Professional Documents
Culture Documents
Pha052 TG 5
Uploaded by
Alcea InguilloOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Pha052 TG 5
Uploaded by
Alcea InguilloCopyright:
Available Formats
PHA 052: Pharmaceutical Analysis 2
Module # 5 Teacher’s Guide
Lesson title: UV-VIS Spectroscopy Materials: Book, pen and notebook,
Learning Targets: index card/class list
At the end of the module, students will be able to:
1. Define the importance of UV-Vis Spectroscopy as a References:
tool in measuring electromagnetic radiation; Watson, David G. (2017).
2. Identify the parts of UV-Vis spectrometry, and Pharmaceuticals analysis: a textbook for
3. Identify the type of wavelength according to its length. pharmacy students and pharmaceutical
chemists, 4th ed. Singapore: Elsevier
A. LESSON PREVIEW/REVIEW
Activity 1
Write T if true, F if false.
_T_1. UV-visible spectroscopy measures the response of a sample to ultraviolet and visible range of
electromagnetic radiation.
_T_2. Beer-Lambert’s Law is a combination of the above laws and relates the power of the incident and
transmitted radiant beams to the thickness and concentration of the solution containing the absorbing chemical
species
_F_3. Wavelength is the number of complete cycles which pass a given point per second (cps). [Frequency]
_F_4. Light source may be designed to split the light beam so that the beam passes through two sample
compartments [Optics]
_T_5. Beer’s Law – states that the power of transmitted radiant beam decreases exponentially as the
concentration of the solution containing the absorbing chemical species increases
B. MAIN LESSON
I. INTRODUCTION
Definition of terms:
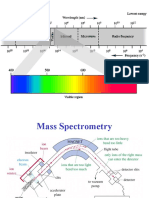
● Electromagnetic spectrum – the complete system of energy propagated in wave form
● Radiant Energy – refers to the energy propagated in the UV, Visible, and IR regions of the
electromagnetic spectrum
● Frequency - the number of complete cycles which pass a given point per second (cps). It may also be
expressed in Hertz, where 1 Hertz= 1cps
● Wavelength – the length of a complete wave or cycle, from the peak of one wave to the peak of the
next. The units used in spectrometry are micrometer (10-4 cm), nanometer (10-7 cm), and less
frequently is angstrom (10-8 cm).
➢ UV – 200 to 380 nm
➢ Vis – 380 to 780 nm
➢ Near IR – 780 to 3000 nm
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 5 Teacher’s Guide
➢ Medium IR – 3.0 to 15 μm
3-8 (group frequency)
8-15 (fingerprint)
➢ Far IR – 15 to 300 μm
Table 1. Summary of wavelengths of different colors in the spectrum
Wavelength of color Color Absorbed Complementary Colors
absorbed (nm) (colors transmitted and seen
by an observer)
380 - 450 Violet Yellow-green
450 - 480 Blue Yellow
480 - 490 Green - blue Orange
490 - 500 Blue - green Red
500 - 570 Green Purple to Red-violet
570 - 590 Yellow Blue
590 - 620 Orange Green-blue
620 - 780 Red Blue-green
IA. UV-Vis Spectroscopy
● Electron Energy Transition
● Used to obtain the absorbance spectra of a compound in solution or as a solid. What is actually being
observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which
excites electrons from the ground state to the first singlet excited state of the compound or material.
● Absorption of photon results in electronic transition of a molecule and electrons are promoted from
ground state to higher electronic states
➢ Principle
UV-visible spectroscopy measures the response of a sample to ultraviolet and visible range of
electromagnetic radiation. Molecules have either π,n,σ electrons. These electrons absorb UV radiation &
undergo transitions from ground state to excited state.
➢ Instrumentation
Parts of UV-Vis Spectrometer
a. Light Source – a deuterium lamp for the UV region from 190 to 350 nm and a quartz halogen or
tungsten lamp for the visible region from 350 to 900 nm.
b. Monochromator – used to disperse the light into its constituent wavelengths, which are further selected
by the slit. The monochromator is rotated so that a range of wavelengths is passed through the sample
as the instrument scans across the spectrum.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 5 Teacher’s Guide
c. Optics – may be designed to split the light beam so that the beam passes through two sample
compartments, and in such a double beam instrument, a blank solution can then be used commonly the
solvent in which the sample is dissolved.
Figure 1. Parts of UV-Vis Spectrometer
➢ Application in Pharmaceutical Analysis:
● A method for the quantification of drugs in formulations where there is no interference from excipients
● Determination of the pKa values of some drugs
● Detection of impurities
● Structure elucidation of organic compounds
● Determination of partition coefficients and solubilities of drugs
● Can be used to monitor the reaction kinetics of drug degradation
● The UV spectrum of a drug is often used as one of a number of pharmacopeial identity checks
IB. LAWS ON SPECTROPHOTOMETRY
❖ Notable Persons
● Pierre Bouguer (1698-1758) – astronomer, Light is diminished as it passes through the atmosphere.
● Johan Lambert (1728-1777) – mathematician, first to prove that π is irrational. No absorption coefficient
● August Beer (1825-1863) – added absorption coefficient and related to concentration in solution
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 5 Teacher’s Guide
❖ Relevant Laws
1. Beer’s Law – states that the power of transmitted radiant beam decreases exponentially as the
concentration of the solution containing the absorbing chemical species increases
2. Lambert’s or Bouguer’s Law – states that the power of a transmitted radiant beam decreases
exponentially as the thickness of the solution containing the absorbing chemical species increases
3. Beer-Lambert’s Law – is a combination of the above laws and relates the power of the incident and
transmitted radiant beams to the thickness and concentration of the solution containing the absorbing chemical
species
This law is expressed through this equation:
A = log10 (I0/I) = εCL
A stands for the absorbance, I0 refers to the intensity of light upon a sample cell, l refers to the intensity of light
departing the sample cell, C stands for the concentration of the solute, L stands for the length of the sample
cell and ε refers to the molar absorptivity.
Based on the Beer-Lambert law, it has been established that the greater the number of the molecules that are
capable of absorbing light at a certain wavelength, the greater the extent of the absorption of light.
C. CHECK FOR UNDERSTANDING
The instructor will prepare 10-15 questions that can enhance critical thinking skills. Students will work by
themselves to answer these questions and write the rationale for each question.
Multiple Choice
(For 1-10 items, please refer to the questions in the Rationalization Activity)
RATIONALIZATION ACTIVITY (DURING THE FACE TO FACE INTERACTION WITH THE STUDENTS)
The instructor will now rationalize the answers to the students and will encourage them to ask questions and to
discuss among their classmates for 20 minutes.
1. Radiant energy is the energy propagated from which regions of the electromagnetic spectrum?
I. UV II. IR III. Radiation IV. Optics
a. I only
b. I and II
c. I and III
d. I and IV
Answer: B
Rationale: Regions of the electromagnetic spectrum that propagate Radiant energy are the UV, Visible, and IR .
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 5 Teacher’s Guide
2. Which part of the UV-Vis Spectrometer is rotated so that a range of wavelengths is passed through the
sample as the instrument scans across the spectrum?
a. Monochromator
b. Optics
c. Light Source
d. Dispersion Device
Answer: A
Rationale: Rotating the Monochromator disperses the light into its constituent wavelengths, which are further
selected by the slit.
3. This law states that as the concentration of the solution containing the absorbing chemical species
increases, the power of the transmitted radiant beam decreases exponentially.
a. Lambert’s Law
b. Beer-Lambert’s Law
c. Bouguer’s Law
d. Beer’s Law
Answer: D
Rationale: Beer’s Law by German Physