEG desynchronization that mechanisms should act efficiently to provide accurate
occurs during a behavioral state (active sleep) associated with discrimination combined with rapid recovery. All these
a sleeping posture and relative unreactivity to stimuli. events have been demonstrated to characterize identified
Towards the end of their paper, however, Vanderwolf & thalamocortical and corticofugal neurons during reticularly
Robinson (V & R) reach the reasonable conclusion that, in induced EEG desynchronization, natural waking, as well as
fact, "brain activity and patterns of motor output during active sleep.
active sleep are quite similar to the patterns that occur during Figure 1A shows the increased rates of spontaneous
waking." In other words, active sleep is not a depressed or discharge and the enhanced responsiveness in a corticothal-
extreme type of slow-wave sleep, but a true yet paralyzed amic cell during waking and active sleep. This is now a
arousal (Jouvet 1972), a state with a high degree of reticular general finding for long-axon neurons in various thalamic
activation (Moruzzi 1972). These views have been tested in nuclei and for corticospinal, corticopontine, and corticothal-
my laboratory and demonstrated to be valid at a cellular amic cells recorded from motor, somatosensory, and associa-
level, as discussed later in this commentary. So, there is no tion areas (see review by Steriade 1981). Also, the time-course
surprise that EEG desynchronization accompanies active of inhibition varies with changes in the state of vigilance. A
(dreaming) sleep, a state to which the qualification of "abject powerful inhibition of cellular responsiveness follows the
mental annihilation" in Eccles's opening remarks to the initial excitation. The duration of the inhibitory period is
symposium on The Nature of Sleep (1961) certainly does not shorter and cyclic inhibitory-rebound sequences are blocked
apply. Along with the same class of EEG-behavioral dissocia- during midbrain reticular stimulation and natural waking, in
tions it is not fair to cite the case of desynchronized EEG both thalamic and cortical long-axon neurons (Fig. IB). It
rhythms of the midpontine pretrigeminal cat in the context of follows that the whole sequence of events triggered by a
deep coma following brainstem injury (V & R's point lc). The stimulus is telescoped during EEG desynchronization (Fig.
transected animals were carefully tested in the studies of the 1C). The fact that inhibition is very sharp in the waking state
Pisa group and "a genuine state of alertness was present when and recovers much faster than in slow-wave sleep provides a
the EEG was desynchronized" (details in Moruzzi 1972, p. mechanism subserving accurate control of thalamic and corti-
27), including the possibility of elaborating conditioning and cal performances, and allows neurons to follow rapidly recur-
differentiation in chronic midpontine pretrigeminal prepara- ring activity (Steriade & Deschenes 1974).
tions (see Zernicki's findings in his review, 1968). Some of the above cellular correlates of activated states,
Is the ascending reticular activation hypothesis invalidated namely the increased firing rate and enhanced somatic
by recent developments? If we discard the unjustified EEG- responsiveness, can be detected in intralaminar thalamocorti-
behavioral extrapolations mentioned above, we find that the cal neurons some 10 seconds before EEG desynchronization
evolution of research has not only confirmed the data of in natural transitions from slow-wave sleep to waking or
Moruzzi & Magoun's paper (1949), but it has fully justified its active sleep (Steriade & Glenn, in preparation). Conversely,
major theoretical proposal: "The presence of a steady back- in the transition from waking to slow-wave sleep, long inhibi-
ground of . . . activity within this cephalically directed brain- tory periods can be seen during drowsiness in precentral
stem system . . . may be an important factor contributing to neurons of the behaving monkey, prior to overt EEG and
the maintenance of the waking state, and absence of such behavioral signs of slow-wave sleep (Steriade et al. 1974).
activity in it may predispose to sleep" (p. 470, italics mine). Long inhibitory pauses provide a mechanism for closing
High levels of reticular activity in rostrally projecting sensory channels. Testing the excitability of lateral thalamic
elements should not be viewed as responsible for all behav- nuclei during the sleep-waking cycle reveals that monosynap-
ioral components of arousal, but may create "the conditions tic responses to afferent volleys are inhibited as of the first
for the organism to be actively interested in the outside sign of drowsiness (Steriade et al. 1969). Such deafferentation
world, . . . the readiness to receive only some kinds of stimuli in thalamocortical systems is a necessary prelude to attaining
490 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary/Vanderwotf & Robinson: Reticulo-cortical activity and behavior
as hypotheses concerning the role of serotoninergic, dopamin-
ergic, or noradrenergic systems in mediating different
components of arousal and waking behavior. Restricting the
discussion to tonic activation processes, several aspects should
be borne in mind:
(a) Electrical stimulation of the raphe system and locus
coeruleus complex, as well as iontophoretic application of
2ms
5QJ U8/S 13.1/S 30.1/s 5-hydroxytryptamine and norepinephrine, induce cellular
phenomena opposite to those observed in forebrain activation
w s D processes and described above. The effects consist of hyper-
B polarization and decreased spontaneous firing in neo- and
allocortical neurons; identified corticospinal neurons are
inhibited by locus coeruleus stimulation, an effect opposite to
that of cholinergic excitation of these elements and to wake-
fulness (Phillis & Kostopoulos 1977; Stone et al. 1975).
(b) The other difficulty in considering monoaminergic
rr • systems responsible for tonic ascending activation phenomena
is the following: Long-axon thalamic and cortical neurons
SYNCHRONIZED display essentially the same activity during both wakefulness
and desynchronized sleep (see above), while raphe and coeru-
leus neurons have opposite behaviors during these two states:
highest discharge rates in waking and lowest in active sleep
(McGinty 1973; Hobson et al. 1975).
(c) Finally, the failure to induce any change in EEG
desynchronization following bilateral destruction of the locus
coeruleus complex with 85-95% depletion of cortical norepi-
nephrine, together with other experimental data, lead to the
conclusion that monoaminergic neurons do not mediate tonic
EEG activation (data also cited in V & R's target article).
Figure 1 (Steriade). Somatic responsiveness and inhibitory While the effects of locus coeruleus stimulation are not
events in thalamic and cortical neurons during states asso- consistent with what is expected from a presumed tonic
ciated with EEG desynchronization and synchronization. A: activating system, their involvement in specific components
A cell in parietal association area 5 of chroncally implanted of activated states should not be discounted. The most inter-
cat, antidromically invaded from the center median thalamic esting action might result in an increased signal-to-noise ratio
nucleus; the antidromic responsiveness was investigated with (Foote & Bloom 1979) or in switching emphasis from one set
a four-shock train at 225/s, with an intensity 30% above of inputs to another (see Dismukes 1979).
threshold allowing fluctuation in response probability; at that Returning to the classical hypothesis of a cephalically
intensity responsiveness increased from the first to successive directed influence arising in the midbrain reticular forma-
shocks in the train; graph depicts the percentage responsive- tion, we find good reasons to concentrate the search for
ness (ordinate) to the first, second, and fourth shock during neuronal candidates underlying tonic activation at this level.
waking (W), synchronized sleep (S), and desynchronized The midbrain reticular formation is characterized by well-
sleep (I)); the mean rate of spontaneous firing (per second) is defined ascending projections and direct routes for transfer-
also indicated. B, 1: Periods of suppressed firing (a, b, and c) ring reticular excitatory influences to cortically projecting
and postinhibitory rebounds in a lateralis intermedius tha- thalamic neurons. Midbrain reticular neurons with identified
lamic neuron following single-shock stimulation of cortical rostrally projecting axons exhibit tonic discharge features and
area 5 (arrow); and 2: the effect of a preceding train of higher firing rates during active behavioral states, compared
high-frequency pulses to the midbrain reticular core; note to slow-wave sleep. It is noteworthy that such neurons show a
reduced duration of the period of silenced firing and disap- statistically significant increase in discharge frequency 15
pearance of the c period. C: Simplified diagram to show the seconds before the end of slow-wave sleep epochs that
whole sequence of excitatory-inhibitory events in thalamus develop into awakening (data in collaboration with Oakson &
and cortex during EEG synchronization and desynchroniza- Ropert, in preparation). Although this analysis discloses a
tion; the changes during states characterized by EEG temporal but not necessarily a causal relation, it suggests that
desynchronization consist of: enhanced primary excitation midbrain reticular neurons play a leading role in the transi-
due to antidromic (or monosynaptic) volleys, diminution of tion from synchronized sleep to activated behavioral states. It
the secondary synaptic excitation (dotted part), sharp inhibi- is obvious that the properties of midbrain reticular neurons
tion but with reduced duration (black part) and blockade of are very unlike those of pontine reticular neurons that prefer-
rhythmic inhibitory-rebound sequences (modified from Ster- entially discharge during movements in waking and during
iade 1981). REM's in active sleep (cited in the target article). We tested
the ascending activating reticular influences for which the
the critical level at which genuine manifestations of sleep criterion of tonic activity is of major importance (Moruzzi
may occur. 1972) and, therefore, discarded periods with phasic motor
As to the question (b) concerning the neuronal aggregates events which may introduce uncontrolled factors into the
subserving activating influences on thalamocortical systems, evaluation of discharge due to the proprioceptive feedback
one should first note the difficulties in interpreting experi- generated by movement itself.
ments involving electrically stimulating and lesioning the From the midbrain reticular core, ascending axons contact
reticular formation (which may have affected passing fibers and excite neurons in medial (centrum medianum-parafas-
arising in nonreticular neuronal aggregates) and the discovery cicularis, CM-Pf; and ventralis medialis, VM) and intralami-
of extensively ramified monoaminergic systems; these have nar (centralis lateralis-paracentralis, CL-Pc) thalamic nuclei
together generated a challenge to the reticular concept as well with direct projections to neocortex (Glenn & Steriade, in
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 491
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
preparation). Inputs to VM are important as this nucleus is a cortex, but that the behavioral significance of the control
source of a contingent that terminates almost exclusively in processes changes from level to level. First, as evidence for
layer I of neocortex in both rat (Herkenham 1980) and cat this view, we can cite the observations that damage to
(Glenn, Hada, Roy, Deschenes & Steriade, in preparation), as ascending catecholamine systems (by 6-OHDA) produces an
expected for a thalamic nucleus transferring reticular influxes akinetic rat (without a circadian rhythm in its postural
and exerting modulatory influences over widespread cortical adjustments). Removal of the neocortex of such a rat produces
areas. The recent findings by Hyvarinen et al. (1980), show- an immediate release from the akinesia and results in hyper-
ing that the effect of attentive behavior is particularly activity; administration of atropine can produce a similar
pronounced in the elements recorded at the junction of effect in these rats (unpublished observations; Schallert,
cortical layers I and II of the monkey, fit well with the Whishaw, Ramirez & Teitelbaum 1978). Removal of both
evidence for an activating superficial thalamocortical projec- ascending and descending pathways together may thus mask
tion. [See also Lynch: "The Functional Organization of Poste- a possibly opposing contribution that each makes to behavior.
rior Parietal Association Cortex" BBS 3(4) 1980.] Second, decortication definitely does have an effect on sleep-
Let me conclude that there is good evidence that ascending waking behavior patterns and phasic motor patterns during
reticular projections provide an important source for poten- the awake state. We have observed that decorticate rats often
tiating thalamic and cortical processes that are prerequisites sleep out in the open when a sheltered spot is available, adopt
for behavioral components of activated states. Moruzzi & unusual postures during locomotion, swimming, eating, etc.,
Magoun's concept is alive and well. do not retain normal tongue or mouth movements, and are
hyperactive in many situations (Vanderwolf, Kolb & Cooley,
1978; Whishaw, Nonneman & Kolb 1980; Whishaw, Schallert
& Kolb, in press). From a Jacksonian perspective of functional
A ghost in a different guise. hierarchies, it is not the case that the "motor score" for
behavior patterns is subcortically represented and that the
R. J. Sutherland, I. Q. Whishaw and B. Kolb
cortex decides when to act, as V & R suggest, but rather that
Department of Psychology, The University of Lethbridge. Lethbridge, the cortical and subcortical mechanisms differ in terms of the
Alberta. Canada T1K3M4
nature of the resident control processes - the environmental
Hebb (1972), in his Textbook of Psychology, makes two triggers and guidances involved in the movements (Whishaw
points about theories of how the nervous system works. The et al. in press).
first is that these ideas are important in determining the kind In regard to the suggestion that the atropine-sensitive
and quality of research conducted; the second is that it is reticulo-cortical system is involved in "stimulus control" it
important that we must not actually believe that these ideas should be noted that this probably cannot be a general or
are correct. Vanderwolf & Robinson (V & R) have done a unitary involvement, although it is easy to find examples of
laudable job of indicating why we should not believe the loss of stimulus control after lesions that damage this system,
traditional account of the reticulo-cortical arousal system and, the following examples indicate the presence of enhanced
perhaps more importantly, how careful descriptions of behav- stimulus control over elicitation, guidance, and reinforcement
ior must play a crucial role in elucidating brain function. mechanisms: (1) the decorticate displays heightened startle
(They should also have mentioned that it is surprising that this responses to a variety of stimuli and exaggerated relaxation
view ever obtained prominence since Goltz - summarized in and activation by warm and cold water, respectively; (2)
Luciani, 1915 - in widely publicized experiments in 1892 had atropine-treated rats show enhanced guidance of locomotion
observed sleep-waking cycles and the various postures and and head movements by snout contact with surfaces (Schal-
phasic motor patterns of the waking state in decorticated lert, De Ryck & Teitelbaum 1980; Whishaw et al. in press,
dogs.) Despite our complete approval of these fundamental and (3); decorticate rats and rabbits show enhanced stimulus
aspects of V & R's paper, we feel the necessity of attending to control and cross-modality discrimination in Pavlovian condi-
Hebb's second point; that is, we would like to indicate why we tioning procedures and are capable of learning in instrumen-
do not believe the "new synthesis." tal conditioning procedures (Oakley 1979).
On a general level, their suggestions that the atropine- A final criticism concerns the notion that the deficits
sensitive reticulo-cortical system plays a role in "stimulus observed in decorticate animals (e.g., in feeding, food hoard-
control" or in making "normal responses to environmental ing, nest building, mating, etc.) indicate that the cortex (or
situations," that the atropine-resistant reticulocortical system some of its input) exhibits a general function. This is only
may play a role in "the control of Type 1 behavior," while the acceptable if these behavioral deficits cannot be doubly
cerebral cortex determines "the occasions on which particular dissociated (Teuber 1955) and associated with specific local-
motor patterns are to be displayed," are hopelessly vague. A ized cortical damage. It is now known, however, that many,
moment's reflection should convince anyone that we could and possibly all, of the deficits observed to follow decortica-
characterize each part of the nervous system, from the tion also follow damage to discrete cortical regions (Kolb &
receptor cells in the retina to the ventral horns of the spinal Whishaw 1978). For example, deficits in food hoarding,
cord, in such a fashion. In order to be helpful in generating feeding, and nest building are produced by frontal, but not
research, the new synthesis must be made more explicit, even posterior, cortex lesions in rats (Kolb & Whishaw 1978; in
though (given Hebb's dictum) we won't believe it. press). If there are actually no deficits after decortication that
More specifically, V & R claim that because sleep, arousal, indicate the loss of some general function (as we suspect),
then there certainly is no compelling reason to search for a
and waking behavior patterns occur in decorticate rats, the
neuroanatomical substrate for it. In fact, through the history
reticulo-cortical projections are not essential for these aspects of neuropsychology, this well-travelled general function has
of behavior. They may not be essential for an animal without migrated from the pineal with Descartes, to the cerebrum
a cortex, but they may be necessary for an animal that still has with Flourens, to the cerebral cortex with Lashley, to the
a cortex. So far, no one has ever observed an animal following right cerebral hemisphere with Semmes and others (Kolb &
removal of only the ascending reticulo-cortical projections; Whishaw, 1980), and now to the reticulo-cortical pathway
decortication removes these ascending systems, as well as the with V & R. We believe that this ghost will finally come to
descending cortical output. Following Hughlings Jackson, we rest only with continued careful behavioral studies concern-
believe that the control over these aspects of behavior is ing the specific functions of each particular neural system.
organized at each level of the nervous system, including the
492 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
Where does the cholinergic modulation of septum through a different pathway, which lacks cholinergic
the EEG take place? synapses. Furthermore, since both "cholinergic" and "non-
cholinergic" RSA is accompanied by increased cholinergic
J. C. Szerb and J. D. Dudar activity in the hippocampus, these terms are misleading.
Department of Physiology and Biophysics, Dalhousie University, Halifax,
V&R refer to the "ascending cholinergic reticular forma-
N.S., Canada B3H4H7
tion" of Shute & Lewis to explain the effect of atropine on the
EEG. However, this concept is now somewhat outdated due
The finding of a relationship between the susceptibility of to the nonspecific distribution of cholinesterase, which was
EEG rhythms to antimuscarinic drugs and two types of used as a marker for cholinergic neurons by Shute & Lewis.
behavior described by Vanderwolf & Robinson (V&R) is an With more sophisticated methods, the origin of the choliner-
important new development in our understanding of brain gic afferents to the neocortex have now been identified in the
function. While the behavioral part of this relationship is well n. basalis magnocellularis located in the ventral pallidum
described, the interpretation of the sources of the rhythms is (Emson & Lindvall 1979; Lehmann, Nagy, Atmadja &
at variance with accepted views. Fibiger 1980). These cholinergic neurons are clearly not
The first point to make is that the EEG rhythms in both the identical with the thalamocortical neurons responsible for
archi- and neocortex are likely to be the result of rhythmic modulating the EEG. Furthermore, the cholinergic input to
inputs which originate in pacemaking areas; the lateral tha- the cortex may be activated independently of the EEG (Szerb
lamic nuclear complex in the case of the neocortex (Andersen 1967). Therefore, the corticopetal cholinergic neurons do not
& Andersson 1968) and the medial septum in the case of the appear to be the ones that mediate the synchronizing effect of
hippocampus (Petsehe, Stumpf & Gogolak 1962). The atropine. A much more likely site for the action of atropine is
evidence for the pacemaking role of the lateral thalamic the cholinoceptive units in the n. reticularis thalami (Ben-Ari,
nuclei consists of their rhythmic activity, which persists after Dingledine, Kanazawa and Kelly 1976), which are inhibited
ablation of the neocortex, and the suppression of rhythmic by acetylcholine through muscarinic receptors. These neurons
activity in the neocortex after ablation of the lateral thalamus. have a well-documented inhibitory projection to the ventro-
In the hippocampus, the essential role of the septum in the basal complex of the thalamus and probably receive choliner-
generation of hippocampal RSA is also well established. gic input from the mesencephalic reticular formation. Thus,
However, this rhythmic activity may require feedback from during Type 2 behavior, activation of the cholinergic tract
the hippocampus through the fimbria (McLennan & Miller from the mesencephalon causes a disinhibition of the thala-
1976), although septal pacemaking burst activity can still be mocortical neurons in the ventrobasal thalamus, leading to a
observed in the absence of hippocampal RSA under certain desynchronized EEG. This disinhibition is readily blocked by
conditions, such as that produced by LSD (Stumpf, Petsehe & atropine acting on the cholinoceptive units in the n. reticularis
Gogolak 1962) or during the electrical silence following thalami. During Type 1 behavior, activation of a noncholiner-
hippocampal seizures (Petsehe et al. 1962). The role of the gic pathway may lead to a disinhibition of the same thalamo-
pacemaker area in regulating the EEG implies that behav- cortical projection.
ioral or pharmacological factors which alter the EEG are In summary, the difference in the susceptibility of EEG
likely to exert their effect on the pacemaker nuclei and not on activation to atropine during Type 1 and Type 2 behaviors is
the cortex via the reticulo-cortical system, as suggested by probably due to different tracts being activated in the dien-
V&R. cephalon and not due to differences in the tracts projecting
In accordance with this view, evidence for the subcortical directly to the archi- and neocortex.
location of the cholinergic link which modulates the EEG in
Type 2 behavior is extensive. With one exception, early
investigators observed drug effects on the EEG following
systemic or intracarotid administration of drugs. The one An atropine-sensitive and a less
exception, Miller, Stavraky & Woonton (1940), applied eser-
ine directly to the cortex and observed EEG desynchroniza- atropine-sensitive system
tion. This was accompanied by twitching of muscles and
vibrissae, indicating that the local application may have Robert P. Vertes
produced a generalized arousal which was reflected in the Department of Physiology, University of Michigan, Medical School, Ann
EEG. Furthermore, when atropine was applied directly to the Arbor, Mich. 48109
cortex of locally anesthetized, curarized cats, it failed to As one who is actively engaged in research investigating the
synchronize the EEG, even though it clearly reached an role of the brainstem in the control of the forebrain, I do not
effective concentration within the cortex because it enhanced find the distinction between atropine-sensitive and atropine-
the release of acetylcholine from the area of application resistant RSA and LVFA (see Glossary in target article)
(Szerb 1964). Yet atropine synchronized the EEG when given extremely useful. I go on record here as a fan of Vanderwolf's
i.v. in this preparation. early hypothesis that in the behaving rat RSA (theta) is
Although hippocampal feedback to the septum may be correlated with Type 1 behaviors and irregular nonrhythmic
necessary for the generation of hippocampal RSA, recent hippocampal activity is associated with Type 2 behaviors. I
evidence indicates that the septo-hippocampal cholinergic admit to a great deal of difficulty with the new two-system
tract is not responsible for the generation of the atropine- theory, especially with respect to the species that I am most
sensitive RSA (Dudar, Whishaw & Szerb 1979). In freely familiar with, the rat. One significant problem I see is that
moving rats, both sensory stimulation and motor activity according to Vanderwolf & Robinson (V&R), rats, unlike
increase the release of acetylcholine from the hippocampus, other species such as rabbits, manifest only one type of RSA
although only sensory RSA is "cholinergic," according to (the atropine-resistant kind) rather than two types during
V&R. Systemic atropine abolishes the increase in acetylcho- waking behavior. The absence of any behavioral manifesta-
line release due to sensory stimulation but not that due to tion of atropine-sensitive RSA in the rat would suggest that in
running. Therefore, both the RSA and the increased septohip- this species, at least, the atropine-sensitive system is of little
pocampal cholinergic activity induced by sensory stimulation importance during behavior.
reaches the septum through a pathway containing a choliner- LVFA. V&R propose that there are two forms of LVFA
gic link. On the other hand, motor activity activates the during behavior and REM sleep in the rat. However, no
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 493
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
electrophysiological criteria are presented by which the two In conclusion, I believe that the results supporting the
types of LVFA can be distinquished. In contrast, the criteria hypothesis of two RSA (LVFA) systems could be explained by
for separating the two types of RSA are clearly stated, i.e., proposing a single ACh system with some components that
atropine-sensitive RSA is slow (5-6 Hz) and atropine-resistant are disrupted by atropine at 25 mg/kg and others which are
is fast (7-12 Hz). The absence of similar criteria for LVFA only affected at doses greater than 25 mg/kg - a highly
would suggest that under natural conditions of waking and atropine-sensitive and a less atropine-sensitive system, if you
REM sleep the cortical EEG does not significantly differ will.
during either Type 1 and 2 behaviors or during phasic vs.
nonphasic periods of REM. In this respect, the situation does
not appear radically changed from the classical association of
LVFA with alert waking behavior and REM sleep.
RSA during active (REM) sleep. It was stated that during Independent forebrain and brainstem
REM sleep there are two naturally occurring forms of RSA,
the slow (6 Hz) atropine-sensitive type associated with controls for arousal and sleep
nonphasic events and the fast (>6 Hz) atropine-resistant type
correlated with the phasic events of REM. My experience Jaime R. Villablanca
does not substantiate this distinction. I do not find that RSA is Mental Retardation Research Center and Departments of Psychiatry and
Anatomy, School of Medicine, University of California, Los Angeles, Calif.
predominantly at 6 Hz during nonphasic REM but rather that
90024
it varies between 6-9 Hz, which is the same frequency
normally present in the waking rat during Type 1 behaviors. The main goals of the Vanderwolf & Robinson (V&R) article
In agreement with V&R, I do observe that during periods of are: (1) To disqualify the reticular formation (RF) theory of
very intense phasic activity during REM, RSA frequency arousal; (2) To propose a new role for the "reticulo-cortical
increases to 9-10 Hz. This, however, appears no different systems." The themes of my commentary will be that the first
from similar conditions during waking in which RSA can purpose is only apparently accomplished and that the authors
increase in frequency during vigorous movements. do not really offer any substantial original view to replace the
In summary, I see no evidence that in the rat under natural theory. In addition, I will criticize the concept of the RF as
conditions (waking behavior and REM sleep) the hippocam- the brain "center" for arousal but, still arguing that the theory
pal RSA or cortical LVFA can be differentiated into two is essentially sound, I will propose that the RF is only a
types on the basis of electrophysiological criteria. As a result I portion of a more complex activating-deactivating system of
am not convinced that for the rat, at least, there are two forms the brain.
of RSA or LVFA. I would acknowledge that the situation may V&R use two clusters of arguments: (a) The lack, under
be entirely different in other species. As an alternative, I many conditions, of correlation between EEG neocortical
would propose that there is a single RSA (LVFA) system that desynchrony and behavioral wakefulness (W) or between
is atropine-sensitive and depends upon the integrity of the EEG synchrony and behavioral sleep (S); (b) The fact that
brainstem-septal-hippocampal axis for its expression. It was sleep-waking (S-W) as well as a variety of other behaviors are
pointed out that the atropine-sensitive system could only be still seen in decorticate and decerebrate animals. Regarding
disrupted by very large doses of atropine (25 mg/kg, ip, in the EEG-behavioral "dissociation" in their examples, there is
the rat). Several explanations for why this might be the case a body of experimental data which, I believe, offers an
were provided, i.e., large doses must be given to extensively adequate explanation for these apparent paradoxes. This
occupy the muscarinic cholinergic receptors; atropine does information will be briefly summarized.
not penetrate the brain well; and normal RSA patterns can In a series of studies (Villablanca 1962, 1965, 1966b, 1966c;
persist with only a very small fraction of the RSA-generating Olmstead & Villablanca 1977) we demonstrated that the
system intact. I believe that each of these explanations is very forebrain of the cat, entirely separated from the brainstem by
reasonable. I would, however, go one step further and suggest a permanent, complete transection of the mesencephalon, i.e.,
that possibly even with these exceptionally large doses of the isolated forebrain, shows the following:
atropine the ACh-mediated RSA (LVFA) system is partially (a) Sustained periods of EEG low-voltage, fast-wave
but not completely blocked. I would propose that in the desynchronized rhythms which, as in the intact animal,
presence of large doses of atropine, RSA could be generated if alternate with periods of sustained high-voltage, slow-wave
an ascending ACh system were highly activated. V&R synchronized patterns. These events are even seen after
supported their claim for an atropine-resistant system by the premesencephalic transection.
demonstration that RSA can be generated in the atropinized (b) EEG desynchrony and synchrony in the isolated fore-
rat during Type 1 behaviors and during the phasic events of brain are functionally equivalent to similar EEG patterns
REM. An equally possible explanation for this phenomenon observed during W and S, respectively, in the intact animals
could be that during Type 1 behaviors and phasic REM the because (i) the EEG of both patterns in the neocortex and
reticular formation is intensely active and capable of driving hippocampus is comparable to those during W and S of intact
some cholinergic RSA-generating sites in the forebrain not cats; (ii) in animals with mesencephalic transections caudal to
completely saturated with atropine. The one-system theory
the third nerve nuclei, pupillary diameter and ocular move-
would be supported by the finding that (1) anatomically
distinct atropine-sensitive and atropine-resistant systems ments follow well the fluctuations of the EEG; (iii) the EEG
could not be identified in brainstem stimulation studies (Rob- synchrony can be interrupted by olfactory stimulation, and
inson & Vanderwolf 1978 and Vertes 1980); (2) discrete this arousal is accompanied by mydriasis.
reticular and hypothalamic lesions were incapable of selec- (c) The effectiveness of an olfactory stimulus for arousing
tively eliminating the atropine-sensitive or atropine-resistant the sleeping isolated forebrain depends on the duration of the
system (Robinson & Wishaw 1974) and (3) the search for a EEG synchrony prior to the stimulus onset. This graded
transmitter for the atropine-resistant system has been largely character of the arousal response suggests a variable tonus of
unsuccessful. One obvious test of this hypothesis would be to an active S mechanism.
inject doses of atropine well in excess of those necessary to (d) Where additional visual and olfactory deafferentations
disrupt the atropine sensitive RSA. This may prove to be are carried out the S-W alternation persists.
futile, however, because it may be impossible to completely (e) The theta rhythm is the predominant EEG pattern of
saturate ACh receptors at any dose of atropine. the hippocampus. Assuming that theta represents a moderate
level of hippocampal activity (Kemp & Kaada 1975; Parmeg-
494 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
giani et al. 1967), we have hypothesized that a moderate level 1974), while the brainstem and body are awake, thereby
of arousal is the dominant state of the isolated forebrain. accounting for the erect posture and locomotion. In patholog-
(f) The S-W patterns of the isolated forebrain are usually ical conditions, a number of etiologies could anatomically
dissociated from those of the decerebrate animal, i.e., the uncouple the rostral from the caudal S-W mechanisms,
animal itself can be sleeping while the forebrain displays thereby creating, depending on the topography as well as the
waking activity and vice versa. time course of the process, a variety of EEG-behavior combi-
(g) The drugs tested in the isolated forebrain, including nations in the rostral brain versus independent combinations
atropine and eserine, produce EEG patterns identical to those in the brainstem and body (Bricolo 1975; see Harner &
seen in the intact brain. Naquet 1971).
(h) In preliminary experiments in kittens, we have Another phenomenon which apparently conflicts with the
observed that the EEG and behavioral patterns of the isolated RF theory is the presence of synchronized EEG activities in
forebrain develop approximately normally even though the the neocortex of awake, performing animals. Those activities
mesencephalic transection is done at a time of immaturity of are not generalized over the entire cortex but consist of
the EEG (10 days of age); this ontogenetic independence specific EEG spindle-like events occurring in discrete regions
further documents the importance of the forebrain processes. which are associated with the display of well-defined behav-
Most of these observations have now been replicated by at iors. Concomitantly, rhythmic field potentials or single unit
least nine laboratories around the world in cats (Batsel 1964, activities occur in the corresponding thalamic nuclei (Rou-
Belardetti et al. 1977; Naneishvili et al. 1975a, Serkov et al. geul-Buser et al. 1978). A reasonable interpretation is that
1966; Slosarska & Zernicki 1973; Sterman 1972), dogs (Batsel these "focal rhythms" represent the replacement of EEG
I960), rats (Gottesmann et al. 1980), and monkeys (Massopust desynchrony of arousal by synchronized oscillations of excit-
et al. 1968). In the monkey, waking EEG rhythms are present ability within a given thalamocortical channel, with the
immediately after transection, whereas in cats, depending on consequence that they "prevent normal sensory or sensory-
the lesion technique, (Olmstead & Villablanca 1977; Villa- motor processing in that particular thalamocortical channel"
blanca 1972) there may be a delay of days (which was the (Buser 1980). Thus, the presence of the rhythms does not
effect that gave rise to the erroneous deafferentation theory contradict the RF theory but would indicate selective deacti-
of sleep following Bremer's (1937) experiments). This fits vation of only portions of a thalamocortical system.
with the demonstration that the more rostral mesencephalo- It has been shown that atropine and eserine can produce
diencephalic components of the RF are more important in electrocortical effects similar to those seen with the same
primates (Bricolo 19/5; Mogoun 1954; Rossi 1980). Thus, the doses in intact animals when injected systemically in prepara-
relatively protracted course of recovery of arousal in the cat tions with an isolated forebrain (Villablanca 1966), or with
may be interpreted as a neurological shock which tends to the cortex totally disconnected from subcortical influences
disappear with encephalization. (see Villablanca 1966c; Villablanca 1966/1967), or following
In another series of reports we demonstrated that cats topical application (see Longo 1971). Furthermore, within a
without the telencephalon (Villablanca & Marcus 1972) or certain range of atropine dosage, external (see Villablanca
even decerebrate animals (Villablanca 1966a) can S or W and 1966c) or RF stimulation (Bradley 1968) can still desynchro-
display a variety of related behaviors. The concomitant nize the EEG such that at relatively low doses the dissociation
polygraphic events also were defined. These findings have is either partial or nonexistent. These data offer an alternative
also been replicated (Houston & Borbely 1974; Jouvet 1962; explanation for the well-known EEG-behavioral dissociation
Sterman 1972) and have been reviewed extensively (Moruzzi produced by these drugs. Indeed, treating the neocortex as an
1972, 1974; Villablanca 1974). effector for the desynchronizing actions of the RF one can
An important conclusion from these data is that the fore- propose that adequate doses of atropine or eserine can effec-
brain possesses intrinsic systems for the control of S and W tively block the cortical circuitry, thus rendering it insensitive
which can function independently of brainstem influences. to the RF physiological inputs. Similar local effects have been
Similar capabilities are also present in the lower brainstem described for other drugs (see Longo 1971 and Villablanca et
separated from descending forebrain controls. A dual system al. 1970) such that ignoring this basic phenomenon is one of
for both arousal and S, one in the forebrain and the other in the pitfalls in interpreting effects of drugs in S-W physiology
the brainstem, can therefore be postulated. Moruzzi (1972, and in EEG control.
1974) has critically reviewed our findings, and additional I do not see how the presence of S-W in decorticate or
evidence, reaching conclusions similar to ours. The multiple decerebrate animals contradicts the RF theory. Although
areas demonstrated to be involved in the control of W and S V&R call it the "reticulo-cortical" theory, there is no doubt
in both segments of the encephalon provide ample structural that the core component is the RF. The forebrain structures
and functional bases for these systems (see Belardette et al. are important as long as they are there to be "awakened" or
1977; Brodal 1969; Moruzzi 1972; Dement & Villablanca enabled by the arousal action to process information and
1974; and other recent reviews). produce outputs. Since the RF is essentially intact in decorti-
We have further hypothesized that the rostral system may cate cats, it should not surprise anyone that such animals can
be uncoupled from brainstem mechanisms either physiologi- display features of W and S to the extent permitted by the
cally or by pathological and pharmacological events (Villa- CNS areas still available. Indeed, in order to validate V&R's
blanca, 1966 a,c). Therefore, considering this potential of the viewpoint it would be necessary to show that eliminating the
two areas for independently displaying intrinsic behavioral RF in the decorticate animal does not impair waking. But this
and EEG manifestations of S and W, the "dissociations" does not seem to be the case. For example, in acute thalamic
mentioned by V&R can be incorporated into the RF theory. cats we demonstrated that following a mesencephalic transec-
For example, in an intact individual REM sleep and cataplexy tion the thalamic EEG spindles and interspindle lulls are
may be conceived, respectively, as resulting from a physiolog- replaced by almost continuous high voltage, slow-wave activ-
ical "disconnection" in which the rostral brain is awake ity (Villablanca & Schlag 1968). Indeed, one would predict
enough to be aware of the contents of dreams (REM sleep) or that destruction of the RF in decorticate or decerebrate
is fully awake, with normal awareness (cataplexy), but with animals would result in a total abolition of the W state.
both states occurring in a "deactivated," sleeping, brainstem The above certainly does not mean that the neocortex or
and body. Conversely, somnambulism may be understood as striatum do not play a role in the control of S-W. We
a state in which the forebrain remains asleep, as evidenced by (Villablanca & Marcus 1972; Villablanca et al. 1976) as well as
the synchronized EEG and lack of awareness (Kales et al. others (Jouvet 1962; Moruzzi 1972) have extensively discussed
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 495
Commentary/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
their role and I refer the reader to those papers. Moreover, I brain state, reflected in EEG changes, must be immediately
have proposed (Dement & Villablanca 1974) that the overall translatable into easily interpretable overt movements for the
brain organization for S- and W-control follows a Jacksonian benefit of an experimenter. While I am sitting immobile,
pattern, and I have defined specific roles for the main CNS gnawing my pen, according to V&R, I am in state II, and
"levels" involved. This hypothesis includes the notion that the synchronization reigns in my neocortex. But I am vigorously
deterioration of both S and W manifestations increases as a thinking about V&R's paper, and I am certain that my
lesion progresses from the "highest" to the "lowest" control neocortex is desynchronized. (This allusion to myself is justi-
"levels" and matches well with our concept of a dual system fied by the fact that animal experimental data in the paper
for CNS activation-deactivation. sandwiched between introduction and conclusion are slightly
I have great difficulty in seeing that V&R clearly establish peppered with human EEG data; this suggests that the
any new function for their two pharmacologically distinct RF proposed ideas are applicable to humans as well.) Although
subsystems. Indeed, these projections may still be conceived animals do not read papers, it is, alas, conceivable, that there
as having a role compatible with the broad functional concept are states (hormonal influences, inner sensations, etc.) which
of the RF theory, i.e., ascending influences which enable their may exert strong influence on brain activity levels (and thus
target areas to carry on their specific functions through the on the EEG) without immediately observable manifestations
activating process which we call arousal. The lengthy discus- in behavior.
sion of the neurotransmitter involved is of little help since, The second pitfall seems to be obvious to the authors
regardless of synaptic mechanisms, the key issue is still the themselves. It is the absence of reliable criteria for differen-
functional role of the RF. Furthermore, in the attempt to tiating voluntary movements from automatic ones by means
show a new role for these subsystems, V&R's emphasis is on of simple observation. After it is multiply stated that hippo-
the functions of the target areas, i.e., the cortices. Or, at best, campal theta and cortical desynchronization occur "if, and
they fail to convince one that the subsystems' role differs from
only if voluntary behavior is being performed," we learn that
that of the corresponding projection areas. Adding to my
hesitation are the perplexing final remarks that "there are, as "there are, as yet, no clear behavioral criteria by which Type
yet no clear behavioral criteria by which Type 1 or Type 2 1 or Type 2 behavior can be classified." The automatic
behavior can be classified" and that the specific roles of their behavior is "defined as behavior with no consistent relations
reticulo-cortical pathways "is a topic for future research." to RSA." Indeed, "it often seems as though "voluntary" and
In conclusion, I feel that we are not being presented with "automated" are defined post hoc by the correlation of
any robust innovative theory. As an alternative, I propose the hippocampal EEG patterns with a response," as Black,
concepts of independent forebrain-brainstem systems for another advocate of the proposed view, wrote in 1975 (p.
arousal and S, and that their organization is Jacksonian. These 154). Thus, first theta is proclaimed to be a correlate of
ideas establish an integrative framework for an overdue voluntary behavior; then voluntary behavior is defined as
theoretical "new synthesis" which: (i) incorporates informa- behavior during which theta is present, and automatic behav-
tion collected after the reticular theory was proposed; (ii) ior as behavior during which theta is absent. I regard this as a
rejects the notion of CNS "centers" for the control of S and W brilliant example of circulus viciosus in logic.
and precludes the interpretation of S as merely a reduction of Third, and maybe most important, is the problem of what
RF activity; and (iii) effectively explains most spontaneous is correlated with theta and desynchronization in voluntary
events as well as experimental and pathological phenomena movements (if we consider such a possibility for a moment.)
related to W-S, including those discussed by V&R. From my early scientific youth I was taught by the late Prof.
A. R. Luria that there is nothing more complex than volun-
tary action. It includes the perception and analysis of the
environment (under certain levels of arousal and attention),
ACKNOWLEDGMENT proper motivation, decision making, the planning of action,
This work was supported by USPHS Grants HD-05958 and HD- its execution, feedback information, and the evaluation of the
04612. result obtained. The complex composition of voluntary action
has also been demonstrated by Anokhin, Pribram, and many
others. From V&R we learn that his major finding is that RSA
Behaviorism and voluntarism is not correlated with environmental circumstances or
psychological processes, but only with overt behavior. What
O. S. Vinogradova does this mean? Are the movements per se so different during
Institute of Biophysics of the USSR Academy of Sciences, Puschino-
voluntary and automatic behaviors? What is the difference
on-Oka 142 292, Moscow District, U.S.S.R. between, for instance, turning the head to attend to a stimulus
and turning the head to lick the skin? What happens with
The progress of science is immense. The "behavioristic" or learned (voluntary) movements, such as lever pressing, after
"ethological approach" proposed by Vanderwolf & Robinson prolonged practice, when they cease to be accompanied by
(V&R) allows us to understand electrophysiological and even theta?
biochemical events through their temporal coincidence with As a result of V&R's proposed principles of analysis, we are
behavior (movements). confronted with such surprising types of "voluntary behav-
V&R's approach is based on the assumption that even such ior" as forced running in the treadmill, immobilization under
events as arousal should be regarded as "inferred processes," curare, and muscle twitches during paradoxical sleep, since
as are perception, attention, motivation, memory and so on; all these states correlate with high-frequency theta. The
thus they should be rejected by the true investigator of example of curarization is used by the authors to show that
behavior. But, strangely enough, the most "inferential," proprioceptive impulses, which are provided by the move-
psychological, and even philosophical concept of all - voli- ment, are not necessary for the production of theta. In fact,
tion - is not only allowed into the behavioristic box (otherwise V&R show only that movement itself is not necessary for
so well sterilized against psychological germs), but is even theta production. The intrinsic similarity between forced
used as basic differentia specified in the analysis provided by running and the curarized state may reside in the fact that
the authors. Aside from the basic inconsistency of such an both are stressful situations with high level of arousal, evoked
approach from the behavioristic point of view, three argu- by strong and unusual bodily sensations. As for paradoxical
ments invalidate it. sleep, it has repeatedly been shown by neurophysiological
First of all, it is a sweet, but deceptive dream, that any methods that this state is in fact in many respects equivalent
496 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Response/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
to the state of high activity (arousal) of the forebrain. As probable that this basic septal rhythmic mechanism is more
Morrison stated recently, ". . . paradoxical sleep can be resistent to anaesthetics, which are known to suppress activity
viewed as a period during which the CNS acts as if there were of the reticular formation. In the normal state the basic
a continual influx of unexpected, or novel, stimuli" (Morrison frequency of the septal pacemaker is always a little higher
1979, p. 572). than in the deafferented septum (not less than 4 per sec in the
In speaking about movement (walking, running, rearing), rabbit), due to the influences of the reticular formation. Any
we should remember that this provides an animal with a high increased level of excitation of the reticular units is recoded
inflow of sensory information. The environment, sliding by into accelerated bursts of septal units, and thus into higher
during the movement, is the source of rapidly changing visual frequency hippocampal EEG theta.
information, of acoustic stimuli with shifting characteristics; In conclusion, I wish to express my sincere regret that after
movement itself produces a strong inflow of somatosensory so much work done by V&R we have not learned anything
and proprioceptive impulses. It is a scholastic undertaking to about the mysterious functions of the hippocampus (which
attempt to segregate "pure motor" and "pure sensory" certainly does not participate in triggering the movements, as
components in the behaving animal [See also Lynch: "The both human and animal lesion data show), or about the
Functional Organization of Posterior Parietal Association functional significance of theta in the machinery of the brain.
Cortex" BBS 3(4) 1980.] For the "ethological" behaviorist it is The proposed "new synthesis" only proves once more that "to
also advisable to take into account the fact that small animals correlate is not necessarily to elucidate an axiom that is most
(mice, rats) have narrow visual angles; hence, in order to apparent in RSA research" (Klemm 1976, p. 23). It certainly
perceive and analyse their environment - to orient and does not shake the classical theory of arousal. The voluntaris-
perform adaptive behavior in it - these animals must move tic attempt to substitute, for neurophysiological realities,
around in this environment much more than do larger vague descriptions based on purely subjective observations
animals. and interpretations, seems outdated and fruitless as a contri-
Thus, it seems that the classical theory of reticulo-cortical bution to our understanding of brain and behavioral prob-
relations has greater potential for explaining various facts, lems.
and much less contradictions, than the "new synthesis."
Many objections against the classical theory of reticulo-
cortical interaction listed by V&R have long ago found their
explanations; some of these objections are just wrong (for Authors' Response
example, the interpretation of the state of the pretrigeminal
animal as "deep coma," just because it cannot move, for Brain-behavioral studies: The importance of
obvious reasons; the prolonged active (aroused) state of the
forebrain in this preparation is demonstrated and well docu- staying close to the data
mented).
C. H. Vanderwolf and T. E. Robinson"
But there is one more specific question regarding the
various theta frequency bands. One band, according to V&R, 'Department of Psychology, University of Western Ontario, London, Ontar-
io, Canada N6A 5C2and"Neuroscience Laboratory, University of Michigan,
is of low frequency and cholinergic pharmacologically; the Ann Arbor, Mich. 48109
other is high frequency and its pharmacological nature is first
proclaimed unknown ("Two types of RSA," para. 13) but For the convenience of the reader, we have summa-
later declared "trace aminergic," whatever that may mean. rized our discussion of the commentaries in Table 1.
There are many investigations, both old and new, which show On the left side of the table are the issues raised; on the
that stimulating one and the same point in the reticular right side the commentaries that raised points relevant
formation can produce theta in its full range of frequencies. It to the issue.
has been shown, moreover, that theta frequency changes as a
linear function of the intensity of reticular stimulation
(Stumpf 1965). Both the frequency of burst discharges and
the number of rhythmically bursting septal cells also grad- Mind and behavior. A very general point raised by a
ually increase. Thus, are we indeed dealing with two number of commentators (Bennett, Carlson, Eichen-
different processes, or with a continuum of frequencies, baum, Ranck) concerns the basic aims and definition
which may be regarded as measures of the levels of afferent of brain-behavior research. Should our main aim be to
input from the reticular formation? discover physiological correlates of psychological
Some relevant data have been obtained in our experiments processes or should we try to understand the neural
with deafferented septum (Vinogradova et al. 1980). It was mechanisms that control behavior (i.e., movement and
shown, that in the basally undercut rabbit septum, completely posture)? It has been pointed out that even if one's long
deprived of MFB (medial forebrain bundle) influences, a
range goal is the elucidation of psychological processes,
relatively large proportion of the septal units (22%) preserve
the ability to discharge in extremely regular rhythmic bursts, some form of behaviorism is a logical necessity (Ratliff
with a mean frequency of 3.3 per sec. Very low frequency 1962). Mind, regarded as the mechanism that controls
theta was found to be present in the hippocampal EEG of behavior, is not accessible to direct examination, appar-
these animals. The rhythmic burst activity was also observed ently not even in oneself (Hebb 1980). Consequently,
in slices of guinea pig septum, incubated in vitro (burst mind can be studied only indirectly, as an inference
frequency was 2-3 per sec). In both conditions the application from behavior. If this is so, we would do well to study
of physostigmine (i.v. or in the bath medium) increased the behavior very thoroughly, regardless of our philosophi-
proportion of rhythmically bursting cells and the density of cal preconceptions. This conclusion is also suggested by
their bursts, and slightly shifted their frequency (by 1-2 per
sec) into the higher range. Thus, we conclude that septal the view that the brain is chiefly a device for regulating
neurons possess the intrinsic ability to produce very low motor output (Sperry 1952). Careful study of motor
frequency rhythmic bursts which can only be slightly acceler- output might, therefore, provide clues to the functional
ated by influences on the septum's own cholinergic mecha- organization of the brain.
nism. To further accelerate this intrinsic rhythmic mecha-
Psychological theories tend to relegate behavior to
nism, ascending brainstem influences are necessary. It is very
the role of a gauge or indicator of mental activity
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 497
Response/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
Table 1. A guide to replies by Vanderwolf and Robinson to
It is possible that atropine-sensitive RSA and LVFA
specific commentators
(see glossary in target article) play some role in the
stimulus control of adaptive behavior, as we suggest.
However, adequate methods for the electrophysiologi-
Section Commentators cal study of this role have not yet been applied in a
behavioral experiment. It is not possible to conclude, as
1. Mind and behavior Bennett, Buzsaki et al., Carl- Bennett does, that atropine-sensitive RSA is directly
son Eichenbaum, Ranck related to attention or cognitive processes since this
2. Voluntary and automatic Bennett, Buzsaki et al., theory is directly contradicted by the well-known
behaviors Eichenbaum, Malmo & experiments of A. H. Black (1975, see also "A reply to
Malmo, Villablanca,
Vinogradova
Vinogradova" below) which Bennett failed to
3. EEG activation as a corre- Buzsaki et al., Eichenbaum, mention.
late of arousal Jasper, Jones, Ranck, Rou-
geul et al., Somjen, Steriade, Voluntary and automatic behaviors. A number of
Sutherland et al., Villablan- authors (Bennett, Buzsaki et al., Eichenbaum, Malmo
ca, Vinogradova & Malmo, and Vinogradova) have referred to our use
4. Two types of RSA and Buzsaki et al., Eichenbaum, of the term "voluntary." The target article discusses a
LVFA Komisaruk, Malmo & Mal- large number of empirical observations which show
mo, Shiromani & Fishbein,
that certain motor activities are consistently accompan-
Vertes, Vinogradova
5. Characteristics of as- Krnjevic, Phillis, Shiromani & ied by distinctive patterns of brain activity while other
cending cholinergic sys- Fishbein, Szerb & Dudar, motor activities and behavioral immobility are gener-
tems Villablanca ally not accompanied by these patterns. These groups
6. Behavior and unit activity Malmo & Malmo, Steriade of motor activities require names. Initially, we chose
7. Possible influences on Calloway, Carlson, Eichen- the terms "voluntary" and "automatic" since: (1) One
brain-behavior research baum, Komisaruk, Malmo & of the groups of motor activities (voluntary) comprises
Malmo, Ranck such behaviors as locomotion and lever pressing which
8. Interpretation of lesion ex- Somjen, Sutherland et al. are familiar as operant or instrumental responses. (2)
periments The other group of motor activites (automatic)
9. A reply to Hirschman Hirschman comprises more reflexive or consummatory behaviors
10. A reply to Vinogradova Vinogradova
such as the startle response, licking and chewing. (3)
The terms voluntary and automatic, used in this way,
are consistent with the usage of J. Hughlings Jackson
(Hebb 1980). Consequently, the psychological ap- (see Taylor 1958), who was an important innovator in
proach has neglected the careful observation and the brain-behavior field. The need to name the behav-
description of behavior, as Lorenz (1973) and ior patterns is equally well served by the terms Type 1
Tinbergen (1963), among others, have pointed out. As and Type 2, although these clearly have less heuristic
an example of this in the brain-behavior field, it is value.
possible that mentalistic preconceptions have delayed In retrospect, using the term "voluntary" may have
by several decades the appearance of accurate descrip- been an error in public relations. For many people
tions of the relation between hippocampal and neocor- "voluntary" has strong metaphysical connotations and
tical slow-wave activity and behavior. is not acceptable simply as a means of referring to a
Consequently, it seems to us that there has not been group of behaviors. For this reason we preferred using
much progress since the beginnings (historically speak- the terms "Type 1" and "Type 2" behavior in the
ing) of scientific investigation of the relation of target article.
mammalian brain activity to behavior. Quite simply, Villablanca and Malmo & Malmo are puzzled by
since behavior was not regarded as a topic of particular our statement that "there are, as yet, no clear behav-
interest, it was not studied adequately in much of the ioral criteria by which Type 1 or Type 2 behavior can
early work. Ranck makes this point very effectively in be classified." Obviously, there is no great difficulty in
his discussion of the necessity of providing a behavioral distinguishing, for example, among walking, face-
definition of arousal. Buzsaki, Isaacson & Hannigan, washing, and immobility. Therefore, at a practical
Eichenbaum, Komisaruk, and Malmo & Malmo also level, it is easy to distinguish Type 1 from Type 2
favor more accurate description of overt behavior. behavior. The difficulty comes in determining whether
The study of cognitive processes in relation to brain there is anything in comon (other than distinctive
activity, recommended by Bennett and by Eichen- patterns of brain electrical activity) among the various
baum, has usually involved testing in rather complex Type 1 behaviors which is not shared by Type 2
situations (in mazes, in discrimination apparatus, or behaviors. A number of characteristics which Type 1
under various reinforcement schedules in a lever box). behaviors may have in common are discussed in the
The stimuli controlling behavior are often not target article (also see Vanderwolf, 1971; and O'Keefe
adequately known and behavior may be described only & Nadel 1978).
in terms of "correct responses," "errors," or "rate of In reply to Buzsaki et al., we are not concerned with
responding" on a lever. Reports based on such studies whether or not we adhere in a dogmatic manner to the
are often uninterpretable (Arnolds et al. 1979c; doctrines of either B. F. Skinner or the Lorenz-
Whishaw & Vanderwolf 1973) since essential informa- Tinbergen school of behavior study, although we do
tion is not included. acknowledge our indebtedness to these behavioral
498 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Response/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
occurring during quiet sleep, but this is not true in all
Table 2. Models of reticulo-cortical activity and behavior
cases. For example, Marczynski et al. (1968, p. 236)
state that "The bursts of PRS (post-reinforcement
Model 1: Traditional arousal synchronization, i.e., slow waves occurring in a cat
theory while drinking) were not discernible from spindle sleep
Ascending reticiilar acti- EEG arousal (LVFA, RSA); ECG (electrocorticogram) recorded from the same
vating system Behavioral waking. cortical region."
Model 2: Vanderwolf and In any case, conventional arousal theory has always
Robinson theory emphasized the difference between "synchrony" and
a) Cholinergic reticulo- Atropine-sensitive LVFA
cortical system
"desynchrony" and has paid scant attention to the
and RSA; Stimulus con-
trol of Type 1 and Type various patterns which large amplitude slow waves can
2 behavior? assume. For example, a cerveau isole preparation
b) Noncholinergic reticu- Atropine-resistant LVFA (Bremer 1935; Moruzzi 1972) displays very rhythmic
lo-cortical system and RSA; control of slow waves which are certainly not identical with the
Type 1 behavior? patterns occurring during quiet sleep. Yet no one, to
c) Descending reticulo- Behavioral waking. our knowledge, has ever objected that this makes the
spinal system (plus cerveau isole preparation irrelevant to the physiology
other descending of sleep! Consequently, an appeal to the particular
systems) properties of various types of slow waves cannot
provide a basis for a defense of conventional arousal
theory.
investigators. What we do claim is that we have Rougeul et al. suggest that more research should be
provided a more accurate descriptive account of the devoted to the study of the various patterns of wave
relations between brain slow-wave activity and behav- morphology and location which neocortical slow-wave
ior than was previously available. It may well be that at activity can assume. We agree with this wholehearted-
some level in the brain there is "a great difference ly. However, it is necessary to point out that we shall
between a foreleg movement in stepping toward a learn very little about the behavioral significance of
receptive female and a similar movement made away such rhythms unless adequate behavioral techniques
from a predator" but distinctions of this type do not are used in conjunction with electrophysiological stud-
seem to be reflected by differences in reticulo-cortical ies. Rougeul et al. are mistaken in their remark that it is
activity. only in the hippocampus that we are concerned about
the correct placement of recording electrodes. In fact,
EEG activation as a correlate of arousal. Table 2 as Fig. 4 (of the target article) shows, we emphasize the
provides a simplified summary of traditional arousal importance of proper electrode location in the record-
theory and of our proposed replacement for it. ing of electrical activity in the neocortex as well as in
A number of commentators (Eichenbaum, Jasper, the hippocampus.
Jones, Rougeul, Bouyer & Buser, Somjen, Steriade, With respect to wave morphology, it should also be
Villablanca, and Vinogradova) question our conclu- mentioned that Somjen is mistaken in his statement
sion that the conventional arousal theory of reticulo- that the slow waves produced by atropinic drugs are
cortical cortical activity is inadequate and should be different from those observed during natural sleep.
abandoned. Rougeul et al. and Villablanca suggest that Montplaisir (1975) reports that neocortical slow-wave
the presence of slow-wave activity during Type 2 activity produced by scopolamine in restrained
behavior in the normal waking animal is compatible monkeys is very similar to the slow-wave activity
with conventional arousal theory since the slow waves present during natural sleep. In addition, interval histo-
do not occur everywhere and LVFA persists in some grams of neocortical unit activity following scopol-
cortical areas. This is true. However, LVFA also occurs amine treatment closely resembled those obtained
in stages I and 2 of normal sleep (Dement & Mitler during natural sleep. Our own data indicate that
1974) and there is undoubtedly a great deal of similar- waking atropinized rats engaged in Type 2 behavior
ity between the EEG activity of a cat standing up display large slow waves (2-6 Hz) and spindles (10-16
drinking milk from a saucer and that of a cat lying Hz) which closely resemble the activity present during
down in an early stage of sleep. When this is considered natural quiet sleep in the same individual animals
together with the other findings mentioned in the (Vanderwolf 1975). However, since LVFA appears
target article (paradoxical sleep, LVFA during anes- during Type 1 behavior in atropinized rats, the slow
thesia and coma, effects of muscarinic blocking drugs) waves and spindles are frequently interrupted. Casual
it seems to us that the conclusion that EEG activation examination of such records may be responsible for the
correlates poorly with the state of arousal is a simple widespread but false impression that atropine does not
reproduce the electrographic phenomena of slow-wave
statement of fact. Ranck provides additional evidence
sleep.
in support of this conclusion.
Rougeul et al. also state that synchronized patterns Another point which should be made is that conven-
occurring in the waking state do not contradict conven- tional arousal theory attempts to relate all ascending
tional arousal theory since such wave patterns are reticulo-cortical activity to a single behavioral dimen-
"completely distinct from slow sleep activities." It is sion extending from deep coma to extreme excitement.
true that some of the slow wave rhythms occurring in If, as we show, there are at least two distinct ascending
the waking state have a morphology distinct from those reticulo-cortical systems with different relations to
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 499
Response/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
behavior, then this simple concept must be false on Using present-day techniques, it could be shown to be
logical grounds alone. either incorrect or probably correct. It is much less
A number of other points are raised by the commen- clear what is meant by statements to the effect that
tators in defense of conventional arousal theory. It atropine alters maze performance by interfering with
seems to us that some confusion results from the use of one or more of: alertness, learning, memory, recall,
such words as "conscious," "waking," "alert," or "vigi- attention, motivation, drive, etc.
lant" to refer to two quite different things: (1) the An incidental point which can be mentioned here is
presence of a waking posture (standing up, head held that Buzsaki et al. are mistaken in their statement that
up against gravity, eyes open, etc.) plus spontaneous we failed to observe changes in grooming behavior in
movement, and (2) an inferred psychological state. For decorticate rats. Such changes were documented quan-
example, Steriade states that "decorticate rats may titatively by Vanderwolf, Kolb & Cooley (1978).
display head movements, walking, and rearing, but I Villablanca suggests that the forebrain and lower
assume that waking processes transcend this reper- brainstem possess potentially independent mechanisms
toire." Nonetheless, since the psychological state is not for sleep and waking which normally operate as a
susceptible to direct measurement, most sleep research- harmonious system but can be dissociated by transect-
ers have been content to define the waking state in ing the brainstem and in other ways. It is very interest-
terms of criterion #1 or even simpler measures such as ing that the neural machinery for generating LVFA
the electro-myogram or electro-oculogram. Let us and RSA is intact in the isolated forebrain. One
follow this sensible convention and use the term "wak- wonders whether these rhythms are totally atropine-
ing behavior" to refer to meaning #1 above. sensitive or not. Atropine-resistant inputs might origi-
Waking behavior is not dependent on ascending nate in the lower brainstem and be severed by a rostral
reticulo-cortical activity, as is shown by the effects of brainstem transection.
decortication. Thus, we can reject Somjen's theory that A separate question, also raised by Villablanca, is
waking behavior survives in atropinized animals whether the presence or absence of LVFA or RSA in
because some cortical neurons are unaffected by atro- the isolated forebrain can be taken as evidence of
pine. However, although atropinized or decorticate waking or sleep in either a behavioral or a psychologi-
animals are undoubtedly capable of waking behavior, cal sense. We think that such conclusions would be
there is also no doubt that adaptive behavior is severely unjustified since cerebral electrographic patterns are
impaired in such animals (i.e., there are impairments in not reliable indices of sleep, waking, or consciousness in
feeding, sexual behavior, performance in mazes, etc.) the intact animal. For example, we do not attribute
Jasper is mistaken in his statement that we do not take "wakefulness" or "consciousness" to an etherized
this into account. In fact, the behavioral effects of animal under light surgical anesthesia simply on the
antimuscarinic drugs provide an important basis for basis of LVFA in the electrocorticogram.
our suggestion that the ascending cholinergic reticular Villablanca's theory encounters other difficulties.
formation plays a role in the stimulus control of behav- For example, contrary to Villablanca's suggestion,
ior (see "Synthesis" section below). One might go on to investigations by Jouvet (1972; 1974), Pompeiano
infer that the alterations in behavior produced by (1967), Siegel (1979), Vertes (1979), and others
decortication or atropinization are due to an impair- (reviewed in target article) show that the brainstem is
ment of "consciousness" in sense #2 above. However, not "deactivated" during active (REM) sleep. In fact,
taking this step leads one inevitably into the quagmire the entire brain seems to be very active but skeletal
of psychological speculation. Is it possible that the motor activity is suppressed by inhibition at a spinal
behavioral effects of atropine are not due to impair- level. Although Villablanca's theory seems to suggest
ment of "alertness," as Jasper suggests, but are instead that the behavior of a high decerebrate human would
the result of impairment in perception, motivation, be approximately equivalent to the behavior of a sleep
attention, learning, memory, recall, or intelligence? walker with an intact brain, this is improbable. Sleep
Questions of this type are, for all practical purposes, walkers can walk about and avoid obstacles, but it is
insoluble. This is due, in part, to the impossibility of doubtful that decerebrate humans would be capable of
defining exactly what the psychological terms mean. It this.
is simpler to stay close to the facts. Decorticate or Finally, contrary to the suggestion of Villablanca,
atropinized animals are capable of waking behavior (in we do not claim that the entire reticular formation is
sense #1 above) but the organization of behavior is irrelevant to waking behavior. The target article states
abnormal. Detailed observation of a wide variety of specifically that descending reticular fibers are of
behaviors under various circumstances should lead to a importance in this but that ascending fibers are not.
comprehensive description of these abnormalities Unfortunately, surgical lesions or electrical stimulation
(Schallert et al. 1980; Vanderwolf, Kolb & Cooley of the reticular formation do not provide a means of
1978). The behavioral abnormalities should then be affecting one or another of these sets of fibers in a
interpreted in terms of measurable neural events and selective manner, as Sutherland, Whishaw & Kolb
not in terms of hypothetical psychological processes. As point out.
an example, one might suggest (without engaging in We agree with Jones that conventional arousal
psychological theorizing of any kind) that the poor theory is anachronistic and oversimplified, but it is
performance of an atropinized rat in a maze test is due certainly no straw man, as is shown by the fact that a
primarily to the blockade of a cholinergic reticulo- number of commentators defend it vigorously. The
cortical pathway. This suggestion may or may not be approach which Jones proposes to refurbish arousal
correct, but it does have a relatively clear meaning. theory is to identify constellations of correlated physio-
500 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Response/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
logical and behavioral events which can then be RSA. The record of an immobile rat shown in the
labelled as "sleep," "waking," etc. Manipulations such upper left corner of Fig. 3 is not claimed to be an RSA
as drugs or brain lesions give rise to new constellations, pattern. We would consider it to be an LIA pattern,
for which (presumably) new names could be invented. even though it undoubtedly contains some power in the
It seems to us that this approach loses sight of the main 5-7 Hz frequency band. The main point is that the
aim of brain-behavior research, which is to give an record becomes decidely more rhythmic when the
adequate account of behavior in terms of the activity of animal walks and can also assume a highly rhythmic
the nervous system. We must aim at a causal analysis pattern during ether anesthesia (Fig. 3, lower left). It
and not content ourselves merely with naming clusters may be worth pointing out that RSA cannot be equated
of correlations. Logically then, we must begin with a simply with activity in a particular frequency band.
description of behavior which can, subsequently, be Factors such as rhythmicity and amplitude must also
accounted for in terms of nervous activity. (This be considered. For example, hippocampal spikes
consideration does not, of course, dictate research strat- contain frequencies that overlap with the upper range
egy. For example, it might be useful to begin with a of RSA frequencies. Nonetheless, the spikes can be
brain eVent, then look to behavior to determine its distinguished from RSA quite easily on the basis of
functional significance.) amplitude and overall morphology. Hippocampal
Jones is certainly right in her contention that patho- spikes and RSA also differ in terms of pharmacology
logical conditions, such as atropinization, do not tell us and relations to behavior.
how brain activity is correlated with behavior in Clear RSA patterns ordinarily do not occur during
normal animals. However, experimental manipulations waking Type 2 behavior in a normal rat. However,
do provide data relevant to a causal analysis. For RSA (atropine sensitive) can be induced during Type 2
example, the effect of atropine shows that LVFA is not behavior by such agents as electrical stimulation of the
essential for the occurrence of waking behavior, as reticular formation, various drugs (including ether),
Wikler (1952) pointed out many years ago. To state, as brain lesions, and prolonged training in learning tasks
Jones does, that "Waking, which is normally character- involving painful electric shock. Since either LIA or
ized by low amplitude electrocortical activity is no RSA patterns can occur during Type 2 behavior, we
longer normal waking when this activity increases in have never claimed (contrary to Eichenbaum's state-
amplitude with alropine" is meaningless in the absence ment) that Type 2 behavior is a "correlate of slow-
of a behavioral definition of waking. As Ranck puts it, RSA," but merely that such RSA "sometimes accom-
"Arousal, or something like it, deserves a clear behav- panies waking immobility or other Type 2 behavior."
ioral definition independent of neural correlates, other- According to Vanderwolf (1975, p. 300) "One input to
wise the correlation (between arousal and neural activi- both hippocampus and neocortex is blocked by atro-
ty) cannot be tested." pine and stimulated by eserine and is essentially unre-
Jones's statement that reticulotelencephalic systems lated to concurrent motor activity."
are necessary for behavioral arousal appears to be An additional point which should be mentioned is
incorrect. Mesencephalic (Woods 1964) and thalamic that the pattern we have referred to as LIA is not
(Wang & Akert 1962) animals which lack a telencepha- identical in all conditions. Records such as those in Fig.
lon are often intensely active and "aroused." If lesions 3 suggest that a normal, waking, motionless rat displays
restricted to the brainstem reticular formation abolish somewhat more 5-7 Hz activity in its LIA pattern than
"behavioral arousal" then one must conclude that the a motionless atropinized rat or a normal rat in slow-
lesions have damaged something (e.g., reticulospinal wave sleep. This impression has recently been
fibers) in addition to the ascending projections to the confirmed by a power spectral analysis of hippocampal
telencephalon. activity in normal and atropinized rats by Leung,
Both Steriade and Vinogradova have objected to Lopes da Silva, and Wadman (Leung, personal
our reference to Batini et al. (1959) in relation to the communication). It may be that a low level of activity
evidence for the presence of LVFA during coma. We is present in ascending cholinergic reticulo-
included this reference since Loeb et al. (1959) hippocampal pathways in a waking, motionless rat.
mention that comatose human patients who display This results in greater power in the 5-7 Hz frequency
normal EEC. activity are often found to have damage band than is present during quiet sleep or in a motion-
to the lower brainstem, including the pons. Since the less atropinized rat. Under some circumstances,
pretrigeminal cat preparation is at least partly similar ascending cholinergic activity becomes sufficiently
to this (and helps to explain the clinical phenomenon) intense to produce a clear (atropine-sensitive) RSA
we included the reference. However, the point was not pattern. Thus, Komisaruk's view that the difference
discussed adequately, and these commentators are between "theta and nontheta activity may be quantita-
justified in their objections. tive rather than qualitative" is partially correct.
However, a qualitatively distinct factor appears to be
Two types of RSA and LVFA. The commentaries by introduced during Type 1 behavior, such as walking,
Eichenbaum and Komisaruk reveal some misunder- when a noncholinergic input produces RSA. Vertes,
standing of the relations of hippocampal activity to Vinogradova, and Shiromani & Fishbein do not agree
behavior which we hope to clarify. While we agree with the latter statement. They propose instead that
with Komisaruk (Malmo & Malnio also refer to this) there is only one reticulo-hippocampal pathway which
produces RSA and that this pathway is cholinergic.
that there is a degree of arbitrariness in classifying RSA
and LIA by inspection from oscillograph records, we Thus, Vinogradova, Brazhnik, Karanov, & Zhadina
do not think there is any ambiguity in our criteria for (1980) propose that low frequency RSA in anesthetized
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 501
Response/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
animals is due to an intraseptal generator which oscil- midbrain reticular formation in a waking rat produces
lates at a low frequency when the reticular formation is an atropine-sensitive RSA of 8-10 Hz during behavioral
depressed as result of the anesthetic. This RSA is immobility. On the other hand, small doses of pento-
sensitive to atropine. In the waking state, ascending barbital reduce the frequency of RSA accompanying
reticular activity drives the generator at a higher climbing behavior (Type 1) to a value below 7 Hz but
frequency, producing the higher frequency RSA the RSA retains its resistance to atropine nonetheless. In
observed during Type 1 behavior. This simple explana- general, it is true that the RSA accompanying Type 1
tion is inadequate since, as pointed out in the target behavior is atropine-resistant while any RSA occurring
article, the RSA which accompanies Type 1 motor during waking Type 2 behavior or anesthesia is atro-
activity in the waking state is resistant to atropinic pine-sensitive, regardless of the frequency of the RSA
drugs and to hemicholinium. Vertes attempts to (Kramis et al. 1975; Vanderwolf 1975).
circumvent this difficulty by assuming that ascending Vertes raises the further question of the significance
cholinergic reticular activity is particularly intense of atropine-sensitive RSA in rats, since ordinarily these
during Type 1 behavior and might therefore be more animals do not display clear RSA independent of
difficult to block with atropine. In this case, larger behaviors such as walking or head movement (Type 1)
doses of atropine might be more effective. However, in the waking state. One situation in which we claim
Vanderwolf (1975) mentions that atropine SO4 in doses normal rats display 5-7 Hz RSA with immobility is
of 100 mg/kg does not block the RSA accompanying during the nonphasic intervals of an episode of active
Type 1 behavior and even doses of 150 mg/kg are sleep. Vertes does not find predominantly low
ineffective (unpublished experiments). A dose of about frequency RSA at this time, although it has been
25 mg/kg is sufficient to abolish any RSA occurring reported by a number of other authors (Robinson et al.
during waking Type 2 behavior or during anesthesia. 1977; Soulairac et al. 1965; Usui & Iwahara. 1977;
Moreover, Vertes s hypothesis cannot account for the Whishaw & Vanderwolf. 1973). Vertes's failure to find
selective effect of hemicholinium, a drug which pre- a clear difference in RSA frequency between phasic
vents the synthesis of brain acetylcholine by blocking and nonphasic periods in active sleep might possibly be
the high affinity uptake of choline by cholinergic due to the use of an insensitive method for monitoring
neurons. If the RSA accompanying Type 1 behavior is phasic activity. The descending phasic excitatory
dependent on high levels of activity in ascending barrages onto motor neurons during active sleep affect
cholinergic neurons, it should be especially sensitive to a wide variety of muscle groups (e.g., limbs, trunk,
the effect of hemicholinium, since acetylcholine stores vibrissae, eyes) but all muscles are not active during
would be rapidly exhausted (Rommelspacher & Kuhar every phasic episode. Consequently, if monitoring is
1974). The low levels of ascending cholinergic reticular not extensive, some phasic events might be missed and
activity invoked to account for the RSA occurring in the associated hippocampal activity might be incor-
anesthetized animals should make such RSA relatively rectly classified as belonging to a nonphasic period.
resistant to the drug, according to Vertes s hypothesis. Robinson et al. (1977) used vibrissal EMG, a movement
In fact, the reverse is found. The RSA accompanying sensor, and close visual observation to determine when
Type 1 behavior is unaffected by intraventricular phasic episodes occurred. Nonphasic episodes were
injections of hemolinium while the RSA occurring in defined as periods when all three measures failed to
urethane anesthetized animals is abolished (Robinson & detect activity at a time at least 1 sec before or after a
Green 1980). Consequently, we think it is necessary to recorded phasic episode. Phasic episodes were defined
postulate the existence of two distinct RSA-generating as periods of motor activity lasting 1 sec or more. With
inputs to the hippocampus, although, as Vertes points these definitions, 6-7 Hz RSA was observed during
out, our information concerning these systems is rather nonphasic periods but RSA of 8 Hz or more was
limited. observed during phasic periods. Robinson et al. (1977)
An important clue to the understanding of the also showed that the RSA correlated with phasic events
atropine-resistant input to the hippocampus (which we is atropine-resistant while nonphasic RSA was atropine-
neglected to mention in the target article) is that large sensitive. Consequently, active sleep is an example of a
hypothalamic lesions abolish atropine-resistant RSA, if normal physiological condition in which rats generate
only temporarily, but "release" atropine-sensitive RSA atropine-sensitive RSA.
so that it occurs more readily than in a normal animal Whether atropine-sensitive RSA occurs in rats under
(De Ryck & Teitelbaum 1978; Kolb & Whishaw 1977; normal conditions in the waking state is less certain.
Whishaw & Kolb 1979). Stimulation of the hypothala- After prolonged shock avoidance training, low-
mus produces atropine-resitant RSA and vigorous Type frequency RSA occurs during immobility in animals
1 motor activity (Bland & Vanderwolf 1972; Kramis et that have not been subjected to drug treatment of
al. 1975). Therefore, ascending atropine-resistant surgical intervention beyond the implantation or
inputs to the hippocampus may arise in or pass through recording electrodes. Such RSA is atropine-sensitive
the hypothalamus. (Vanderwolf 1969, 1975; Whishaw 1972). It is also
In relation to the comments of Buzsaki et al., Vertes, possible that a cholinergic input to the hippocampus is
and Vinogradova, it must be pointed out that although active concurrently with a noncholinergic input during
atropine-sensitive RSA usually has a frequency of Type 1 behavior (Leung & Vanderwolf 1980). This
about 4-7 Hz and atropine-resistant RSA usually has a hypothesis is supported by the finding of Dudar,
frequency of 7-12 Hz, frequency in itself is not an Whishaw & Szerb (1979) that high levels of acetylcho-
infallible predictor of the response of a given RSA line release occur in the hippocampus in rats during
pattern to atropinic drugs. Thus, stimulation of the locomotion in a treadmill.
502 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
Response/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
Finally, Vertes suggests that LVFA, as well as RSA, but in a later paper they, and others, show that pontine
is controlled by a single ascending cholinergic reticular reticular nuclei, such as the nucleus pontis oralis or
system. The evidence which we have to support the nucleus pontis caudalis, contain low levels of musca-
concept of two distinct inputs to the neocortex which rinic receptors (Rotter, Birdsall, Field & Raisman 1979;
are independently capable of producing LVFA, is Wamsley, Lewis, Young & Kuhar 1981).
similar to the evidence supporting the concept of two
inputs to the hippocampus. Essentially, the LVFA Behavior and unit activity. As pointed out by Malmo &
correlated with Type 1 behavior is pharmacologically Malmo we omitted many studies on the relation of
distinct from the LVFA which often occurs during reticular unit activity to behavior, although it is not
Type 2 behavior. However, we note that neocortical true that we referred only to Vertes (1979). Our aim
evoked potentials vary iivrelation to hippocampal RSA was to confine the review primarily to reticulo-cortical
and Type 1 behavior in rats (Racine, Tuff & Zaide inputs involved in EEG activation.
1975; Schwartzbaum & Kreinick 1973). Consequently, However, studies on the behavioral relations of retic-
there may be electrophysiological criteria by which ular formation cells have been reviewed recently by
atropine-sensitive and atropine-resistant LVFA can be Siegel (1979a, 1979b) as we pointed out. Siegel reviews
distinguished without the use of drugs. studies which have related unit or multi-unit activity in
the reticular formation to a wide variety of processes.
Characteristics of ascending cholinergic systems. In These include: sensory processes, pain, conditioning,
reply to the points raised by Szerb & Dudar, we did not habituation, arousal, complex behavioral states, active
mean to imply that atropine necessarily exerts all of its sleep, eye movements, respiration, locomotion, and
effects on slow-wave activity by a direct action on the specific movements. Siegel shows that different func-
neocortex or hippocampus. In fact, we specifically tions have been ascribed to the same units by different
mentioned the possibility that subcortical cholinergic investigators. Consequently, many of these functions
synapses may play a role in the production of atropine- may have something in common. What is this common
sensitive RSA. However, this topic should have been factor? Arousal level might be such a common factor,
discussed more extensively and we thank Szerb & and many investigators have related reticular forma-
Dudar for doing this. Similarly, we thank Krnjevic and tion unit activity to arousal, as Malmo & Malmo and
Phillis for providing additional insights into the mech- Siegel point out. However, others have stressed the lack
anisms by which ascending cholinergic systems affect of a correlation between reticular unit activity and
hippocampal and neocortical activity. arousal. For example, in a study of multi-unit activity
We think that the sites at which atropine acts to in the mesencephalic reticular formation and thalamus,
abolish one form of neocortical LVFA and hippocam- Goodman & Mann (1967) concluded that activity levels
pal RSA have not been adequately identified. There were sometimes as high during anesthesia as in the
are many indications that acetylcholine normally waking state. It would be interesting to know whether
influences hippocampal and neocortical neurons, as the the electrophysiological phenomena Steriade describes
target article and several of the commentaries point as occurring during waking LVFA might not also occur
out. (Consequently, one might think that atropine exerts during LVFA periods in etherized animals. That is, are
its effects on slow-wave activity by a direct action in the single unit phenomena related to "arousal" level, as
the neocortex and hippocampus. Thus, Villablanca Steriade suggests, or are they related to an EEG wave-
refers to a study (Villablanca 1966/1967) showing that form independent of "arousal" level?
atropine increases slow-wave activity, while eserine From his studies in cats, Siegel (1979a) has suggested
decreases it, in a cerebral hemisphere isolated from the that the activity of most cells located in the medial
thalamus. In opposition to this, Szerb (1964) showed reticular core is actually correlated with the activity of
that topically applied atropine increases the efflux of specific muscle groups. Vertes (1979) has reported
acetylcholine from the neocortex but does not produce similar findings in rats. In rats many reticular forma-
slow waves. However, acetylcholine efflux from the tion units increase their rate of firing during specific
cortex may originate from more than one source (corti- behaviors and active sleep (phasic MOV-REM cells).
cal cholinergic afferents and intracortical cholinergic Vertes (1979) also described tonic MOV-REM cells
neurons). Therefore, it seems possible than an which increase their firing rate during active sleep or
increased acetylcholine efflux might appear when atro- whenever a Type 1 behavior occurs. Although Malmo
pine is applied topically even though deeply situated & Malmo suggest that the activity of these cells, "was
cholinergic synapses remain unblocked. Such synapses not specific to the kind of movement," it is specific in
might be sufficient to permit the persistence of LVFA. the sense that their activity is highly correlated with
Type 1, but not Type 2 behaviors (Vertes, personal
Kxperiments of the type discussed by Shiromani &
communication). Others have reported similar rela-
Fishbein suggest that systemically administered cho- tions to behavior using multi-unit recording techniques
linergic agonists or antagonists might affect cortical (Malmo & Malmo 1977; Schwartzbaum 1975).
activity and behavior by actions at a number of sites. It
is obvious that there is a great deal remaining to be In agreement with the conclusions drawn in the
learned. target article, the foregoing studies show that the
We note in passing that Shiromani & Fishbein are activity of many reticular formation units is related to
mistaken in claiming that Lewis et al. (1980) found a specific aspects of overt behavior and not to arousal in
high concentration of muscarinic receptor sites in the the traditional global sense. We suggest that some cells
pontine reticular formation. Lewis et al. do not (e.g., Vertes's [1979] tonic MOV-REM cells) may be
mention the pontine reticular formation specifically, involved in the activation of the hippocampus or the
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 503
Response/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
neocortex while others have primarily descending cal techniques can be more successfully applied to the
axons and are directly involved in specific movements. analysis of both brain activity and behavior.
No doubt many additional types of units will be found A final point that may be worth mentioning is that a
as well. large number of processes are simultaneously active
Malmo & Malmo also suggested that our conclusions during any natural behavior. If one wishes to study
about the relations of atropine-resistant neocortical and sniffing in relation to RSA for example, as recom-
hippocampal slow-wave activity to ongoing motor mended by Eichenbaum and by Komisaruk, it would
behavior would be strengthened if other recording be a mistake to restrict one's observations to the pattern
techniques yielded similar results. Although we did not of respiration, ignoring head movements, stepping, etc.
review such studies extensively, many investigators Respiration entrains itself readily to the occurrence of
have reported similar correlations using different tech- ongoing movement patterns (Wilke, Lansing & Rogers
niques. Ranck (Ranck 1973; Feder & Ranck 1973) was 1975). Therefore, it is possible that an apparent
the first to relate hippocampal unit activity to ongoing entrainment of sniffing to RSA may be a consequence
motor behavior. One class of cells (Ranck's theta cells) of a more fundamental entrainment of head-move-
is reported to show exactly the same relations to behav- ment to RSA. This would be missed if head-movement
ior as hippocampal RSA. More recently, O'Keefe and were not monitored accurately, perhaps using the
others (O'Keefe & Nadel 1978) have described the device recommended by Malmo & Malmo (Mundl &
same behavioral relations at the single-unit level, Malmo, 1979). In general, if one restricts one's observa-
although cells which fire in close relation to Type 1 tions unnecessarily, incorrect conclusions are likely to
movements are given different names by different result.
investigators (e.g., Ranck's "theta cell" is O'Keefe's
"displace cell"). Although many "nontheta" hippo- Interpretation of lesion experiments. While reticulo-
campal cells do not fire in relation to motor activity, cortical projections are not essential for sleep and
some do fire in relation to specific movements, or to waking when the entire cerebral cortex is removed,
movements which occur in a particular place (O'Keefe Sutherland et al. state, it is nonetheless possible that a
& Nadel 1978; Ranck 1973). In addition to hippocam- selective lesion of ascending reticulo-cortical fibres
pal slow-wave and unit activity, hippocampal evoked would produce an impairment of sleep or waking. We
potentials also vary as a function of motor activity agree. However, it is worth pointing out that if a
(Leung 1980; Winson & Abzug 1978). This is also true particular brain lesion results in the disappearance of
of some types of evoked potentials in the neocortex, as
some function, one cannot conclude that the damaged
was mentioned above (Racine, Tuff & Zaide 1975;
Schwartzbaum & Kreinick 1973). [See also O'Keefe & structures are essential to the function in question. It is
Nadel: "The Hippocampus as a Cognitive Map" BBS always possible that neurons in some other area, which
2(4) 1979.] are essential to the function, are depressed or inhibited
as a result of the lesion. However, if some function
Malmo & Malmo refer to a hypothesis of Teitel- survives despite the presence of a large lesion, one can
baum (1976), which assumes that atropine-sensitive conclude that none of the neurons which were
and atropine-resistant RSA might be related selectively destroyed are essential to that function. [See also
to the CA1 and dentate RSA generators. This is incor- Denenberg: "Hemispheric Laterality in Animals and
rect, since both types of RSA are present in both the Effects of Early Experience" BBS 4(1) 1981.]
generators (Whishaw et al. 1976). Therefore, it appears that there are no neural elements
in the cerebral cortex (including the elements that
generate the EEG) which are essential for the behav-
Possible influences on brain-behavior research. Ways ioral phenomena of sleep, arousal, waking, and circa-
in which our analysis of reticulo-cortical activity in dian rhythms. We do not dispute the fact that sleep and
relation to behavior might be applied to other topics in waking behaviors may be altered in various ways by
the broad field of behavioral research are suggested by decortication. However, the fact that decorticate
Calloway and by Carlson. Naturally, we welcome this animals often sleep in the open without walking to a
development. However, we would caution against any sheltered place first suggests a deficit in the stimulus
uncritical acceptance of Type 1 and Type 2 behaviors control of locomotion rather than a deficit in the
mechanisms of sleep.
as totally distinct groups. We agree entirely with
Sutherland et al. that our synthesis does not go very far Somjen suggests that the LVFA states which we
in providing an account of how the brain controls describe may not be dependent on reticular input to
behavior. Type 2 behaviors, in particular, are unlikely the forebrain since large lesions of the reticular forma-
to be a homogeneous class. We think that the most tion do not produce large slow waves in the neocortex,
general lesson which can be derived from our experi- provided that the lesions are produced gradually rather
ence is that brain-behavior research requires an induc- than suddenly. While we agree that this phenomenon is
tive approach involving extensive observation and of great interest from the point of view of recovery of
description of behavior as well as brain activity. As function, it is unlikely to be a reliable guide to the
Banck points out, our methods have been extremely function of the reticular formation under normal
simple, involving little more than visual inspection of conditions. The possibilities of neuronal sprouting
both behavior and brain electrical activity. Initially this (Lynch & Cotman 1975) and of denervation supersen-
was justified, since the possibilities of simple observa- sitivity (Stavraky, 1961) mean that the surviving parts
tion had not been adequately explored. When a good of a chronically damaged nervous system may not be
observational basis has been laid, we hope that analyti- functioning in the same way as they would under
504 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
References/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
normal circumstances. This difficulty does not apply to 3. We do not claim that "any brain state—must be
the effects of decortication discussed above. After a immediately translatable into easily interpretable overt
one-stage removal of the cerebral cortex, rats display movements." Neither do we state that "synchroniza-
waking behavior within a few hours. Sprouting and tion" is necessarily present during behavioral immobil-
denervation supersensitivity presumably would not ity or "gnawing on a pen." A large part of the target
have occurred in so short a time. article discusses atropine-sensitive RSA and LVFA
which, we have been at pains to show, has no immedi-
ate correlation with any type of motor activity. Vino-
A reply to Hirschman. Two main suggestions are made gradova seems to have overlooked this completely.
by Hirschman: (1) that hippocampal RSA is not of 4. Vinogradova is certain that her cortex is "de-
biological origin, but arises as a result of movement of synchronized" while she thinks about our paper. In the
the brain relative to the recording electrode, and (2) absence of direct evidence, we are unconvinced. It may
that the presence of RSA indicates that the hippocam- be that the neocortex is desynchronized much of the
pus is inactive. time when people claim to be thinking, but we should
With respect to the first point, the possibility of not forget that the presence of considerable alpha
recording RSA both extracellularly and intracellularly activity (synchronization) is compatible with the
under conditions in which the mechanical stability of performance of a variety of intellectual tasks (Creutz-
the electrode in the hippocampus is sufficient for feldt, Griinewald, Simonova & Schmiz 1967) and that
prolonged intracellular recording (Fujita & Sato 1964) thinking or dreaming can occur during slow-wave
shows that RSA can be recorded in the absence of sleep (Foulkes 1962). Perhaps slow waves can also
significant brain movement. In other words, movement occur during thinking in the waking state.
is not necessary for the occurrence of RSA. It can also 5. According to Vinogradova, perception, attention,
be shown that any brain movement which may occur motivation, decision making, etc. occur during volun-
during spontaneous motor activity in a freely moving tary movement but thinking can also occur during
animal is not sufficient to produce RSA. Thus, RSA behavioral immobility. Most people would agree with
cannot be recorded from the rat hippocampus during this. It seems to follow that if a given brain electrical
the isoelectric period following a hippocampal seizure pattern is found to be consistently present during
even though the rat walks about actively, and its brain, movement and absent during the absence of move-
presumably, continues to move to the same extent as it ment, then it must be related to the movement in some
does in the normal state. The same point can be made way and not to those psychological processes which are
with respect to the absence of RSA in a behaving presumed to occur both during movement and during
animal following a septal lesion. Consequently, we do immobility. An analysis based on this consideration was
not regard movement artifacts as an important prob- used extensively by Black (1975) to show that RSA in
lem in recording brain activity, provided that a stable, dogs and rats is related to motor activity rather than
well-designed recording system is used. However, inferred psychological processes. We recommend this
there is no question but that movement artifacts can be work to Vinogradova.
a tremendous problem if adequate methods are not 6. Contrary to Vinogradova s statement, theta (RSA)
used. does not necessarily disappear during prolonged prac-
With respect to the idea that the hippocampus is tice in a lever-pressing task (Frederickson & Whishaw
inactive during the presence of RSA, we suggest that it 1977).
is unlikely that large brain structures function in an 7. We do not know how to evaluate Vinogradova's
"off" and "on" manner. Research must be aimed at statement that curarized animals are highly aroused,
establishing the conditions under which various classes but we wish to point out that curarization produces a
of neurons are more or less active and the significance sharp increase in large amplitude slow-wave activity in
of such activity to brain function. We note that hippo- the neocortex (Hodes 1962).
campal pyramidal cells are not necessarily inactive 8. The statement that mice and rats have "narrow
when RSA is present (Bland, Andersen, Ganes & Sveen visual angles" puzzles us, since these animals have
1980; O'Keefe & Nadel 1978; Ranck 1975). almost circumferential visual fields.
9. Concerning trace amines, we recommend such
recent reviews and symposia volumes as Boulton
A reply to Vinogradova. The commentary by Vino- (1979), Mosnaim & Wolf (1978) and Usdin & Sandier
gradova includes a number of points which were also (1976).
raised by other commentators and have been answered
in the preceding sections. In addition, however, Vino-
gradova's commentary contains a number of References
unfounded assumptions and factual inaccuracies which
require detailed point-by-point corrections. Adam, H. M. & Hye, H. K. A. (1966) Concentration of histamine in different
parts of brain and hypophysis of cat and its modification by drugs. British
1. We do not state that arousal should be regarded Journal of Pharmacology and Chemotherapy 28:137-52. [CH V]
as an inferred process, but merely that some authors Adametz, J. H. (1959) Rate of recovery of functioning in cats with rostral retic-
(not all) appear to use it in this way. We recommend an ular lesions. Journal of Neurosurgery 16:85-98. [GGS]
objective descriptive approach to behavior. Adams, P. R. & Brown, D. A. (1980) Luteinizing hormone-releasing factor and
muscarinic agonists act on the same voltage-sensitive K* current in bull-
2. "Volition" is not discussed in the target article. frog sympathetic neurones. British Journal of Pharmacology 68:353-
The term "voluntary" is used as a descriptive label and 55. [KK]
does not refer to an inferred process. Adey, W. R. (1966) Neurophysiological correlates of information transaction
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 505
References/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
and storage in brain tissue. hv.Progress in phijsiological psychology, vol. 1, (1979) A gating function for the hippocampus in working memory. Behav-
ed. E. Stellar & J. M. Sprague, pp. 1-43, New York: Academic ioral and Brain Sciences 2:322-33. [TLB]
Press. [TLB] Berntson, G. G. & Micco, D. J. (1976) Organization of brain stem behavioral
Albanus, L.; Hammarstrom, L., Sundwall, A., Ulberg, S. & Vangbo, B. (1968) systems. Brain Research Bulletin 1:471-83. [CHV]
Distribution and metabolism of a H-atropine in mice. Acta Physiologica Biscoe, T. J. & Straughan, D. W. (1966) Micro-electrophoretic studies of neu-
Scandinaoica 73:447-56. [CHV] rones in the cat hippocampus. Journal of Physiology (London) 183:341-
59. [CHV|
Albanus, L.; Sundwall, A.; Vangbo. B. & Windbladli, B. (1968) The fate of
Black, A. H. (1975) Hippocampal electrical activity and behavior. In: The Hip-
atropine in the dog. Acta Pharmacotogica et Toxicologica 26:571-
pocampus, vol. 2: Neurophysiology and behavior, ed. R. L. Isaacson &
82. [CHV]
K. H. Pribram, pp. 129-167. Plenum Press, New York. [CHV, OSV]
Altman, J.; Brunner, H. L. & Bayer, S. A. (1973) The hippocampus and behav- Black, A. H.; Young, G. A. & Batenchuk, C. (1970) Avoidance training of hip-
ioral maturation. Behavioral Biology 8:557-96. [GH| pocampal theta waves in flexedilised dogs and its relation to skeletal
Anden, N. E.; Dahlstrcim, A.; Fuxe, K.; Larsson, K.; Olson, L. & Ungerstedt, U. movement. Journal of Comparative and Physiological Psychology 70:15-
(1966) Ascending monamine neurons to the telencephalon and diencepha- 24. [GH]
lon. Acta physiologica Scanclinavica 67:313-26. [CHV) Bland, B. H. & Vanderwolf, C. H. (1972a) Electrical stimulation of the hippo-
Andersen, I'. & Andersson, S. A. (1968) The physiological basis of the alpha campal formation: Behavioral and bioelectrical effects. Brain Research
rhythms. New York: Appleton-Century-Crofts. [JCS] 43:89-106. [GH]
(1972b) Diencephalie and hippocampal mechanisms of motor activity in the
Amiable, A. & Wearden, J. H. (1979) Grooming movements as operants in the
rat: Effects of posterior hypothalamic stimulation on behavior and hippo-
rat. Journal of the Experimental Analysis of Behavior 32:297-
campal slow wave activity. Brain Research 43:67-88. [CHV]
304. [CHV]
Bland, B. H. & Whishaw, I. Q. (1976) Generators and topography of hippo-
Arnolds, D. E. A. T.; Lopes da Silva, F. H ; Aitink.J. W. & Kamp, A. (1979a) campal theta (RSA) in the anesthetized and freely moving rat. Brain Re-
Hippocampal EEC and behavior in dog. I. Hippocampal EEC correlates search 118:259-80. [CHV]
of j;ross motor lx*havior, Electrocncephalof>raphy and Clinical Neuro- Bland, B. H.; Andersen, P. & Ganes, T. (1975) Two generators of hippocampal
physiology 46:552-70. [CHV] theta activity in rabbits. Brain Research 94:199-218. [CHV]
(1979b) Hippoeampal EEC and behavior in dog. II. Hip[)ocampal EEC cor- Bland, B. H. ; Andersen, P.; Ganes, T. & Sveen, O. (1980) Automated analysis
relates with elementary motor acts. Electrocncephalography and clinical of rhythmicity of physiologically identified hippocampal formation neu-
Neurophysiology 46:571-80. [CHV) rons. Experimental Brain Research 38:205-19. [GH]
(1979c) Hippocampal EEC and behavior in dog. 111. Hippocampal EEC cor- Bolme, P.; Fuxe, K. & Lidbrink, P. (1972) On the function of central catechol-
relates of stimulus-response tasks and of sexual behavior. Electro- amine neurons - their role in cardiovascular and arousal mechanisms. Re-
encephalography and clinical Neurophysiology 46:581-91. [CHV] search Communications in Chemical and Patlwlogical Pharmacology
Assaf, S. Y. & Miller, J. J. (1978) The role of a raphe serotonin system in the 4:657-97. [CHV]
control of septal unit activity and hippocampal desychronization. Neuro- Bonnet, V. & Bremer, F. (1937) Action de potassium, du calcium, et de
science 3:539-50. [CHVJ l'acetylcholine sur les activites electriques, spontanees et provoquees de
Azmitia, E. (1978) The serotonin-producing neurons of the midbrain median l'ecorce cerebrale. Comptcs Rendus de la Societe de Biologic (Paris)
and dorsal raphe nuclei. In: Handlxwk of psychopharmacologlj, o. 9, 126:1271-75. [CHV]
Chemical pathways in the lyrain, ed. L. L. Iverson, S. D. Iverson & S. H. Boulton, A. A. (1979) Trace amines in the central nervous system. Interna-
Snyder, pp. 233-314, New York: Plenum Press. [CHV] tional Review of Biochemistry, 26:179-206. [CHV]
Bard, P. & Hioch, D. Mck. (1937) A study of four cats deprived of neocortex Boulton, A. A.; Juorio, A. V.; Philips, S. R. & Wu, P. H. (1977) The effects of
and additional portions of forebrain. Bulletin of the Johns Hopkins Hos- reserpine and 6-hydroxydopamine on the concentration of some arylalkyl-
pital 60:73-147. [CHV] amines in rat brain. British Journal of Pharmacology 59:209-14. (CHV]
Bartus, R. T.; Dean, R. L.; Coas, J. A. & Lippa, A. S. (1980) Age-related Bouyer, J. J.; Montaron, M. F. & Rougeul, A. (1981) Fast fronto-parietal
changes in passive avoidance retention: Modulation with dietary choline. rhythms during combined focused attentive behaviour and immobility in
Science 209:301-03. [CHV) cat: Cortical and thalamic localizations. Electroencephalography and
Batini, C ; Moruzzi, G.; Palestini, M.; Rossi, G. F. & Zanchetti, A. (1959) Ef- Clinical Neurophysiology, In press. [AR]
fects of complete pontine transections on the sleep-wakefulness rhythm:
Bowker, R. M. & Morrison, A. R. (1977) The PGO spike: An indicator of hy-
The mid)X>ntine pretrigeminal preparation. Archives italiennes de Bio-
peralertness. European Congress on Sleep Research, 3d, Montpcllier,
logie 97:1-12. [CHV)
1976. ed. W. P. Koella and P. Levin. Sleep, 1976, memory, environment,
Batsel, H. L. (1960) Electroencephalographic synchronization and desynch-
epilepsy, sleep staging, pp. 23-27. Basel, New York, Karger. [CHV)
ronization in the chronic "cerveau isole" of the dog. Electroencephalogram
Bradley, P. B. (1968) The effect of atropine and related drugs on the EEG and
phy and Clinical Neurophysiology 12:421-30. [JRV]
behavior. Progress in Brain Research 28:3-13. [JRV)
(1964) Spontaneous desynchronization in the chronic cat. "cerveau isole."
Bradley, P. B. & Elkes, J. (1953) A technique for recording the electrical activ-
Archives Italiennes de Biologic. 102:281-306. [JRV]
ity of the brain in the conscious animal. Electroencephalography and clin-
Baust, W.; Niemczyk, H. & Vieth, J. (1963) The action of blood pressure on
ical Neurophysiology 5:451-56. [CHV]
the ascending reticular activating system with special reference to adren-
aline-induced EEC arousal. Electroencephalography and clinical Neuro- (1957) The effects of some drugs on the electrical activity of the brain. Brain
physiology 15:63-72. [CHV) 80:77-117. (CHV)
Baxter, B. L. (1969) Induction of both emotional behavior and a novel form Bradley, P. B. & Hance A. J. (1957) The effect of chlorpromazine and metho-
of REM sleep by chemical stimulation applied to cat mesencephalon. Ex- promazine on the electrical activity of the brain in the cat. Electroence-
perimental Neurology 23:220-30. [PS] phalography and clinical Neurophysiology 9:191-215. [CHV]
Beecher, H. K. & McDonough, F. K. (1939) Cortical action potentials during Bradley, P. B. & Nicholson, A. N. (1962) The effect of some drugs on hippo-
anesthesia. Journal of Neurophysiology 2:289-307. (CHV] campal arousal. Electroencephalography and clinical Neurophysiology
Bein, H. J. (1956) The pharmacology of Rauwolfia. Pharmacological Review 14:824-34. [CHV]
8:435-83. [CHV] Bremer, F. (1935) Cerveau "isole" et physiologie du sommeil. Comptcs Ren-
Belardetti, F.; Borgia, R. & Maneia, M. (1977) Prosencephalic mechanisms dus de la Societe de Biologic (Parts) 118:1235-41. (CHV)
of EEC desynchronization in "cerveau isole" of the cat. Electroencepha- (1936) Action de differents narcotiques sur les activites electriques spontanee
lography and Clinical Neurophysiology 42:213-25. (JRV] et reflexe du cortex cerebral. Comptes Rendus de la Societe de Biologic
Ben-Ari, Y.; Dingledine, R.; Kanazawa, I. & Kelly, J. S. (1976) Inhibitory ef- (Paris) 121:861-66. [CHV]
fects of acetylcholine on neurones in the feline nucleus reticularis thalami. (1937) L'activite cerebrale au cours du sommeil et de la narcose. Contribu-
Journal of Physiology 261:647-71. (JCS] tion a l'etude du mecanisme du sommeil. Bulletin de la Academic Royale
Ben-Ari, Y.; Krnjevic, K.; Reinhardt, W. & Ropert, N. (1981) Intracellular de Medicine de Belgique 4:68-86. [JRV]
observations on the disinhibitory action of acetylcholine in the hippocam- Bremer, F. & Chatonnet, J. (1949) Acetylcholine et cortex cerebral. Archives
pus. Neuroscicnce. In press. [KK] Internationales de Physiologie 57:106-9. [CH V]
Bennett, T. L. (1973) The effects of centrally blocking hippocampal theta Bricolo, A. (1975) Neurosurgical exploration and neurological pathology as a
activity on learning and retention. Behavioral Biology 9:541-52. [GH] means for investigating human sleep semiology and mechanisms. In: Ex-
(1975) The electrical activity of the hippocampus and processes of attention. perimental study of human sleep: Methodological problems, ed. G. C.
In: The hippocampus, vol. 2, ed. R. L. Isaacson & K. H. Pribram, pp. 7 1 - Lairy & P. Salzarulo, pp. 51-82. Amsterdam: Elsevier Scientific Publish-
99. New York: Plenum Press. [TLB, CHB] ing Company. (JRV]
506 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
References/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
Brimblecombe, l(. W. (1974) Drug actions on cholinergic systems. Baltimore: T. B. Mulholland, pp. 148-68. London: Butterworths. [CHV]
University Park Press. [CUV] Davies, P. & Maloney, A. J. F. (1976) Selective loss of central cholinergic neu-
Broadbent, 13. K. (1977) The hidden prealtentive processes. American Psychol- rons in Alzheimer's disease. Lancet 1403. [CHV]
ogist 32(2):109-I8. [EC] Davis, K. L.; Mohs, R. C ; Tinklenberg, J. R.; Pfefferbaum, A.; Hollisler, L. E.
Brodal, A. (1969) Neurological anatomy, pp. 304-344. New York: Oxford Uni- & Kopell, B. S. (1978) Physostigmine: Improvement of long-term memory
versity Press. [JRV] processes in humans. Science 201:272-74. [CHV]
Delgado, J. M. R. (1969) Physical control of the mind: Toward a psychocivil-
Brown, D. A. & Adams, P. R. (1980) Muscarinic suppression of novel voltage-
ized society. New York: Harper & Row. [NRC]
sensitive K* current in a vertebrate neurone. Nature, 283:673-76. [KK]
Delacour, J. (1980) Conditioned modifications of arousal and unit activity in
Brugge, J. F. (1965) An electrographic study of the hippocampus and neocor-
the rat hippocampus. Experimental Brain Research 38:95-101. [GH]
tex in unrestrained rats following septal lesions. Electroencephalography
Dell, P. C. (1958) Humoral effects on the brain stem retieular formations. In:
and clinical Neurophysiology 18:36-44. [CHV]
Retieular Formation of the Brain, eds. H. H. Jasper, L. D. Proctor, R. S.
Buchwald, N. A.; Horvath, K. E.\ Wyers, E. J. & Wakefleld, C. (1964) Electro- Knighton, W. C. Noshay fit R. T. Costello, Little, Brown & Co.: Boston,
encephalogram rhythms correlated with milk reinforcement in cats. Na- pp. 365-79. [CHV]
ture, (London) 201:830-31. [CUV]
(1958) Some basic mechanisms of the translation of bodily needs into behav-
Buser, P. (1980) Attention: A brief survey of some of its electrophysiological iour. In: Neurological basis of behaviour, ed. G. E. W. Wolstenholmc &
correlates. In: Functional states of the brain: Their determinants, ed. M. M. O'Connor, pp. 187-203. London: Churchill. [MS]
Koukkou, D. Lehmann & J. Angst, pp. 175-188. Amsterdam: Elsevier/ Dement, W. (1958) The occurrence of low voltage fast electroencephalogram
North Holland Biomedical Press. [JRV] patterns during behavioral sleep in the cat. Electroencephalography and
Buzsaki, C; Crastyan, K.; Czopf, J.; Kellenyi, L. & Prohaska, O. (1981 in press) clinical Neurophysiology 10:291-96. [CHV]
Changes of neuronal transmission in the rat hippocampus during behav- Dement, W. C. & Kleitman, N. (1957) Cyclic variations in EEC during sleep
ior. Brain Research. [BRK] and their relation to eye movements, body motility and dreaming. Elcc-
Buzsaki, ('..; Crastyan, E.; Tveritskaya, I. N. & Czopf, F. (1979) Hippocampal troencephalography and clinical Neurophysiology 9:673-90. [Cl IV]
evoked |)otcntials and EEC changes during classical conditioning in the Dement, W. C. & Miller, M. M. (1974) An introduction to sleep. In: Basic sleep
rat. Electroencephalography and Clinical Neurophysiology 47:64- mechanisms, ed. O. Petre-Quadens & J. D. Schlag. pp. 271-96. New York:
74. [CB] Academic Press. [CHV]
Buzsaki, C ; llaulx'nreiser, F.; Crastyan, E.; Czopf, F. & Kellenyi, L. (1981) Dement, W. C. & Villablanca, J. (1974) Clinical disorders in man and animal
Ilippocampal slow wave activity changes during conditioning in the cat. model experiments. In: Basic sleep mechanisms, ed. O. Petre-Quadens fit
Electroencephalography and Clinical Neurophysiology. In press. [GB] J. D. Schlag, pp. 328-31. New York and London: Academic Press. [JRV]
Callaway, E. & Band, H. 1. (1958) Some psychopharmacological effects of atro- De Ryck, M. & Teitelbaum, P. (1978) Neocortical and hippocampal EEC; in
pine: Preliminary investigation of broadened attention. AM A Archives of normal and lateral hypothalamic-damaged rats. Physiology and Behavior
Neurology 6 Psychiatry 79:91-102. [EC] 20:403-9. [CHV]
Dismukes, R. K. (1979) New concepts of molecular communication among
Callaway, E. & Dcmho, D. (195H) Narrowed attention: A psychological phe-
neurons. Behavioral and Brain Sciences, 2:309-448. [MS]
nomenon that accompanies a certain physiological change. AM A Archives
of Neurology O Psychiatry, 79:74-90. [EC] Dodd, J.; Dingledine, R. & Kelly, J. S. (1981) The excitatory action of acetyl-
choline on hippocampal neurones of the guinea pig and rat maintained in
Carlson, N. R., F.l-Wakil. F. W.; Standish, L. J. & Ormond, D. (1976) DRL |>er-
vitro.Brain Research 208: In Press. [KK]
formance, extinction, and secondary reinforcement: Effects of apjxMitive
Donchin, E. (1979) Event-related brain potentials: A tool in the study of hu-
value of fcxid in mice with septal lesions. Journal of Comparative and
man information processing. In: Evoked brain potentials and Ix'havior,
Physiological Psychology 90:780-89. (NRC)
ed. II. Begleiter. New York: Plenum Press. [EC]
Carlsson, A.; Lind(|vist, M. & Magnusson, T. (1957)3-4 Dihydroxyphenylala- Douglas, R. J. (1967) The hippocampus and behavior. Psychological Bulletin
nine and 5-liytlroxytryptophan as reserpine antagonists. Nature, (London)
67:416-42. [GH]
180:1200. [C1IV|
Dudar, J. D. (1975) The effect of septal nuclei stimulation on the release of
Celesia, G. C. fit Jasper, H. II. (1966) Acetylcholine released from cerebral cor- Ach from the rabbit hippocampus. Brain Research 83:123-33. [CHV]
tex in relation to state of activation. Neurology 16:1053-64. [HHJ.CHV] Dudar, J. D.; Whishaw, I. Q. fit Szerb, J. C. (1979) Release of acelylcholinc
Chase, M. 11. (1974) Somatic reflex activity during sleep and wakefulness: In: from the hip|)ocainpus of Irecly moving rats during sensory stimulation
Basic sleep mechanisms, ed. C). Petre-Quadens & J. D. Schlag, pp. 249- and running. Neuropharmacology 18:673-78. [JCS, C'HV]
67. [CHV] Eccles, J. (1961) Chairman's opening remarks. In: The nature of sleep, ed.
Chase, M. 11. & Har|x¥r, R. M. (1971)Somatomotor and visuomotor correlates G. E. W. Wolstenholme fit M. O'Connor, pp. 1-3. London: Church-
of o|x i rantly conditioned 12-14 c/sec sensorimotor cortical activity. Elec- ill. [MS]
troencephalography and clinical Neurophysiotogy 31:85-92. [CHV] Edstrom, J. P. & Phillis, J. W. (1980) A cholinergic projection from the glohus
Chatrian. C. E.; Petersen, M. C:. & Lazarte, J. A. (1959) The blocking of the ro- pallidus to cerebral cortex. Brain Research 189:524-29. [ J WP]
landic wicket rhythm and some central changes related to movement. Eibl-Eibesfeldt, I. (1970) Ethology: The biology of behavior. New York: Holt,
Electraenccphalography and Clinical Neurophysiology 11:497-510. Rinehart and Winston. [CHV]
[AH] Eichenbaum, II. B.; Macrides, F. it Shedlack, K. J. (1979) Relationship be-
('how, K. I.. & Randall, W. (1964) Learning and retention in cats with lesions tween hippocampal RSA and sniffing during reversal odor discrimination
in retieular formation. Psychonomic Science 1:259-60. [CCS] learning. Neurosciences Abstracts 5:273. [HE, BRK]
Elul, R. (1972) The genesis of the EEC. International Review of Ncuwlnology
Cocnen, A. M. L. (1975) Frequency analysis of rat hippocampal electrical ac-
15:227-72. [GGS]
tivity. Physiology and Behavior 14:391-94. [CHV]
Emson, P. C. & Lindvall, O. (1979) Distribution of putative neurolransmilters
Coleman, J. R. & Lindsley, I). B. (1975) Hippocampal electrical correlates of
in the neocortex. Neurosciencc 4:1-30. [JCS]
free Ix'havior and Ix'havior induced by stimulation of two hypothalamic-
Feder, R. & Ranck, J. R, Jr. (1973) Studies on single neurons in dorsal hippo-
hipixxampal systems in the cat. Experimental Neurology 49:506-28.
campal formation and septum in unrestrained rats. Part II. Hippoeampal
[CUV]
slow waves and theta cell firing during bar pressing and other Ix^haviors.
Cordeau, J. P.; dcChamplain, J. fit Jacks, B. (1971) Excitation and prolonged Experimental Neurology 41:532-55. [CHV]
waking produced by catecholamines injected into the ventricular system
Feldman, S. M. & Waller, H. J. (1962) Dissociation of electrocortical activation
of cats. Canadian Journal of Physiology and Pharmacology 49:627-31.
and behavioural arousal. Nature 196:1320-22. [CHV]
[CHV]
Foote, S. L. & Bloom, F. E. (1979) Activity of norepinephrine-containing locus
Covello, A.; De Barros-Kerreira, M. fit Lairy, G. C. (1975) Etude telemetrique coeruleus neurons in the unanesthelized squirrel monkey. In: Catechol-
des rythmes centraux chez 1'enfant. Electroencephalography and Clinical amines: Clinical basic interactions, vol. 1, ed. E. Usdin, pp. 625-27. New
Neurophysiology 38:307 19. [AR] York: Pergamon Press. [MS]
Creery, B. 1.. fit Bland, B. 11. (1980) Ontogeny of fascia dentata electrical activ- Foulkes, W. D. (1962) Dream reports from different stages of sleep. Journal of
ity and motor Ix'havior in the Dutch belted rabbit (Oryctolagus cunicu- Abnormal and Social Psychology 65:14-25. [CHV]
lus). Experimental Neurology 67:554-72. [CHV] Fowles, D. C. (1980) The three arousal model: Implications of Gray's two-
Creutzfeldt, O.; Criinewald. C ; Simonova, O. & Schmiz, H. (1967) Changes of factor learning theory for heart rate, electrodermal activity, and psycho-
tlie basic rhythms of the EEC during the performance of mental and vi- pathy. Psychophysiology 17:87-104. [RBM]
suomotor tasks. In: Attention in neurophysiology, ed. C. R. Evans and Fox, S. E. & Ranck, J. B. (1975) Localization and anatomical identification of
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 507
Re/erences/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
theta and complex spike cells in dorsal hippocampal formation of rats. Ex- during instrumentally conditioned appetitive behavior in the cat. Brain
perimental Neurology 49:299-313. [CH] Research 15:447-64. [CHV]
Frederickson, C. J. & Whishaw, I. Q. (1977) Hippocampal EEC during Halgren, E.; Babb, T. L. & Crandall, P. H. (1978) Human hippocampal forma-
learned and unlearned behavior in the rat. Physiology and Behavior, tion EEC desynchronizes during attentiveness and movement. Electroen-
18:597-603. [CHVJ cephalography and Clinical Neurophysiology 44:778-81. [RBM]
Frederickson, C. J.; Miczek, K. A.; Zurawin, R. A. & Frederickson, M. H. Harner, R. & Naquet, R. (1971) Altered states of consciousness, coma, cerebral
(1977) Hippocampal EEC correlates of intraspecies aggressive behavior in death. In: Handbook of electroencephalography and clinical neuro-
the rat. Brain Behavior and Evolution 14:352-67. [CHV] physiology, Vol. 12, ed. A. Remond. Amsterdam: Elsevier Publishing Co.
Fried, P. A. (1972) Septum and behavior: A review. Psychological Bulletin [JRV]
78:292-310. [NRC] Harper, R. M. (1971) Frequency changes in hippocampal electrical activity
Friedman, L. & Jones, B. E. (1981) Computerized classification by cluster anal- during movement and tonic immobility. Physiology and Behavior 7:55-8.
ysis of sleep-waking states in the cat. Abstract submitted to: Association for [CHV]
the Psychophysiological Study of Sleep. [BEJ] Hebb, D. O. (1955) Drives and The C.N.S. (Conceptual Nervous System). Psy-
Friedman, M. M. & Wikler, A. (1970) The effect of intrahypothalamic micro- chological Review 62:243-54. [CHV]
injection of hemicholinium (HC-3) on the hippocampal theta rhythm of (1972) Textbook of psychology. Philadelphia: W. B. Saundors Co. [RJS]
cats. Psychopharmacologia (Berlin) 17:345-53. [CHV] (1980) Essay on mind. Hillsdale, New Jersey: Lawrence Erlbaum Associates.
Fujita, Y. & Sato, T. (1974) Intracellular records from hippocampal pyramidal [CHV]
cells in rabbit during theta rhythm activity. Journal of Neurophysiology Hebb, C. O.; Krnjevic, K. & Silver, A. (1963) Effect of undercutting on the
27:1011-25. [CHV] acetylcholinesterase and choline acetyl transferase activity in the cat's
Funderburk, W. H. & Case, T. J. (1951) The effect of atropine on cortical po- cerebral cortex. Nature 198:692. [CHV]
tentials. Electroencephalography and clinical Neurophysiology 3:213- Hendricks, J. C ; Bowker, R. M. & Morrison, A. R. (1977) Functional charac-
23. [CHV] teristics of cats with pontine lesions during sleep and wakefulness and
Fuxe. K.; Grobecker, II. & Jonsson, J. (1967) The effect of /3-phenylethylamine their usefulness for sleep research. European Congress on Sleep Research,
on central and peripheral monamine-containing neurons. European Jour- 3d, Montpellier, 1976. In: Sleep, 1976; memory, environment, epilepsy,
nal of Pharmacology 2:202-7. [CHV] sleep staging, ed. VV. P. Koella & P. Levin, pp. 207-10. Basel, New York,
Fu.xe, K.; Hokfelt, T. & Ungerstedt, U. (1970) Morphological and functional Karger. [CHV]
aspects of central monoamine neurons. International Review of Neuro- Hendricks, J. C ; Morrison, A. R. & Mann, G. L. (1980) Pontine tegmental le-
Inology 13:93-126. [CHV] sions release similar behaviors in both wakefulness and paradoxical sleep.
Fuxe, K.; Lidbrink, P.; Hokfelt, T.; Bolme, P. & Goldstein, M. (1974) Effects of Abstracts, Society for Neurosdence 6:203. [CHV]
piperoxane on sleep and waking in the rat. Evidence for increased waking Henley, K. & Morrison, A. R. (1974) A re-evaluation of the effects of lesions of
by blocking inhibitory adrenaline receptors on the locus coeruleus. Acta the pontine tegmentum and locus coeruleus on phenomena of paradoxical
physiologica Scandinavica, 91:566-67. [CHV] sleep in the cat. Acta Neurohiologica Experimental 34:215-32. [CH V]
Gastaut, II.: Jus, A.; Jus, C ; Merrell, F.; Storm Van Leeuwen, W.; Dongier, S.; Henwood, R. W., Boulton, A. A. & Phillis, J. W. (1979) lemtophnretic studies of
Naquet. R.; Regis, H.; Roger, A.; Bekkering, D.; Kamp, A. & Werre, J. some trace amines in the mammalian CNS. Brain Research 164:347-51.
(1957) Etude topographique des reactions electroencephalographiques [JWP]
conditionnees chez 1'homme. Electroencephalography and Clinical Neu-
Herkenham, M. (1980) Laminar organization of thalamic projections to the rat
rophysiology 30:1-34. [AR]
neocortex. Science 207:532-35. [MS]
Gault, F. P. & Leaton, R. N. (1963) Electrical activity of the olfactory system.
Hess, R. (1964) The electroencephalogram in sleep. Electroencephalography
Electroencephalography and Clinical Neurophysiology 15:299-304.
and clinical Neurophysiology 16:44-55. [CHV]
[GH]
Hinde, R. A. (1970) Animal behavior: A syntlicsis of ethology and compara-
George, R.; Haslett, W. & Jenden, D. (1964) A cholinergic mechanism in the
tive psychology. 2nd Ed. New York: McGraw-Hill. [CUV]
brainstem reticular formation: Induction of paradoxical sleep. Interna-
tional Journal of Neuropliarmacology, 3:541-52. [PS] Hobson, J. A. & McCarley, R. W. (1977) The brain as a dream state generator:
An activation-synthesis hypothesis of the dream process. American Jour-
Glotzbach, S. F. (1975) Correlation of hippocampal theta activity and move-
nal of Psychiatry 134:1335-48. [PS]
ment during slow-wave sleep in cats. Behavioral Biology 15:485-90.
[CHV] Hobson, J. A.; McCarley, R. W. & Wyzinski, P. W. (1975) Sleep cycle oscilla-
tion: Reciprocal discharge by two brain stem neuronal groups. Science
Goodman, S. J. (1968) Visuo-motor reaction times and brain stem multiple-unit
189:55-58. [MS]
activity. Experimental Neurology 22:367-78. [RBM]
Goodman, S. J. & Mann, P. E. G. (1967) Reticular and thalamic multiple unit Hodes, R. (1962) Electrocortical synchronization resulting from reduced pro-
activity during wakefulness, sleep, and anesthesia. Experimental Neurol- prioceptive drive caused by neuromuscular blocking agents. Electroen-
ogy 19:11-24. [CHV] cephalography and Clinical Neurophysiology, 14:220-32. [CHV]
Cosselin, R. E.; Gabourel, J. D.; Kaiser, S. C. & Wills, J. H. (1955) The metabo- Hoover, D. B.; Muth, E. A. & Jacobowitz, D. M. (1978) A mapping of the dis-
lism of C " labelled atropine and tropic acid in mice. Journal of Pharma- tribution of acetylcholine, choline acetyltransferase and acetylcholinester-
cology and Experimental Therapeutics 115:217-29. (CHV] ase in discrete areas of rat brain. Brain Research 153:295-306. [CHV]
Gottesmann, C. L.; User, P. & Gioanni, H. (1980) Sleep: A physiological "cer- Houston, J. P. & Borbely, A. A. (1974) The thalamic rat: General behavior,
veau isole" stage. Waiting and Sleeping 4:111-19. [JRV] operant learning with rewarding hypothalamie stimulation, and effects of
Grandstaff, N. VV. (1969) Frequency analysis of EEC during milk drinking. amphetamine. Physiology and Beliavior 12:433-48. [JRV]
Electroencephalography and clinical Neurophysiology 27:57-65. [CHV] Hrdina, P. & Ling, C. M. (1973) Effects of desipramine and rcserpine on
Graslyan, E.; Karmos, G.; Vereczkey, L. & Kellenyi, L. (1966) The hippocam- "free" and "bound" acetylcholine in rat brain. Journal of Pharmacy and
pal electrical correlates of the homeostatic regulation of motivation. Elec- Pharmacology 25:504-07. [CHV]
troencephalography and Clinical Neurophysiology 2\ .34-53. [TLB] Hunter, B.; Zornetzer, S. F.; Jarvik, M. E. & McGaugh, J. L. (1977) Modulation
Grastyan, E.; Lissak, K.; Madarasz, 1. & Donhoffer, 11. (1959) Hippocampal of learning and memory: Effects of drugs influencing neurotransmitters.
electrical activity during the development of conditioned reflexes. Elec- In: Handbook of psychopharmacology, v. 8 Drugs neurotransmitters and
troencephalography and Clinical Neurophysiology 11:409-30. [GH] behavior, ed. L. L. lverson, S. D. Iverson & S. II. Snyder, pp. 531-77. New
York: Plenum Press. [CHV]
Green, J. D. & Arduini, A. (1954) Hippocampal electrical activity in arousal.
Journal of Neurophysiology 17:533-57. [CH, CHV] Hyviirinen, J.; Poranen, A. & Jokinen, Y. (1980) Influence of attentive behavior
Grill, H. J. & Norgren, R. (1978) Neurological tests and behavioral deficits in on neuronal responses to vibration in primary somatosensory cortex of the
chronic thalamic and chronic decerebrate rats. Brain Research 143:299- monkey. Journal of Neurophysiology 43:870-82. [MS]
312. [CHV] Isaacson, R. L. (1974) The limbic system. New York: Plenum Press. [TLB,
Grossman, S. P. (1976) Behavioral functions of the septum: A reanalysis. In: NRC]
The septal nuclei, ed. J. F. DeFrance, pp. 366-422. New York: Plenum Jacobowitz, D. M. & Palkovits, M. (1974) Topographical atlas of catechola-
Press. [NRC] mine- and acetylcholinesterase-containing neurons in the rat brain. 1.
(1978) An experimental dissection of the septal syndrome. Ciba Symposium Forebrain (telencephalon; diencephalon). Journal of Comparative Neu-
on Functions of the Septo-Hippocampal System 58:227-60. [NRC] rology 157:13-28. [CHV]
llaekett, J. T. & Marczynski, T. J. (1969) Postreinforcement electrocortical Jacobs, B. L. & Jones, B. E. (1978) The role of central monoamine and acetyl-
synchronization and enhancement of cortical photic evoked potentials choline systems in sleep-wakefulness states: Mediation or modula-
508 THE BEHAVIORAL AND BRAIN SCIENCES (1981). 4
References/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
lion? In: Cholincrgic-monoamincrgic interactions in the brain, ed. L. L. the adrenal medulla. Journal of Biological Chemistry 237:2311-17.
Butcher, pp. 271-90. New York: Academic Press. [BEJ, CHV] [CHV]
Jasper, H. H. (1966) Pathophysiological studies of brain mechanisms in dif- Klatzky, R. L. (1980) Human memory structures and processes. New York:
ferent states of consciousness. In: Brain and conscious experience, ed. J. C. W. H. Freeman & Co. [EC]
Eccles, pp. 256-82. New York: Springer-Verlag. [HHJ) Klemm, W. R. (1971) EEC and multiple-unit activity in limbic and motor sys-
tems during movement and immobility. Physiology and Behavior 7:337-
Jasper, H. H. & Koyama, I. (1967) Rate of release of acetylcholine and glu-
43. [RBM]
tamic acid from the cerebral cortex during reticular activation. Federa-
(1976) Physiological and behavioral significance of hippocampal rhythmic
tion Proceedings 26:373. [HHJ]
slow activity ("theta rhythm"). Progress in Neurobiology 6:23-47.
Jasper, H. H. & Krnjevic, K. (1969) Cholinergic mechanisms and amino acids
[CHV, OSV]
in cortical activation and arousal. American Physiological Society Sympo-
Klingberg, F. (1971) Hypersynchrony and learning. In: Biology of memory,
sium, "Neurohumoral Aspects of Sleep and Wakefulness," Atlantic City.
ed. G. Adam, pp. 299-305. New York: Plenum Press. [CHV]
[HHJ]
Knook, H. L. (1965) The fibre-connections of the forebrain. Assen: Van Gor-
Jasper, H. H. & Penfield, W. (1949) Electrocorticograms in man: Effect of vol- cum & Comp., N. V. [CHV]
untary movement upon the electrical activity of the precentral gyrus. Kobayashi, R. M.; Palkovits, M.; Kopin, I. J. & Jacobowitz, D. M. (1974) Bio-
Archives of Psychiatry and Neurology 183:163-74. [AR] chemical mapping of noradrenergic nerves arising from the rat locus
Jasper, H. H. & Tessier, J. (1971) Acetylcholine liberation from cerebral cortex coeruleus. Brain Research, 77:269-79. [CHV]
during paradoxical (REM) sleep. Science 172:601-02. [HHJ, CHV] Kolb, B. & Whishaw, I. Q. (1977) Effects of brain lesions and atropine on hip-
Jones, B. E. & Friedman, L. (1981) Disruption of the sleep-waking cycle fol- pocampal and neocortical electroencephalograms in the rat. Experimental
lowing large lesions of the pontine gigantocellular tegmental field in the Neurology, 56:1-22. [CHV]
cat. Abstract submitted to: Association for the Psychophysiological Study (1978) Decortication? Was Flourens correct? Society for Neuroscience Ab-
of Sleep. [BEJ] stracts 4:76. [RJS]
(1980) Fundamentals of human neuropsychology. San Francisco: W. H.
Jones, B. E.; Bobillier, P. & Jouvet, M. (1969) Effets de la destruction des neu-
Freeman and Co. [RJS]
rones contenant des catecholamines du mesencephale sur le cycle veille-
Komisaruk, B. R. (1970) Synchrony between limbic system theta activity and
sommeils du chat. Comptcs Rendus des Seances de la Societe de Biologie,
rhythmical behavior in rats. Journal of Comparative and Physiological
163:176-80. [CHV]
Psychology 70:482-92. [GH, BRK]
Jones, 15. E.; Bobillier, P.; Pin, C. & Jouvet, M. (1973) The effect of lesions of (1973) Hypothalamic influences on motor patterns. Neurosdences Research
catecholamine-containing neurons upon monoamine content of the brain Program Bulletin 11:376-81. [BRK]
and EEC and behavioral waking in the cat. Brain Research 58:157-77.
(1977) The role of rhythmical brain activity in sensorimotor integration.
[BEJ]
Progress in Psychobiology and Physiological Psychology 7:55-90.
Jones, IV E.; Harper, S. T. & Halaris, A. E. (1977) Effect of locus coeruleus le- [BRK]
sions upon cerebral monoamine content, sleep-wakefulness states and the Kopin, I. J. (1972) Metabolic degradation of catecholamines. The relative im-
response to amphetamine in the cat. Brain Research 124:473-96. [CHV] portance of different pathways under physiological conditions and after
Jouvet, M. (1962) Recherches sur les structures nerveuseset les mechanismes the administration of drugs. In: Handbook of experimental pharmacolo-
responsables des differentes phases du sommeil physiologique. Archives gy, v.33, Catecholamines. ed. H. Blaschko & E. Muscholl, pp. 270-82.
Italicnncs de Biologic 100:125-206. [JRV] New York: Springer-Verlag. [CHV]
(1967) Mechanisms of the states of sleep: A neuropharmacological approach. Kramis, R. & Vanderwolf, C. H. (1980) Frequency-specific RSA-like hippo-
In: Sleep and altered states of consciousness. Research Publications: As- campal patterns elicited by septal, hypothalamic, and brain stem electrical
sociation for Research in Nervous and Mental Disease, ed. S. S. Kety; E. stimulation. Brain Research 192:383-98. [CHV]
V. Evarlsand II. L Williams, Vol. 45, pp. 86-126. Baltimore: Williams Kramis, R.; Vanderwolf, C. H. & Bland, B. H. (1975) Two types of hippocam-
and Willcins. ICHV] pal rhythmical slow activity in lx>th the rabbit and the rat: Relations to be-
(1972) The role of monoamines and acetylcholine containing neurons in the havior and effects of atropine, diethyl ether, urethane and pentobarbilal.
regulation of the sleep waking cycle. Ergebnisse der Physiologic 64:166- Experimental Neurology 49:58-85. [CHV]
307. [CHV] Krnjevic, K. (1974) Chemical nature of synaptic transmission in vertebrates.
(1974) The role of monoaminergic neurons in the regulation and function of Physiological Reviews 54:418-540. [CHV]
sleep. In: Basic sleep mechanisms, eds. O. Petre-Quadens & J. D. Schag, (1977) Effects of substance P on central neurones in cats. In: Suljstancc P,
pp. 207-32. New York: Academic Press. [CHV] ed. U.S. von Euler, pp. 217-30. New York: Raven Press. [KK]
(1977) Neuropharmacology of the sleep-waking cycle. In: Handbook of Psy- Krnjevic, K. & Phillis, J. W. (1963a) Acetylcholine-sensitive cells in the cere-
chopharmacology, o. S. Drugs, Neurotransmilters and Behavior, ed. L. L. bral cortex. Journal of Physiology (London) 166:296-327. [CH V]
Iverson; S. D. Iverson & S. 11. Snyder, pp. 233-93. New York: Plenum (1963b) Pharmacological properties of acetylcholine sensitive cells in the
Press. [CHV] cerebral cortex. Journal of Physiology (London), 166:328-50. [CHV]
Jouvet, M. & Delorme, !•'. (1965) Locus coeruleus et sommeil paradoxal. Krnjevic, K.; Pumain, R. & Renaud, L. (1971) The mechanism of excitation by
Comptcs Rendus de la Societe dc Biologie (Paris) 159:895-99. [CH V] acetylcholine in the cerebral cortex. Journal of Physiology 215:247-68.
[KK]
Kales, A.; Bixler, E. & Kales, J. (1974) Role of the sleep research and treatment
Krnjevic, K.; Reiffenstein, R. J. & Ropert, N. (1980) Disinhibitory action of
facility: Diagnosis, treatment and education. Advances in Sleep Research
acetylcholine in the hippocampus. Journal of Physiology 308:73-741'.
1:391-415. [JRV]
[KK]
Kanai, T. & Szerb, J. C. (1965) Mesencephalic reticular activating system and
(1981) Disinhibitory action of acetylcholine in the rat's hippocampus: Extra-
cortical aeetylcholine output. Nature 205:80-82. [CHV]
cellular observations. Neuroscience. In Press. [KK]
Kandel, E. R. (1979) Simple forms of modulation: Attention, arousal and sensi- Kuhar, M. J. (1975) Cholinergic neurons: Seplal hippocampal relationships. In:
li/ution. Introduction. Neuroscicnccs Research Program Bulletin 17:539- The Hippocampus: Structure and development, ed. R. L. Isaacson &
41. [CHV] K. H. Pribram, Vol. 1, pp. 269-83. Plenum: New York. [CHV]
Kawamura, II. & Oshima, K. (1962) Effect of adrenaline on the hypothalamic Kuhar, M. J.; Sethy, V. H.; Roth, R. H. & Aghajanian, G. K. (1973) Choline: Se-
activating system. Japanese Journal of Physiology 12:225-33. [CHV] lective accumulation by central cholinergic neurons. Journal of Ncuro-
Kemp, I. R. & Kaada, B. R. (1975) The relation of hippocampal theta activity chemistry 20:581-93. [CHV]
to arousal, attentive behavior, and somato-motor movements in unre- Kuhlman, W. (1978) Functional topography of the human mu rhythm. Elec-
strained cats. Brain Research 95:323-42. [TLB, JRV, CHV] troencephalography and Clinical Neurophysiology 440:83-93. [AR]
Kinible, D. P. (1968) Hippocampus and internal inhibition. Psychological Bul- Kuypers, H. G. J. M. (1964) The descending pathways to the spinal cord, their
letin 70:285-95. [GH] anatomy and function. Progress in Brain Research 11:178-202. [CHV]
Larsson, L. E. (1960) Correlation between the psychological significance of
Kimura, D. (1962) Multiple response of visual cortex of the rat to photic stimu-
stimuli and the magnitudes of the startle blink and evoked EEC potentials
lation. Elcctrocnccpfialography and clinical Neurophysiology 14:115-22.
in man. Acta physiologica Scandinavica 48:276-94. [CHV]
[CHV]
Lashley, K. S. (1929) Brain mechanisms and intelligence. Chicago: University
Kimura, II.; McCeer, P. I..; Peng, F. & McCeer, E. G. (1980) Choline acetyl- of Chicago Press. [CHV]
transferace-containing neurons in rodent brain demonstrated by immuno- (1935) Studies of cerebral function in learning XI. The behavior of the rat in
histochemistry. Science 208:1057-59. [CHV] latch box situations. Comparative Psychology Monographs XI (#2),
Kirschner, N. (1962) Uptake of catecholamines by a participate fraction of 1-42. [CHV]
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 509
References/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
Lehmann, J.; Nagy, J. I.; Atmadja, S. & Fibiger, H. C. (1980) The nucleus ba- (1965) Physiological gradients and behavior. Psychological Bulletin 64:225-
salis magnocellularis: The origin of a cholinergic projection to the neucor- 34. [RBM]
tex of the rat. Neurosdcnce 5:1161-74. (JCS] Malmo, R. B. & Belanger, D. (1967) Related physiological and behavioral
Leung, L. S. (1980) Behavior-dependent evoked potentials in the hippocampal changes: What are their determinants? Sleep and altered states of con-
CA1 region of the rat. I. Correlation with behavior and E E C Brain Re- sciousness. Research Publications: Association for Research in Nervous
search 198:95-117. [CHV] and Mental Disease, Baltimore: Williams & Wilkins, 45:288-313.
Leung, L. S. & Vanderwolf, C. H. (1980) Behavior-dependent evoked poten- [CHV]
tials in the hippocampal CA1 region of the rat. II. Effect of eserine, atro- Malmo, H. P. & Malmo, R. B. (1977) Movement-related forebrain and mid-
pine, ether and pentobarbital. Brain Research 198:119-33. [CHV] brain multiple unit activity in rats. Elcctroencephalography and Clinical
Lewis, P. R. & Shute, C. C. D. (1967) The cholinergic limbic system: Projection Neurophysiology 42:501-09. [RBM]
to hippocampal formation, medial cortex, nuclei of the ascending cholin- Mantegazzini, P.; Poeck, K. & Santibanez, G. (1959) The action of adrenalin
ergic reticular system, and the subfornical organ and supra-optic crest. and nor-adrenaline on the cortical electrical activity of the encephale isole
Brain 90:521-40. [CHV] cat. Archives italicnncs dc Biologic 97:222-42. [CHV]
(1978) Cholinergic pathways in CNS. In: Handbook of Psychopharma- Marczynski, T. J. & Hackett, J. T. (1969) Postreinforcement electrocortical
cology, v. 9, Chemical pathways in the brain, ed. L. L. Iverson; S. D. Iver- synchronization and facilitation of cortical somato-sensory evoked poten-
son & S. H. Snyder, pp. 315-55. New York: Plenum Press. [CHV] tials in appetitive behavior in the cat. Elcctroencephalography and clini-
Lewis, P. R.; Shute, C. C. D. & Silver, A. (1967) Confirmation from choline cal Neurophysiology 26:41-9. [CHV]
acetylase analyses of massive cholinergic innervation to the rat hippocam- Marczynski, T. J.; Rosen, A. J. & Hackett, J. T. (1968) Postreinforcement
pus. Journal of Physiology (London) 191:215-24. [CHV] electro-cortical synchronization and facilitation of cortical auditory
Lewis. M.; Wamsley, J. K.; Young, W. S. & Kuhar, M. J. (1980) Autoradiogra- evoked potentials in appetitive instrumental conditioning. Electrocnce-
phic localization of muscarinic cholinergic receptors in rat brain stem. So- phalography and clinical Neurophysiology 24:227-41. [CHV]
ciety for Ncuroscience Attracts 6:515. [PS]
Massopust, L. C , Jr.; White, R. J.; Albin, M. S.; Yashoun, D. & Talitz, N. (1968)
Li. C.-L. & Jusper, H. (1953) Microelectrode studies of electrical activity of Electrical activity of the isolated macaque brain. Experimental Neurol-
cerebral cortex in the cat. Journal of Physiology 121:117-40. [GH]
ogy 22:303-25. (JRV]
Lidbrink. P. (1974) The effect of lesions of ascending noradrenaline pathways
Mays, L. E. it Best, P. J. (1975) Hippocampal unit activity to tonal stimuli dur-
on sleep and waking in the rat. Brain Research 74:19-40. [CHV]
ing arousal from sleep and in awake rats. Experimental Neurology
Lidsky, T. 1.; Levine, M. S. & MacGregor, S. (1974) Hippocampal units during
47:268-79. [GH]
orienting and arousal in rabbits. Experimental Neurology 44:171-86.
McLennan, H. & Miller, J. J. (1976) Frequency-related inhibitory mechanisms
[CH]
controlling rhythmical activity in the septal area. Journal of Physiology
Lindevall, O. & Bjorklund, A. (1978) Organization of catecholamine neurons
254:827-41. [JCS]
in the rat contra! nervous system. In: Handlxtok of psychopharmacology,
v. 9, Chemical pathways in the brain, ed. L. L. Iverson, S. D. Iverson & S. McGinty, D. J. (1973) Neurochemically defined neurons: Behavioral correlates
of unit activity of serotonin-containing cells. In: Brain unit activity dur-
H. Snyder, pp. 139-231. New York: Plenum Press. [CHV]
ing behavior, ed. M. I. Phillips, pp. 244-287. Springfield, Illinois: Ch. C.
Lindsley, D. B. (1951) Emotion. In: Handbook of experimental psychology,
Thomas. [MS]
ed. S. S. Stevens, pp. 473-516. New York: Wiley. [CHV]
(1960) Attention, consciousness, sleep and wakefulness. In: Handbook of McLean, J. P. & Shulman, G. L. (1978) On the construction and maintenance
Physiology. Section I: Neurophysiology, o. 3., ed. J. Field; H. W. Magoun of expectancies. Quarterly Journal of Experimental Psychology 30:441-
& V. E. Hall, pp. 1553-93. Washington, D.C.: American Physiological So- 54. [EC]
ciety. [CHV] Melamed, E.; Lahav, M. & Atlas, D. (1977) 0-Adrenergic receptors in rat cere-
Lippold, O.C.J. (1973) The origin of the alpha rhythm. London: Churchill bral cortex: Histochemical localization by a fluorescent /3-blocker. Brain
Livingston. [CH] Research 128:379-84. [CHV]
Loeb, C ; Rosadini, G. & Poggio, G. F. (1959) Electroencephalograms during Mercer, L. F ; Remley, N. R. & Oilman, D. P. (1978) Effects of urethane on
coma: Normal and borderline records in 5 patients. Neurology 9:610-18. hippocampal unit activity in the rat. Brain Research Bulletin 3:567-70.
[CHV] (GH]
Longo, V. C. (1971) Effects of drugs on the EEC. In: Handbook of electroen- Miller, R. F.; Stavraky, C. W. & Woonton, G. A. (1940) The effects of eserine,
eephalography and clinical nettrophijsiology. Vol. 7 (pt. C), ed. A. Re- acetylcholine, and atropine on the electrocorticogram. Journal of Neuro-
mnnd. Amsterdam: Elsevier Publishing Co. [JRV] physiology 3:131-38. [JCS, CHV]
(1962) Rabbit Inain research, v. 2. Amsterdam: Elsevier. (CHV] Miller, S. W. & Groves, P. M. (1977) Sensory evoked neuronal activity in the
(1966) Behavioral and electroecephalographic effects of atropine and related hippocampus before and after lesions of the medial septal nuclei. Physiol-
compounds. Pharmacological Review 18:965-96. [CHV] ogy and Behavior 18:141-46. [GH]
Longo, V. C. & Silvestrini, C. (1957) Action of eserine and amphetamine on Mink, W. D.; Best, P. J. & Olds, J. (1967) Neurons in paradoxical sleep and mo-
the electrical activity of rabbit brain. Journal of Pharmacology and Ex- tivated behavior. Science 158:1335-37. (GH]
perimental Therapeutics 120:160-70. [CHV] Mirsky, A. F. & Pragay, E. B. (1967) The relation of EEC and performance in
Lorenz, K. Z. (1973) The fashionable fallacy of dispensing with description. altered states of consciousness. Research Publications, Association for Re-
Die Naturwissenschaften 60:1-9. [CHV] search in Nervous and Mental Disease 45:514-34. [CHV]
Luciani, L. (1915) Human physiology. London: Macmillan and Co., Ltd.
Monmaur, P.; Houcine, O. & Delacour, J. (1979) Experimental dissociation be-
[HJS] tween wakefulness and paradoxical sleep hippocampal theta. Physiology
Oakley, D. A. (1979) Neocortex and learning. Trends in NeuroSciences 2:149- and Behavior 23:471-79. [CHV]
52. [HJS]
Monnier, M. & Romanowski, W. (1961) Les systemes cholinoceptifs cere-•
Lynch, C. & Cotman, C. W. (1975) The hippocampus as a model for studying
braux - actions de I'acetylcholine, de la physostigmine, pilocarpine et de
anatomical plasticity in the adult brain. In: The hippocampus, vol. 1: GABA. Electroencephalography and clinical Neurophysiology 14:486-
Structure and development, ed. R. L. Isaacson & K. H. Pribram, pp. 123- 500. [CHV]
54. New York: Plenum Press. [CHV]
Montplaisir, J. Y. (1975) Cholinergic mechanisms involved in cortical activa-
Macadar, A. W.; Chalupa, L. M. & Lindsley, D. B. (1974) Differentiation of
tion during arousal. Electroencephalography and clinical Neurophysi-
brainstem loci which affect hippocampal and neocortical electrical activi-
ology 38:263-72. [CHV]
ty. Experimental Neurology 43:499-514. [GH, CHV]
Moore, R. Y. (1975) Monamine neurons innervating the hippocampal forma-
Macrides, F. (1975) Temporal relationships between hippocampal slow waves
tion and septum: Organization and response to injury. In: The hippocam-
and exploratory sniffing in hamsters. Behavioral Biology 14:295-308.
pus, vol. 1, Structure and development, ed. R. L. Isaacson & K. H. Pri-
(HE, GH, BRK)
bram, pp. 215-237. New York: Plenum Press. [CHV)
Magoun, H. W. (1954) The ascending reticular system and wakefulness. In:
Morris, R. G. M. & Black, A. H. (1978) Hippoeampal electrical activity and be-
Brain mechanism and consciousness, ed. J. F. Delafresnaye, pp. 1-53.
havior elicited by nonreward. Behavioral Biology 22:524-32. [CHV]
Oxford: Blackwell Scientific Publications. [JRV]
Malhotra, C. L. & Pundlik, P. G. (1959) The effect of reserpine on the acetyl- Morrison, A. R. (1979) Relationship between phenomena of paradoxical asleep
choline content of different areas of the central nervous system of the dog. and their counterparts in wakefulness. Ada Neurohiologiac Experimen-
British Journal of Pharmacology and Chemotherapy 14:46-50. [CHV] tal 39:567-83. [OSV]
Malmo, R. B. (1959) Activation: A neuropsychological dimension. Psychologi- Moruzzi, G. (1972) The sleep-waking cycle. Ergclmissc der Physiologic 64:1—
cal Review 66:367-86. [CHV] 165. [JRV]
510 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
References/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
(1972) The sleep-waking cycle, Ergelmissc der Physiologic 64:1-165. [CHV, Phillis, J. W. (1908) Acelylcholine release from the cerebral cortex: Its role in
JRV] cortical arousal. Brain Bescarch 7:378-89. [JWP, CHV)
(1974) Neural mechanisms of the sleep-waking cycle. In: Basic sleep mecha- (1974) Evidence for cholinergic transmission in the cerebral cortex. Ad-
nisms, ed. O. Petre-Quadens it J. D. Schlag, pp. 13-31. New York and vances in Behavioral Biology 10:57-77. [JWP]
London: Academic Press. [JRV] (1974) Evidence for cholinergic transmission in the cerebral cortex. In: Neu-
Mnruzzi, C. 4 Mugoun, II. VV. (19-19) Brainstem reticiilar formal ion and acti- rohumoral coding of brain function, ed. R. D. Myers & ft. R.
vation of the KEG. Eleetrovncephalography and clinical Neurophysi- Drucker-Colin, pp. 57-77. New York: Plenum. [CHV]
ology 1:455-73. [CllVj (1976) Acetylcholine and synaptic transmission in the central nervous sys-
Mosivaim, A. D. & Wolf. M. E. (1978) Noncatecholic phcnylethylamines. Part tem. In: Chemical transmission in the mammalian central nervous sys-
I. Phcnylethylaminc: Biological mechanisms and clinical aspects. New tem,ed. C. H. Hockman & D. Bieger, pp. 159-213. Baltimore: University
York: Marcel lJekker. Inc. [CHV] Park Press. [CHV]
Mulholland, T. B. (1969) The concept of attention and the electroencephalo- Phillis, J. W. it Jhamandas, K. (1971) The effects of chlorpromazine and etha-
graphic alpha rliylhm. In: Attention in neurophysiology, ed. C. H. Evans nol on in vivo release of acetylcholine from the cerebral cortex. Compara-
& T. B. Mnlhollund, pp. 100-27. London: Buttcrworths. [CHV] tive and General Pharmacology 2:306. [CH V]
Miillin, VV. J. & Phillis, J. W. (1975) The effect of graded forelimb afferent vol- Phillis, J. W. & Kirkpatrick, J. R. (1980) The actions of motilin, luteini/.ing hor-
leys on acetyleholine release from cal sensorimotor cortex. Journal of mone, releasing hormone, cholecystokinin, somatostatin, vasoactive intes-
Physiology (London) 244:741-56. [ J WP] tinal peptide, and other peptides on rat cerebral cortical neurons. Cana-
dian Journal of Physiology and Pharmacology 58:612-23. [ J W P |
Mundl, W. J. it Malmo, II. P. (1979) An accelerometer for recording head
movement of lalxiratory animals. Physiology and Behavior 23:391-93. Phillis, J. W. & Kostopoulos, G. K. (1977) Activation of a noradrenergic path-
[RBM] way from the brain stem to rat cerebral cortex. General Pharmacology
8:207-11. [MS]
Naneishvili, T. I..; Bakuradzc, A. N.; Noselidze, A. G.; Aragveli, R. I. & Dash-
niani, M. C. (1975a) Kleclrical activity of the cat neocorlex after premes- Phillis, J. W. & York, D. H. (1968) Pharmacological studies on a cholincrgic in-
encephalic transection of llie brain stem in chronic experiments. Filmo- hibition in the cerebral cortex. Brain Besearch 10:297-306. [JWP]
logicheskii Zhumal SSSR imeni I.M. Sechenova (Moskva) 61:1273-80. Pickenhain, L. & Klingberg, F. (1965) Behavioural and electrophysiological
[JRV] changes during avoidance conditioning to light flashes in the rat. Elcctro-
encephalography and clinical Neurophysiology 18:464-76. [CHV]
Naneishvili, T. L.; Bakurudze, A. N.; Noselidize, A. G. & Dashniani, M, G.
(19751)) The role of the posterior hypothalamus in the activation of neo- Pompeiano, O. (1967) The neurophysiological mechanisms of the postural and
cortex in the chronic premesencephalic cats. Izccstiia Akademix Nauk motor events during desynchronized sleep. Research Publications. Asso-
SSSR.. Biological Series 1:215-16. [JRV] ciation for Besearch in Nervous and Mental Disease 45:351 -423.
[CHV]
Noda, M.; Manohar, S. & Adey, W. R. (1969) S|X)iitaneous activity of cat hip-
pocampal neurons in sleep anil wukefillness. Experimental Neurology Pompeiano, O. (1973) Beticular Formation. In: Handbook of sensory physiol-
24:217-31. [CM] • ogy, vol. 2, ed. A. Iggo, pp. 381-488. Berlin: Springer-Verlag. [JWP]
O'Keefe, J. (1976) Place units in the hippocampus of the freely moving rat. Ex- Posner, M. I. (1978) Chronometric explorations of mind. Hillsdalc, N.J.: Law-
perimental Neurology 51:78-109. [HE] rence Erlbaum Associates, Inc. [EC]
Potkin, S. G.; Karoum, F.; Cluiang, L. W.; Cannon-Spoor, H. E.; Philips, I. bi
O'Keefe, J. & Dostrovsky, J. (1971) Tlu* hippocampus as a spatial map. Prelim-
Wyatt, R. J. (1979) Phenylethylamine in paranoid chronic schizophrenia.
inary evidence from unit activity in the freely moving rat. Brain Research
Science, 206:470-71. [CH V)
3-4:171 75. [CI1]
Pribram, K. H. (1977) Modes of central processing in human learning and re-
O'Keefe, J. & Nadel, L. {1978) 77M' hippocampus as a cognitive map. Oxford:
membering. In: Brain and learning, ed. T, H. Teyler. Connecticut: Grey-
Clarendon Press. [CUV]
lock Publishers. [EC]
(1979) Precis of O'Keefe & NadeTs The hippocampus as a cognitive map. Pribram. K. H. & McCiiinness. D. (1975) Arousal, activation, and effort in the
The Behavioral and lira in Sciences 2:487-533. [KBM]
control of attention. Psychological Beview 82:116-49. (RBM]
Ohnstead, C. K. & Villahlanca, J, H. (1977) Hippocampal theta rhythm persists
in the permanently isolated forebrain of the cat. Brain Research Bulletin Rabbitt, P. (1979) Some experiments and a model for changes in attcntional se-
2:93-100. [GB, JRV] lectivity with old age. Bayer-symposium VII Inain function in old age.
pp. 82-94, New York: Springer-Verlag, Inc. [EC]
Ott, T.; Malisch, R. & Krug, M (1977) Pharmacological analysis of hippocam-
Racine, R.; Tuff, L. & Zaide, J. (1975) Kindling, unit discharge patterns and
pal "theta rhythm." Ada Neurolriologica Experimental 36:720.
neural plasticity. Canadian Journal of Neurological Sciences, 2:395-405.
(CHV]
[CHV)
Purmeggiani, V. I,.; Azzaroni, A. At Kenzi, P. (1967) On the functional signifi-
Radii-Weiss, T.; Zernicki, B. & Michalski, A. (1976) Hippoeampal theta activ-
cance of the hippocampal theta-rhythm. In: Structure and function of
ity in acute pretrigeminal cat. Ada Neurolnologie Experimentalis
the limlric system. Progress in Itrain research. Vol. 24, ed. VV, R. Adey &
36:517-34. [GB]
T. Tokizane, pp. 413-41. Amsterdam: Elsevier Publishing Co. [JRV]
Ramm, P. (1979) The locus coeruleus, catecholamines and REM sleep: A criti-
Penfield, VV, & Jasper, M. (1954) Epilepsy and the functional anatomy of the
cal review. Behavioral and Neural Biology 25:415-48. [CH V]
human itrain, p. 188, Boston; Little, Brown & Co. [CHV]
Ranck, J. B. (1973) Studies on single neurons in dorsal hippocampal formation
Pepeu, G. bi Bartolini, A, (1968) Effect of psychoactive drugs on the output of
and septum in unrestrained rats. Part I. Behavioral correlates and firing
acetylcholine from the cerebral cortex of the cat. European Journal of
repertoire. Experimental Neurology 40:461-555. [GH, CH V]
Pharmacology 4:254-63. [CM V]
(1973) Studies on single neurons in dorsal hippocampal formation and sep-
IV[X*ut C ; Mantovani, P. & Pedatu, F. (1978) Drug stimulation of acetylcho- tum in unrestrained rats. Part I. Behavioral correlates and firing reper-
line output from the cerebral cortex. In: Cholincrgic mechanisms and toires. Experimental Neurology 41:461-531. [HE, CHV]
psychopharmacology, ed. D. J. Jenden, pp. 605-614. New York: Plenum
(1975) Behavioral correlates and firing repertoires of neurons in the dorsal
Press. [CMV]
hippocampal formation and septum of unrestrained rats. In: The hippo-
Perry, E. K.; Perry, R.; Blessed, G. & Tomlinson. B. (1977) Necropsy evidence campus: Vol. 2: Neurophysiology and behavior, ed. R. L. Isaacson it K. H.
of central cholinergic deficits in senile dementia. Lancet Jan. 22, p. 189. Pribram, pp. 207-44. New York: Plenum. [PS, CHV]
[CHV] Ratliff, F. (1962) Some interrelations among physics, physiology, and psychol-
Perry, E. K.; Perry. It. II,; Gibson, P. H.; Blessed, G. & Tomlinson, B. E. (1977) ogy in the study of vision. In: Psychology: A study of science. Study II.
A cholinergic connection Ix'tueen normal aging and senile dementia in Empirical substructure and relations with other sciences, vol. 4: Biologi-
the human hip|xx?ampus. Neuroscience Letters 6:85-9. [CHV] cally oriented fields: Their place in psychology and biological science, ed.
Perry, K. K.\ Tomlinson, B. E.; Blessed, G.; Bergman, K.; Gibson, P. H. & Per- S. Koch, pp. 417-82. New York: McGraw-Hill. [CHV]
ry, B. II. (1978) Correlation of cholinergic abnormalities with senile Reader, T.; Ferron, A.; Descarries, L. it Hasper, H. (1979) Modulatory role for
plaques and mental test scores in senile dementia. British Medical Journal biogenic amines in the cerebral cortex. Microiontophoretic studies. Brain
2:1457-9. [CI!V| Besearch 160:217-29. [HHJ]
Petsche, 11.; Stumpf, Cli. tt Gogolak. G. (1962). The significance of the rabbit's Reinstein, D. K.; Hannigan, J. H., Jr. & Isaacson, R. L. (1981) Behavioral
septum as a relay station Ix'tween the midbrain and the hippocampus. 1. changes after hippoeampal destruction involve forebrain dopaminergic
The control of hippocampus arousal activity by the septum cells. Electro- systems. Submitted. [GB]
encephalography and Clinical Neurophysiology 14:202-11. [JCS] Reisine, T. D.; Yamamura, H. I.; Bird, E. D.; Spokes, E. & Enna, S. J. (1978)
THE BEHAVIORAL AND BRAIN SCIENCES (1981). 4 511
References/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
Pro- and postsynaptic neurochemical alterations in Alzheimer's disease. epinephrine from rat neocortical slices. In: Noncatecholic phenylethyl-
Brain Research 159:477-81. [CHV] amines, Part 1, ed. A. D. Mosnaim & M. E. Wolf, pp. 159-77. New York:
Richter, C. P. (1967) Sleep and activity: Their relation to the 24-hour clock. Marcel Dekker. [CHV]
Research Publications, Association for Research in Nervous and Mental Sandier, M.; Ruthven, C. R. J.; Goodwin, B. L. & Coppen, A. (1979) Decreased
Disease 45:8-27. [CHV] cerebrqspinal fluid concentration of free-phenylacetic acid in depressive
Rinaldi, F. & Himwich, H. E. (1955) Alerting response and actions of atropine illness. Clinica Chemica Ada 93:169-71. [CHV]
and cholinergie drugs. Archives of Neurology and Psychiatry (Chicago) Santini, M. & Berl, S. (1972) Effects of reserpine and monoamine oxidase inhi-
73:387-95. (CHV] bition on the levels of amino acids in sensory ganglia, sympathetic ganglia
Robinson. T. E. (1980) Hippocampal rhythmical slow activity (RSA; theta): A and spinal cord. Brain Research 47:167-76. [CHV]
critical analysis of selected studies and discussion of possible species-dif- Sastre, J. P. & Jouvet, M. (1977) Les schemes moteurs du sommeil paradoxal.
ferences. Brain Research Reviews 2:69-101. [CHV] In: European Congress on Sleep Research, 3d, Montpellier, 1976. Steep;
Robinson, T. E. & Green, D. G. (1980) Effects of hemieholinium-3 and choline 1976; memory, environment, epilepsy, sleep staging, ed. W. P. Koella &
on hippocampal electrical activity during immobility vs movement. Elec- P. Levin, pp. 18-23. Basel, New York, Karger. [CHV]
troencephalography and clinical Neurophysiology 50:314-23. [CHV] Schallert, T.; De Ryck, M. & Teltelbaum, P. (1980) Atropine stereotypy as a
Robinson, T. E. & Winshaw, 1. Q. (1974). Effects of posterior hypothalamic le- behavioral trap: A movement subsystem and electroencephalographic
sions on voluntary behavior and hippocampal electroencephalograms in analysis. Journal of Comparative and Physiological Psychology 94:1-24.
the rat. Journal of Comparative and Physiological Psychology 86:768-86. [RJS, CHV]
[RI'V] Schallert, T.; Whishaw, I. Q. ; Ramirez, V. D. & Teitelbaum, P. (1978) Compul-
Robinson, T. E.; Kramis, R. C. & Vanderwolf, C. H. (1977) Two types of cere- sive, abnormal walking caused by anticholinergics in akinetic,
bral activation during active sleep: Relations to behavior. Brain Research 6-hydro.xydopamine-treated rats. Science 199:1461-63. [RJS]
124:544-9. [CHV] Schaul, N.; Gloor, P.; Ball, G. & Gotman, J. (1978) The electromicrophysiology
Robinson, T. E. & Vanderwolf, C. H. (1978) Electrical stimulation of the brain of delta waves induced by systemic atropine. Brain Research 143:475-86.
stem in freely moving rats. II. Effects on hippocampal and neocortical [CHV]
electrical activity and relations to behavior. Experimental Neurology Schwartzbaum, J. S. (1975) Interrelationship among multiunit activity of the
61:485-515. [CHV, RPV] midbrain reticular formation and lateral geniculate nucleus, thalamoeorti-
Robinson, T. E.; Vanderwolf, C. H. & Pappas, B. A. (1977) Are the dorsal nora- cal arousal, and behavior in rats. Journal of Comparative and Physiolog-
drenergic bundle projections from the locus coeruleus important for neo- ical Psychology 89:131-57. [RBM]
cortical or hippocampal activation? Brain Research 8:75-98. [GH, CHV] Schwartzbaum, J. S. & Kreinick, C. J. (1973) Interrelationships of hippocampal
Rommelspacher, H. & Kuhar, M. J. (1974) Effects of electrical stimulation on electroencephalogram, visually evoked response, and behavioral reactivity
acetylcholine levels in central cholinergic nerve terminals. Brain Research to photic stimuli in rats. Journal of Comparative and Physiological Psy-
81:243-51. [CHV] chology, 85:479-90. [CHV]
Ropert. N.; Ben-Ari., Y.; Krnjevic, K. & Pole, P. (1980) Septo-hippocampal Segal, M. (1978) A correlation between hippocampal responses to interhemi-
pathway and cholinergic disinhibition of pyramidal cells. Canada Physi- spheric stimulation, hippocampal slow rhythmic activity and behaviour.
ology \ I MS. [KK] Electroencephalography and Clinical Neurophysiology 45:409-11.
Rossi, C. F. (1980) Neural regulation of sleep. Experientia 36:19-20. [JRV] [GH]
Rossi. G. F. & Zanchetti, A. (1957) Brain stem reticular formation: Anatomy Segal, M. & Bloom, F. E. (1974) The action of norepinephrine in the rat hippo-
and physiology. Archives ltaliennes de Biologic 95:199-435. [CHV] campus. I. Iontophoretic studies. Brain Research 72:79-97. [CHV)
Rossi, G. F. & Zirondoli, A. (1955) On the mechanism of the cortical desynch- (1976) The action of norepinephrine in the rat hippocampus. 111. Hippocam-
ronization elicited by volatile anaesthetics. Electroencephalography and pal cellular responses to locus coeruleus stimulation in the awake rat.
clinical Neurophysiology 7:383-90. [CHV] Brain Research 107:499-511. [GH]
Roth, S. R.; Stennan, M. B. & Clemente, C. D. (1967) Comparison of EEC cor- Semba, K. & Komisaruk, B. R. (1978) Phase of the theta wave in relation to dif-
relates of reinforcement, internal inhibition and sleep. Electroencepha- ferent limb movements in awake rats. Electroencephalography and Clini-
lography and clinical Neurophysiology 23:509-20. [AR, CHV] cal Neurophysiology 44:61-71. [BRK]
Rothballer, A. IV (1956) Studies on the adrenaline-sensitive component of the Semba, K.; Szeehtman, H. & Komisaruk, B. R. (1980) Synchrony among rhyth-
reticular activating system. Electroencephalography and clinical Neuro- mical facial tremor, neocortical "alpha" waves, and thalamic non-sensory
physiology 8:603-21. [CHV] neuronal bursts in intact awake rats. Brain Research 195:281-91. [BRK,
Rotter, A.; Birdsall, N. J. M.; Field, P. M. & Raisman, G. (1979) Muscarinic re- CHV]
ceptors in the central nervous system of the rat. II. Distribution of binding Serkov, F. N.; Makaulin, R. F. & Tychina, D. N. (1966) Electrical activity of
of [3H] propylbenzyleholine mustard in the midbrain and hindbrain. the brain following mesencephalic transection under chronic experiment.
Brain Research Reviews 1:167-83. [CHV] (Russ) Scchnov Physiological Journal of the USSR 52:837-46. [JRV]
Rougeul, A. (1958) Observations electrographiques au cours du conditionne- Shapovalov, A. I. (1975) Neural organization and synaptic mechanisms of su-
ment instrumental alimentaire chez le chat. Journal de Physiologie praspinal motor control in vertebrates. Review of Physiology, Biochemis-
(Paris) 50:494-96. [CHV] try and Pharmacology 72:1-54. [CHV]
Rougeul, A.; Letalle, A. & Corvisier, J. (1972) Activite rhythmique du cortex Shettleworth, S. J. (1975) Reinforcement and the organization of behavior in
somesthesique primaire en relation avec I'immobilite chez le chat libre ev- golden hamsters: Hunger, environment, and food reinforcement. Journal
eille. Electroencephalography and clinical Neurophysiology 33:23-39. of Experimental Psychology: Animal behavior processes 1:56-87. [ C H V ]
[CHV] Shiffrin, R. M. & Schneider, W. (1977) Controlled and automatic human infor-
Bougeul-Biiser, A.; Boyer, J. J. & Buser, P. (1975) From attentiveness to sleep. mation processing: II. Perceptual learning, automatic attending and a gen-
A topographical analysis of localized "synchronized " activities on the cor- eral theory. Psychological Reviews, 84:127-89. [EC]
tex of normal cat and monkey. Ada Neurobiologiae Experimental^ Shiromani, P. & Fishbein, W. (1980) Cholinergic stimulation of pontine brain-
35:805-19. [AR] stem cells by mini-pump infusion augments paradoxical sleep for five
(1978) Transitional states of awareness and specific attention neurophysio- days. Society for Neuroscicnce Abstracts 6:708, (PS]
logical correlates and hypothesis. International Symposium on Cerebral Shute, C. C. D. & Lewis, P. R. (1967) The ascending cholinergic reticular sys-
Correlates of Conscious Experience, Senanque 1977:215-32. [AR] tem: neoeortical, olfactory and subcortical projections. Brain 90:497-520.
(1978) Transitional states of awareness and short-term fluctuations of selec- [CHV]
tive attention: Neurophysiological correlates and hypotheses. In: Cerebral Sie, G.; Jasper, H. H & Wolfe, L. (1965) Rate of Ach release from cortical sur-
correlates of conscious experience, ed. P. Buser & A. Rougeul-Buser, pp. face in encephale and cerveau isole preparations in relation to arousal and
215—32. Amsterdam: Elservier/North Holland Biomedical Press. [JRV] epileptic activation of the ECG. Electroencephalography and clinical
Rudell, A. P.; Fox, S. E. & Ranck, J. B. (1980) Hippocampal excitability phase- Neurophysiology 18:206. [CHV]
locked to the theta rhythm in walking rats. Experimental Neurology Siegel, J. M. (1979a) Behavioral functions of the reticular formation. Brain Re-
68:87-96. [BRK] search Reviews 1:69-105. (JBR, CHV]
Rusak, B. & Zucker, I. (1975) Biological rhythms and animal behavior. Annual (1979b). Unit activity in the reticular formation and REM sleep. In: The
Review of Psychology 26:137-71. [CHV] functions of sleep, ed. R. Drucker-Colin; M. Shkurovich & M. B. Stennan,
Sailer, S. & Stuinpf, C. (1957) Beeinflussbarkeit der rhinencephalen Tatigkeit pp. 31-77. New York: Academic Press. [CHV]
des kaninchens. Naunyn-Schmiedeherg's Archiv fur die Experimentelle Siegel, J. M.; McGinty, D. J. & Breedlove, S. M. (1977) Sleep and waking activ-
Pathologic und Pharmakologie 231:63-77. [CHV] ity of pontine gigantocellular field neurons. Experimental Neurology
Saldate, M. C. & Orrego, F. (1978) Differences in the electrically induced re- 56:553-73. [RBM]
lease of 3 H-labelled 0-phenylethvlamine, tyramine, octopamine, and nor- Sitaram, N.; Weingartner, H. & Gillin, J. C. (1978) Human serial learning: En-
512 THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4
References/Vanderwolf & Robinson: Reticulo-cortical activity and behavior
liatK'nntMit with arecholine and choline and impairment with scopol- tioning of hippocampal theta patterns in the rat. Journal of Comparative
amine. Science 201:27-4-76. [CUV] and Physiological Psychology 91:674-81. [RBM]
Skinner, B. F. (1938) The Ix'havior of organisms. New York: Appleton- Teuber, H. L. (1955) Physiological psychology. Annual Review of Psychology
Ct-ntiiry-Crnfts. [CUV] 6:267-96. [RJS]
(1950) Are theories nf learning necessary? Psychological Review 57:193-216. Thayer, R. E. (1978) Toward a psychological theory of multidimensional acti-
[CUV] vation (arousal). Motivation and Emotion 2:1-34. [RDM]
(1969) Contingencies of reinforcement: A theoretical analysis. New York: Thompson, R. F. (1980) Neuronal substrates of classical conditioning in a
Appleton-Century-Crofls. [CIIV] mammalian model system. Paper presented at Society of Neuroscience
(1071) Almil Miaviorism. New York: Alfred A. Knopf. [CHV] Symposium. [HE]
Slosarska, M. & Zernieki, B. (1973) Sleep-waking eyele in the "cerveau isole" Tinbergen, N. (1951) The study of instinct. New York: Oxford University
eat. Archives ltalienncs de Biologic 111:138-55. [JRV] Press. [CHV]
Sterman, M. B. (1972) The basie resl-aetivity cycle and sleep: Developmental (1963) On the aims and methods of ethology. Zeitschrift fiir Tierpsycho-
considerations in man and cats. In: Sleep and the maturing nervous sys- logie 20:410-33. [CHV]
tem, ed. C. I). Clemenlc; D. P. Piirpuru & F. E. Mayer, pp. 175-79. New (1972) The animal in its world: Explorations of an ethologist, vol. 1. Lon-
York. Academic Press. [JRV] don: Allen & Unwin. [CHV]
Smith, C. M (1972) The release of aeetylcholine from rabbit hippocampus. (1973) The animal in its world: Explorations of an ethologist, vol 2 London:
British Journal of Pharmacology 45:l72p. [CIIV] Allen & Unwin. [CHV]
Soulairae, A.; Gottesman, Cl. & Thangapregassam, J. (1965) Etude electrophy- Tfinnesen, M. (1948) The absorption and distribution of atropine in rats. Ada
siologiquc des differentes phases de sommeil chez le rat. Archives Hal- Pharmacologica 4:367-78. [CHV]
ienne.i de Biologic 103:469-82. [CUV] Torii, S. & Wikler, A. (1966) Effects of atropine on electrical activity of hippo-
Sperry, R. W. (1952) Neurology and the mid-brain problem. American Scien- campus and cerebral cortex in cat. Psychopharmacoloqica (Berlin) 9:189-
tist.M>2ti\ -312. [CHV] 204. [CHV]
Stavraky, G. W. (1961) Supcrsensitivity following lesions of the nervous sys- Ungerstedt, U. (1971) Stereotaxic mapping of the monoamine pathways in the
tem. Toronto: University of Toronto Press. [Cl IV] rat brain. Acta physiologica Scandinavica Supplcmentum 367:1-48.
Slephan, F. K. 4 Zucker, 1. (1972) Orcadian rhythms in drinking behavior and [CHV]
locoinotor activity of rats are eliminated by hypothalamic lesions. Pro- Usdin, E. & Sandier, M. (1976) Trace amines and the brain. New York: Marcel
ceedings of the National Academy of Sciences of the United States of Dekker, Inc. [CHV]
America 69:1583-86. [CHV] Usui, S. & Iwahara, S. (1977) Effects of atropine upon the hippocampal elec-
trical activity in rats with special reference to paradoxical sleep. Electro-
Steriade, M. (1978) Cortical long-axoned cells and putative interneurons dur-
encephalography and clinical Neurophysiology, 42:510-17. [CH V|
ing the sleep-waking cycle. Behavioral and Brain Sciences 3:465-514.
Valenstein, E. S. (1973) Brain control. New York: Wiley-Interscience. [NRC]
[I'M. KK, PS)
VanderMaelen, C. P. & Aghajanian, G. K. (1980) Intracellular studies showing
(1981) Mechanisms underlying cortical activation. Neuronal organization
modulation of facia] motoneurone excitatility by serotonin. Nature
and projxTties of the midbrain reticular core and intralaminar thalamic
287:346-47. [KK]
nuclei. In: Brain mechanisms of perceptual awareness and purposeful
Vanderwolf, C. H. (1969) Hippocampal electrical activity and voluntary
hhavior. ed. C). Pompeiano & C. Ajmone-Marsan. New York: Raven
movement in the rat. Electroencephalography and clinical Neurophysi-
Press, in press. IMS]
ology 26:407-18. [CHV]
Steriade, M. & Dcschenes, M. (1974) Inhibitory processes and interneuronal
(1971) Limbic-diencephalic mechanisms of voluntary movement. Psycho-
apparatus in motor cortex during sleep and waking. II Recurrent and af-
logical Review, 78:83-113. [CHV]
lerent inhibition of pyramidal tract neurons. Journal of Neurophysiology
(1975) Neocortical and hippocampal activation in relation to behavior: Ef-
37:1093 113. [MS]
fects of atropine, eserine, phenothiazines, and amphetamine. Journal of
Steriade, M.; Deschenes, M.; Wyzinski, P. & Halle, J. Y. (1974) Input-output
Comparative and Physiological Psychology 88:300-23. [CHV]
organization of the motor cortex and it.s alterations during sleep and wak-
Vanderwolf, C. H. & Cooley, R. K. (1974) Hippocampal electrical activity dur-
ing. In: Basic sleep meclianisms. ed. O. Petre-Quadens & J. Schlag, pp.
ing long-continued avoidance performance. Effects of fatigue. Physiology
144 200 New York: Academic Press. [MS]
and Behavior 13:819-23. [CHV]
Steriade, M ; losif, G. & Apostol, V. (1969) Responsiveness of thalamic and m o
Vanderwolf, C. H. & Pappas, B. A. (1980) Reserpine abolishes movement-
tor relays during arousal and various stages of sleep. Journal of Neuro-
correlated atropine-resistant neocortical low voltage fast activity. Brain
physiology 32:251-65. [MS]
Research 202:79-94. [CHV]
Stone, T. \V.; Taylor, 1). A. & Bloom, F. K. (1975) Cyclic AMP and cyclic CMP
Vanderwolf, C. H.; Bland, B. H. & Whishaw, 1. Q. (1973) Diencephalic, hippo-
may mediate opposite neuronal responses in the rat cerebral cortex.
campal, and neocortical mechanisms in voluntary movement. In: Efferent
Science 187:845-47. [MS]
organization and the integration of behavior, ed. J. D. Maser, pp. 229-
Straughan, I). W. (1975) Neurotransmitters and the hippocampus. In: The hip- 62. New York: Academic Press. [CHV]
pocampus, vol. I. Structure and development, ed. R. L. Isaacson and
Vanderwolf, C. H.; Kolb, B. & Cooley, R. K. (1978) Behavior of the rat after
k' II Pribrani, pp. 239-68. New York: Plenum Press. [CHV]
removal of the neocortex and hippocampal formation. Journal of Com-
Stumpf, C. (1965) Drug action on the electrical activity of the hippocampus. parative and Physiological Psychology 92:156-75. [RJS, CHV]
International Review of Neurobiology 8:77-138. [CHV, OSV] Vanderwolf, C. H.; K'ramis, R.; Gillespie, L. A. & Bland, B. H. (1975) Hippo-
Stumpf, C ; Pctsche, II. & Gogolak, C. (1962) The significance of the rabbit's campal rhythmic slow activity and neocortical low-voltage fast activity:
septum as a relay station between the midbrain and the hippocampus. II. Relations to behavior. In: The Hippocampus, vol. 2: Neurophysiology and
The differential influence of drugs upon both the septal cell firing pattern Behavior, ed. R. L. Isaacson & K. H. Pribram, pp. 101-28. New York: Ple-
and the hipixx'umpus theta activity. Electroenccphalography and Clini- num Press. [HE, CHV]
cal Neurophysiology 14:212-19. [JCS] Vanderwolf, C. H.; Kramis, R. & Robinson, T. E. (1978) Hippocampal elec-
Stilton, S.; Spring, B. J. & Tenting, P. (1978) Modality shift at the crossroads. trical activity during waking behavior and sleep: Analyses using centrally
In: The nature of schizophrenia: New approaches to research and treat- acting drugs. Function of the Septo-hippocampal System. Ciba Founda-
ment, ed. 1,. C. Wynne, R. L. Cromwell & S. Matthysse. New York: John tion Symposium 58 (new series). Elsevier, Excerpta Medica, North-
Wiley & Sons, Inc. (EC] Holland, Amsterdam. [CHV]
S/erb, J, C. (1964) The effect of tertiary and quaternary atropine on cortical Vanderwolf, C. H.; Robinson, T. E. & Pappas, B. A. (1980) Monoamine re-
aeetylcholine output and on the electroencephalogram in cats. Canadian placement after reserpine: Catecholaminergic agonists restore motor ac-
Journal of Physiology and Pharmacology 42:303-14. [JCS] tivity but phenylethylamine restores atropine-resistant neocortical low
(1967) Cortical acetylcholine release and electroencephalographic arousal. voltage fast activity. Brain Research 202:65-77. [CHV]
Journal of Physiology (London) 19:329-43. [JCS, CIIV] Vanegas, H. & Flynn, J. P. (1968) Inhibition of cortically elicited movements
Szerb, J. C ; Malik, II. & Hunter, E. G. (1970) Relationship between acelyl- by electrical stimulation of the hippocampus. Brain Research 11:489-506.
choline content and release in the cat's cerebral cortex. Canadian Journal [CH]
of Physiology and Pharmacology 48:780-90. [CHV] Vertes, R. P. (1979) Brain stem gigantocellular neurons: Patterns of activity
Taylor, J. ed. (1958) The selected writings of John Hughlings Jackson, 2 vol. during behavior and sleep in the freely moving rat. Journal of Ncuro-
London: Staples Press. [CHV] physiology 42:214-28. [PS, CHV]
Teitelbaum, H. (1976) Hipixx?ampal activity and scopolamine. Science (1980) Brain stem activation of the hippocampus: A role for the magnoeellu-
192:914-15. [RUM] lar reticular formation and the MLF. Electroencephalography and Clini-
Teitelbaum, II.; McFarland, W. L. & Mattson, J. I.. (1977) Classical condi- cal Neurophysiology 50:48-58. [RPV]
THE BEHAVIORAL AND BRAIN SCIENCES (1981), 4 513
References/Vander-wotf & Robinson: Reticulo-cortical activity and behavior
Villablanca, J. (1962) Electroencephalogram in the permanently isolated fore- systems and neocortical desynchronization. Brain Research Bulletin
brain of the cat. Science 138:44-46. [ JRV] 1:573-81. [CHV]
(1965) The electrocorticogram in the chronic "cerveau isole" of the cat. Whishaw, I. Q. & Kolb, B. (1979) Neocortical and hippooampal EEC in rats
Electroencephalography and Clinical Neuwphysiology 19:576-86. during lateral hypothalamie lesion-induced hyperkinesia: Relations to lx'-
[JRV] havior and effects of atropine. Physiology and Behavior, 22:1107-13.
(1966a) Behavioral and polygraphic study of "sleep" and "wakefulness" in [CHV]
chronic decerebrate cats. Etectroencephatography and Clinical Neuro- Whishaw, I. Q.; Nonneman, A. & Kolb, B. (1980) Environmental constraints on
physiology 21:562-77. [CHV, JRV] the motor abilities of deneocorticate rats. Society for Neuroscience Ab-
(1966b) Ocular behavior in the chronic "cerveau isole" cat. Brain Research stracts. 6:419. [RJS]
2:99-102. (JRV] Whishaw, I. Q.; Schallert, T. & Kolb, B. (1981) An analysis of feeding and sen-
(1966e) Effects of atropine, eserine and adrenaline in cats with mesence- sorimotor abilities of rats after decortication. Journal of Comparative and
phalic transection and in the "isolated hemisphere" cat preparation. Ar- Physiological Psychology. 95: In press. [RJS]
chivos de Biologia y Medidna Expcrimentales 3:118-29. [JRV] Whishaw, 1. Q.; Robinson, T. E., Schallert, T.; De Ryck, M. & Ramirez. V. D.
(1966/1967) Electrocorticogram in the chronic "isolated hemisphere" of the (1978) Electrical activity of the hippocampus and neocortex in rats de-
cat. Effect of atropine and eserine. Brain Research 3:287-91. [CHV, pleted of brain dopamine and norepinephrine: Relations to Ijehavior and
JRV] effects of atropine. Experimental Neurology 62:748-67. [CHV]
(1972) Specialized lesions: Cerveau isole and encephale isole. In: Methods of Whishaw, 1. Q. & Vanderwolf, C. H. (1971) Hippocampal EEC and behavior:
psycholtiology: Laboratory techniques in neuropsychotogy, Vol. 2, ed. R. Effects of variation in body temperature and relation of EEC to vibrissae
D. Myers, pp. 285-302. London: Academic Press. [JRV) movements, swimming, and shivering. Physiology and Behavior 6:391-
(1974) Role of the thalamus in sleep control: Sleep-wakefulness studies in 97. [CHV]
chronic diencephalie and athalamic cats. In: Basic sleep mechanisms, ed. (1973) Hippocampal EEC and behavior: Changes in amplitude and fre-
O. Petre-Quadens & J. D. Sehlag, pp. 51-81. New York and London: quency of RSA (theta rhythm) associated with spontaneous and learned
Academic Press. [JRV] movement patterns in rats and cats. Behavioral Biology 8:461-84.
Villahlanca, J. & Marcus, R. (1972) Sleep-wakefulness, EEC and behavioral [CHV]
studies of chronic cats without neocortex and striatum: The 'diencephalie Wikler, A (1952) Pharmacologic dissociation of behavior and EEC "sleep pat-
cat. Archives Italiennes de Biologic 110:348-82. [CHV, JRV] terns" in dogs: Morphine, N-allylnormorphine, and atropine. Proceedings
Villablanca, J & Schlag, J. (1968) Cortical control of thalamic spindle waves. of the Society for Experimental Biology and Medicine 79:261-65.
Experimental Neurology 20:432-44. [JRV] [CHV]
Villablanca, J.; Marcus, R. J. & Olmstead, C. E. (1976) Effects of caudate nu- Wilke, J. T.; Lansing, R. W. & Rogers, C. A. (1975) Entrainment of respiration
clei or frontal cortical ablations in cats. II. Sleep-wakefulness, EEC and to repetitive finger tapping. Physiological Psychology, 3:345-49. [CHV]
motor activity. Experimental Neurology 53:31-50. [JRV] Winson, J. (1972) Interspecies differences in the occurrence of theta. Behav-
Villablanca, J.; Schlag, J. & Marcus, R. (1970) Blocking of experimental spike ioral Biology 7:479-87. [CHV]
and wave by a localized forebrain lesion. Epitepsia 11:163-67 [JRV] (1974) Patterns of hippocampal theta rhythm is the freely moving rat. Elec-
Vinogradova, O. S.; Brazhnik, E. S.; Karanov, A. M. & Zhadina, S. D. (1980) troencephalography and clinical Ncurophysiology 36:291-301. [CH V]
Analysis of neuronal activity in rabbit's septum with various conditions of (1976a) Hippocampal theta rhythm. 1. Depth profiles in the curarized rat.
deafferentation. Brain Research 187:354-68. [CHV, OSV] Brain Research 103:57-70. [CHV]
Votaw, C. L. (1960) Study of septal stimulation and ablation in the maeque (1976b) Hippocampal theta rhythm. II. Depth profiles in the freely moving
monkey. Neurology 10:202-09. [CH] rabbit. Brain Research 103:71-9. [CHV]
Walsh, R. R. (1956) Single cell spike activity in the olfactory bulb. American Winson, J. & Abzug, C. (197/J) Neuronal transmission through hippocampal
Journal oj Physiology 186:255-57. [GH] pathways dependent on behavior. Journal of Neuwphysiology, 41:716-
Wamsley. J. K.; Lewis, M. S.; Young, W. S. Ill & Kuhar, M. J. (1981) Autora- 32. [CHV]
diographic localization of muscarinic cholinergic receptors in rat brain- Woodruff, M. L.; Cage, K. H., Ill & Isaacson, R. L. (1973) Changes in focal
stem. Journal of Neuroscience 1:176-91. [CHV] epileptic activity produced by brainstem section in the rabbit. Electroen-
Wang, C. H. & Akert, K. (1962) Behavior and reflexes of chronic striatal cats. cephalography and Clinical Ncurophysiology 35:475-86. [CB]
Archives Italiennes de Biologie, 100:48-85. [CHV] Woods, J. W. (1964) Behavior of chronic decerebrate rats. Journal of Ncuro-
Weiss, P. (1941) Self-differentiation of the basic patterns of co-ordination. physiology 27:635-44. [CHV]
Comparative Psychology Monographs 17:1-96. [CHV]
Yamamura, H. I.; Kuhar, M. J.; Greenberg, D. & Synder, S. H. (1974) Musca-
Welker. W. 1. (1964) Analysis of sniffing in the albino rat. Behaviour 22:223-
rinic cholinergic receptor binding: Regional distribution in monkey brain.
244. [HE]
Brain Research 66:541-46. [CHV]
Whishaw, 1. Q. (1972) Hippocampa] electroencephalographic activity in Mon-
Yamamura, H. I.; Kuhar, M. J. & S. H. Snyder (1974) In vivo identification of
golian gerbil during natural behaviours and wheel running and in the rat
muscarinic cholinergic receptor binding in rat brain. Brain Research
during wheel running and conditioned immobility. Canadian Journal of
80:170-76. [CHV]
Psychology, 26:219-39. [CHV]
(1976) The effects of alcohol and atropine on EEC and behavior in the rab- Yokota, T. & Fujimori, B. (1964) Effects of brain-stem stimulation upon hippo-
bit. Psychopharmacoloqica (Berlin) 48:83-90. [CHV] campal electrical activity, somatomotor reflexes and autonomic functions.
Whishaw, I. Q.; Bland, B. H. & Bayer, S. A. (1978) Postnatal hippocampal Electroencephalography and Clinical Neurophysiology 16:375-82.
granule cell agenesis in the rat: Effects on two types of rhythmical slow [GH]
activity (RSA) in two hippocampal generators. Brain Research 146:249- Zernicki, B. (1968) Pretrigeminal cat. Brain Research 9:1-14. [MS]
68. [CH, CHV] Zieglgansberger, W. ; French, E. D ; Siggins, G. R. & Bloom, F. E. (1979)
Whishaw, 1. Q.; Bland, B. H.; Robinson, T. E. & Vanderwolf, C. H. (1976) Opioid peptides may excite hippocampal pyramidal neurons by inhibiting
Neuromuscular blockade: The effects on two hippocampal RSA (theta) adjacent inhibitory interneurons. Science 205:415 17. [KK]
514 THE 8EHAVIORAL AND BRAIN SCIENCES (1981), 4
CAMBRIDGE
Mind in Science The Meaning of Things
Richard L. Gregory Symbols in the Development of Self
Gregory traces the development of modern Mihaly Cslkszentmihalyl and
scientific concepts from the Sumerians and the Eugene Rochberg-Halton
Greeks on, and charts the growth of psychol- A remarkable study of the significance of mate-
ogy in man's attempts to relate Mind to Matter. rial possessions in contemporary urban Ameri-
He ultimately suggests that such recent efforts can life, and the ways people find meaning in
as attempts to design intelligent machines may their domestic environments.
provide new answers to the riddle of the phe- "The book has no parallels. It is highly original
nomenon of knowledge. $29.95 and important... moves surefootedly over a
vast intellectual terrain."-M. Brewster Smith,
Human Groups and University of California, Santa Cruz $22.50
Social Categories Behavioral Development
Studies in Social Psychology
The Bielefeld Interdisciplinary Project
Henri Tajfel
Klaus Immelmann, George W. Barlow,
A volume chronicling the progress of Tajfel's
work on relations among, and conflicts
Lewis Petrinovich, and Mary Biggar
between, groups. Tajfel has played a central Main, Editors
role in the development of social psychology in This collection of essays by a distinguished
Europe over the last twenty-five years. group of psychologists, zoologists, neurolo-
gists, and psychiatrists represents the first
"These essays are more than an expression of major attempt to remove disciplinary barriers to
the creative talents of Tajfel. They are also a create a common framework for the analysis of
sensitive testament to the times in which he behavioral development in humans and other
has lived and in which the social sciences have animals.
grown."-from the foreword by Jerome Bruner Hardcover $59.50 Paper $18.95
Hardcover $54.50 Paper $17.50
of methodological interest
Time-Series Analysis Estimating the Effects of
A Comprehensive Introduction
for Social Scientists Social Interventions
John M. Gottman Charles M. Judd and David A. Kenny
A practical and complete presentation of A comprehensive introduction to methods for
all the major time-series techniques, both measuring the impact of social interventions in
time-domain and time-frequency, for social a variety of experimental and field settings-for
scientists who need to analyze data over time. example, education, mental health care, and
Presupposes only basic introductory statistics; income maintenance.
requires no calculus. $24.95 Hardcover $32.50 Paper $12.95
Other Books of Interest
Sigmund Freud Social Contexts of Health,
Richard Wollheim Paper $7.95 Illness, and Patient Care
Elliot G. Mishler, Editor
Child Language Hardcover $24.95 Paper $10.95
Alison Elliot Hardcover $29.95 Paper $8.95
The Development of
Children's Friendships Social Cognitive Development
Steven R. Asher and John M. Gottman, Frontiers and Possible Futures
Editors John H. Flavell and Lee Ross, Editors
Hardcover $34.50 Paper $12.95 Hardcover $32.50 Paper $12.95
all prices subject to change
Cambridge University Press
~ " — ^ 32 East 57th Street. New York. N Y 10022 ^~~"M
You might also like
- Cote 2002Document15 pagesCote 2002Chandan ShandilNo ratings yet
- Anafi Kayser and Raizen Nature Reviews Neuroscience 2018Document8 pagesAnafi Kayser and Raizen Nature Reviews Neuroscience 2018Francisco MtzNo ratings yet
- Jurnal 3 CDocument36 pagesJurnal 3 CCintya RambuNo ratings yet
- Sleep, Emotions, and Visceral Control: I. N. Pigarev and M. L. PigarevaDocument12 pagesSleep, Emotions, and Visceral Control: I. N. Pigarev and M. L. PigarevaMariaNo ratings yet
- Applied Physiology: Jownd ofDocument10 pagesApplied Physiology: Jownd ofdoraNo ratings yet
- Caie As Level Psychology 9990 Four Psychological Approaches v4Document22 pagesCaie As Level Psychology 9990 Four Psychological Approaches v4Catriona 37100% (1)
- Mental Activity at Sleep Onset: &. Kleitman, 19s7a, 19S7b) Has EstablishedDocument13 pagesMental Activity at Sleep Onset: &. Kleitman, 19s7a, 19S7b) Has EstablisheddoraNo ratings yet
- Dreaming and The Brain: From Phenomenology To NeurophysiologyDocument13 pagesDreaming and The Brain: From Phenomenology To NeurophysiologyemailannujNo ratings yet
- SleepDocument4 pagesSleepBekir Can ÇelikNo ratings yet
- Sleep and Dreaming: R. T. PivikDocument30 pagesSleep and Dreaming: R. T. PivikLina Pulido AvellaNo ratings yet
- Physiological Basis: How NREM Sleep Components Can Promote and REM Sleep Components Can Suppress Seizure Discharge PropagationDocument10 pagesPhysiological Basis: How NREM Sleep Components Can Promote and REM Sleep Components Can Suppress Seizure Discharge PropagationMukernas Perdossi 2021No ratings yet
- Mecanismos Genéticos Que Median La Regulación Circadiana Del SueñoDocument10 pagesMecanismos Genéticos Que Median La Regulación Circadiana Del SueñoDiego Soto ChavezNo ratings yet
- The Study of Human Sleep: A Historical Perspective: William C DementDocument6 pagesThe Study of Human Sleep: A Historical Perspective: William C DementKrisha ann MesanaNo ratings yet
- C134 1945-1960 PDFDocument16 pagesC134 1945-1960 PDFstein godoy pachecoNo ratings yet
- Core StudiedDocument21 pagesCore StudiedsuuNo ratings yet
- EEG Normal Sleep - StatPearls - NCBI BookshelfDocument7 pagesEEG Normal Sleep - StatPearls - NCBI BookshelfChandan ShandilNo ratings yet
- Wang 2021Document9 pagesWang 2021Aldo GuascoNo ratings yet
- Fpsyg 11 01662Document16 pagesFpsyg 11 01662Aldo GuascoNo ratings yet
- TMP 5 FADocument12 pagesTMP 5 FAFrontiersNo ratings yet
- Sleep and Synaptic Homeostasis: A Hypothesis: Giulio Tononi, Chiara CirelliDocument8 pagesSleep and Synaptic Homeostasis: A Hypothesis: Giulio Tononi, Chiara CirelliGabriela Cristina García FacioNo ratings yet
- Are Corticothalamic Up' States Fragments of Wakefulness?Document9 pagesAre Corticothalamic Up' States Fragments of Wakefulness?WhichLilithNo ratings yet
- The Nural Correlates of DreamingDocument24 pagesThe Nural Correlates of DreamingDaniel Naranjo RebolledoNo ratings yet
- Voss Ursula Induction of Self Awareness in Dreams 2014Document5 pagesVoss Ursula Induction of Self Awareness in Dreams 2014Mike JonnyNo ratings yet
- Science Abi8372Document5 pagesScience Abi8372Mateo MendozaNo ratings yet
- Control of BreathingDocument31 pagesControl of BreathingAchmad Hafidz BaraqbahNo ratings yet
- Neurobiology of Sleep: Madhu Kalia4Document5 pagesNeurobiology of Sleep: Madhu Kalia4Julian ReyesNo ratings yet
- 2001 Atlas, Rules, and Recording Techniques For The Scoring of Cyclic Alternating Pattern (CAP) in Human SleepDocument17 pages2001 Atlas, Rules, and Recording Techniques For The Scoring of Cyclic Alternating Pattern (CAP) in Human SleepNHUNG Hoàng HuyềnNo ratings yet
- Cortical State and AttentionDocument31 pagesCortical State and AttentionJesica AlvarezNo ratings yet
- Fpsyg 11 567618Document8 pagesFpsyg 11 567618christinaceciliaaNo ratings yet
- Chapter 9 (Biopsych)Document4 pagesChapter 9 (Biopsych)Bella SpiralNo ratings yet
- Letters To The Editor Related To New TopicsDocument12 pagesLetters To The Editor Related To New TopicsFrontiersNo ratings yet
- The Neurologyof Sleep 2012 Published FormDocument18 pagesThe Neurologyof Sleep 2012 Published FormNeurologie PediatricaNo ratings yet
- Science Abi8375Document5 pagesScience Abi8375Mateo MendozaNo ratings yet
- Is Sleep Essential - 2008Document7 pagesIs Sleep Essential - 2008visapusiska_05No ratings yet
- Sleep and The BrainDocument5 pagesSleep and The BrainjelenaNo ratings yet
- Neurobiology of The Sleep-Wake Cycle: Sleep Architecture, Circadian Regulation, and Regulatory FeedbackDocument12 pagesNeurobiology of The Sleep-Wake Cycle: Sleep Architecture, Circadian Regulation, and Regulatory FeedbackRoberto Rafael Ramírez FelipeNo ratings yet
- Sllep InertiaDocument11 pagesSllep InertiaWina Non RegulerNo ratings yet
- 1961 ReynoldsDocument7 pages1961 Reynoldscarlos tNo ratings yet
- Sleep and Epilepsy Strange Bedfellows No MoreDocument26 pagesSleep and Epilepsy Strange Bedfellows No Morerifki irsyadNo ratings yet
- Sleep 1Document40 pagesSleep 1Sst YadavNo ratings yet
- TwoProcessModel PPSChapter BorbleyDocument16 pagesTwoProcessModel PPSChapter Borbleysuresh2250No ratings yet
- Optogenetics in Psychiatric DiseasesDocument6 pagesOptogenetics in Psychiatric DiseasesolivukovicNo ratings yet
- Sleep Memory Processing The Sequential Hypothesis (Theoretical Lens)Document8 pagesSleep Memory Processing The Sequential Hypothesis (Theoretical Lens)Sebastian CrayNo ratings yet
- Epilepsy: Ever-Changing States of Cortical Excitability: R. A. B. Badawy, D. R. Freestone, A. Lai AND M. J. CookDocument11 pagesEpilepsy: Ever-Changing States of Cortical Excitability: R. A. B. Badawy, D. R. Freestone, A. Lai AND M. J. CooknurhikmahNo ratings yet
- Sleep Medicine 2015 (304 529)Document226 pagesSleep Medicine 2015 (304 529)Guillermo MoranteNo ratings yet
- From Spontaneous Event To Lucidity PDFDocument20 pagesFrom Spontaneous Event To Lucidity PDFEloued SiryneNo ratings yet
- Functions and Mechanisms of SleepDocument38 pagesFunctions and Mechanisms of SleepFrancisco MtzNo ratings yet
- NIH Public Access: Control of Sleep and WakefulnessDocument172 pagesNIH Public Access: Control of Sleep and WakefulnessPrima Akli SetyoNo ratings yet
- Control of Sleep and WakefulnessDocument101 pagesControl of Sleep and WakefulnessLevente BalázsNo ratings yet
- 523 FullDocument3 pages523 FulldesckygamerNo ratings yet
- Out-of-Body Experiences, Dreams, and REM SleepDocument11 pagesOut-of-Body Experiences, Dreams, and REM SleepSelenaNo ratings yet
- Sleep Function and Synaptic Homeostasis - Giulio Tononi y Chiara CirelliDocument14 pagesSleep Function and Synaptic Homeostasis - Giulio Tononi y Chiara CirelliGabriela Cristina García FacioNo ratings yet
- About Sleep's Role in MemoryDocument87 pagesAbout Sleep's Role in MemoryAlex BorroelNo ratings yet
- Why We SleepDocument6 pagesWhy We SleepJustine MenesesNo ratings yet
- The Functional Utility of Consciousness Depends On Content As Well As On StateDocument73 pagesThe Functional Utility of Consciousness Depends On Content As Well As On StatePusat Bahasa NasionalNo ratings yet
- Neural Circuitry of Wakefulness and SleepDocument40 pagesNeural Circuitry of Wakefulness and Sleepribamar CoutinhoNo ratings yet
- Slow Wave Synchronization and Sleep State TransitionsDocument11 pagesSlow Wave Synchronization and Sleep State TransitionsPrakash N KNo ratings yet
- Exam 4 Study Guide Fall 2017: I. Cognition & Consciousness: The Nature of ConsciousnessDocument7 pagesExam 4 Study Guide Fall 2017: I. Cognition & Consciousness: The Nature of ConsciousnessAnonymous C6dXawSNo ratings yet
- Malmo 1982Document50 pagesMalmo 1982Víctor FuentesNo ratings yet
- Malmo 1960Document24 pagesMalmo 1960Víctor FuentesNo ratings yet
- Malmo 1963Document38 pagesMalmo 1963Víctor FuentesNo ratings yet
- Malmo 1983Document24 pagesMalmo 1983Víctor FuentesNo ratings yet
- Malmo 1975Document15 pagesMalmo 1975Víctor FuentesNo ratings yet
- Malmo 1957Document12 pagesMalmo 1957Víctor FuentesNo ratings yet
- Malmo 1949Document16 pagesMalmo 1949Víctor FuentesNo ratings yet
- Mac Do Nough 1973Document8 pagesMac Do Nough 1973Víctor FuentesNo ratings yet
- Malmo 1954Document20 pagesMalmo 1954Víctor FuentesNo ratings yet
- Malmo 1951Document3 pagesMalmo 1951Víctor FuentesNo ratings yet
- Maes 1996Document15 pagesMaes 1996Víctor FuentesNo ratings yet
- Lynch 1966Document14 pagesLynch 1966Víctor FuentesNo ratings yet
- Malmo 1942Document14 pagesMalmo 1942Víctor FuentesNo ratings yet
- Leland Crafts Recent Experiments in PsychologyDocument532 pagesLeland Crafts Recent Experiments in PsychologyVíctor FuentesNo ratings yet
- Linds Ley 1962 ADocument8 pagesLinds Ley 1962 AVíctor FuentesNo ratings yet
- Libet 1991Document28 pagesLibet 1991Víctor FuentesNo ratings yet
- Leo M. Chalupa, Robert W. Williams - Eye, Retina, and Visual System of The Mouse-MIT Press (2008)Document815 pagesLeo M. Chalupa, Robert W. Williams - Eye, Retina, and Visual System of The Mouse-MIT Press (2008)Víctor FuentesNo ratings yet
- Liberty 1966Document14 pagesLiberty 1966Víctor FuentesNo ratings yet
- Linds Ley 1964Document20 pagesLinds Ley 1964Víctor FuentesNo ratings yet
- Lilli Crap 2013Document13 pagesLilli Crap 2013Víctor FuentesNo ratings yet
- Lila R. Gleitman An Invitation To Cognitive Science Visual Cognition MIT Press 1995Document805 pagesLila R. Gleitman An Invitation To Cognitive Science Visual Cognition MIT Press 1995Víctor FuentesNo ratings yet
- Lila Gleitman and Barbara Landau (Editors) - The Acquisition of The Lexicon - The MIT Press (1994)Document510 pagesLila Gleitman and Barbara Landau (Editors) - The Acquisition of The Lexicon - The MIT Press (1994)Víctor FuentesNo ratings yet
- Lei Gland 1984Document5 pagesLei Gland 1984Víctor FuentesNo ratings yet
- Leung 1986Document12 pagesLeung 1986Víctor FuentesNo ratings yet
- Li 2012Document8 pagesLi 2012Víctor FuentesNo ratings yet
- Lerea 1964Document8 pagesLerea 1964Víctor FuentesNo ratings yet
- (Essential Psychology) Stephen Walker - Learning and Reinforcement-Methuen (1975)Document110 pages(Essential Psychology) Stephen Walker - Learning and Reinforcement-Methuen (1975)Víctor FuentesNo ratings yet
- (Cognition and Language) O. Hobart Mowrer (Auth.), O. Hobart Mowrer (Eds.) - Psychology of Language and Learning-Springer US (1980)Document297 pages(Cognition and Language) O. Hobart Mowrer (Auth.), O. Hobart Mowrer (Eds.) - Psychology of Language and Learning-Springer US (1980)Víctor FuentesNo ratings yet
- (Essays in Cognitive Psychology) Susanna Millar - Space and Sense (Essays in Cognitive Psychology) - Psychology Press (2008)Document244 pages(Essays in Cognitive Psychology) Susanna Millar - Space and Sense (Essays in Cognitive Psychology) - Psychology Press (2008)Víctor FuentesNo ratings yet
- Stephen M. Gavazzi (Auth.) Families With Adolescents - Bridging The Gaps Between Theory, Research, and Practice PDFDocument217 pagesStephen M. Gavazzi (Auth.) Families With Adolescents - Bridging The Gaps Between Theory, Research, and Practice PDFMarvins Free Underground100% (2)
- Clinical Psychiatry (Kraepelin, 6th Ed. 1902)Document478 pagesClinical Psychiatry (Kraepelin, 6th Ed. 1902)Ricardo R. Ricardo100% (1)
- Academic Writing For Graduate Students Essential Tasks and SkillsDocument430 pagesAcademic Writing For Graduate Students Essential Tasks and SkillsJonathan Zhou95% (20)
- Dubois Gadde 2002Document11 pagesDubois Gadde 2002Radu PanaitescuNo ratings yet
- What Is Public WritingDocument6 pagesWhat Is Public WritingPublicWriting100% (1)
- The Neuroscience of Mindfulness MeditationDocument13 pagesThe Neuroscience of Mindfulness MeditationBárbara SáNo ratings yet
- Task Based Language TeachingDocument13 pagesTask Based Language TeachinglittlesandNo ratings yet
- Topical Approach To Lifespan Development 7th Edition Santrock Test Bank DownloadDocument50 pagesTopical Approach To Lifespan Development 7th Edition Santrock Test Bank DownloadWilliams Stjohn100% (21)
- Rene Guenon - Introduction ToDocument355 pagesRene Guenon - Introduction Toegor_egorovich100% (2)
- Soal Latihan Snbt-Utbk 2024 SharedDocument7 pagesSoal Latihan Snbt-Utbk 2024 SharedrevanandaoktaviarNo ratings yet
- Raymond Kuhn - Political Journalism - New Challenges, New Practices (Routledge Ecpr Studies in European Politicalscience) (2002)Document268 pagesRaymond Kuhn - Political Journalism - New Challenges, New Practices (Routledge Ecpr Studies in European Politicalscience) (2002)Rafael ParenteNo ratings yet
- General de Jesus College: Vallarta Street, San Isidro, 3106 Nueva EcijaDocument23 pagesGeneral de Jesus College: Vallarta Street, San Isidro, 3106 Nueva EcijaMelody Jane CastroNo ratings yet
- Consumer Behavior: Buying, Having, and Being: Thirteenth EditionDocument37 pagesConsumer Behavior: Buying, Having, and Being: Thirteenth EditionArinaNo ratings yet
- Module 16 Part 2Document19 pagesModule 16 Part 2lawNo ratings yet
- Chapter 3 - Learning. Motivation, and PerformanceDocument14 pagesChapter 3 - Learning. Motivation, and PerformanceCarl Johnave Manigbas Monzon91% (11)
- Metu Neter Volume 1 by Ra Un Amen Nefer TextDocument452 pagesMetu Neter Volume 1 by Ra Un Amen Nefer Textsovereignone95% (43)
- Managing Wellbeing Survey GuideDocument28 pagesManaging Wellbeing Survey Guide0poi890No ratings yet
- Listening Skills Assignments 5Document11 pagesListening Skills Assignments 5Don Baraka Daniel92% (12)
- CW Cookbook Web PDFDocument76 pagesCW Cookbook Web PDFAmanda Judit GyarmatiNo ratings yet
- Attention Restoration Theory II A Systematic Review To Clarify Attention Processes Affected by Exposure To Natural EnvironmentsDocument43 pagesAttention Restoration Theory II A Systematic Review To Clarify Attention Processes Affected by Exposure To Natural EnvironmentsbobNo ratings yet
- Chapter 12 Surveillance OperationsDocument12 pagesChapter 12 Surveillance OperationsMares BaysanNo ratings yet
- Handbook of Developmental Cognitive NeuroscienceDocument702 pagesHandbook of Developmental Cognitive NeuroscienceClaudia Patricia Aponte Restrepo100% (2)
- Advertising Appeals IMC 9Document46 pagesAdvertising Appeals IMC 9m_dattaias100% (2)
- Brief Strategic Family TherapyDocument13 pagesBrief Strategic Family TherapyJoana CarvalhoNo ratings yet
- David Rock On Neuroscience, Coaching and LeadershipDocument9 pagesDavid Rock On Neuroscience, Coaching and LeadershipGodeliva Rina Rahayu100% (1)
- Oral Communication in Context: Principles of Speech WritingDocument23 pagesOral Communication in Context: Principles of Speech Writingbernadette domoloanNo ratings yet
- The Pali Line PDFDocument359 pagesThe Pali Line PDFGuillermo MendezNo ratings yet
- Phosphenic Mixing BrochureDocument7 pagesPhosphenic Mixing BrochureDeris Bogdan IonutNo ratings yet
- Attending SkillsDocument7 pagesAttending SkillsRoy MonNo ratings yet
- Obsession PhrasesDocument242 pagesObsession Phrasesalicia stoddard81% (59)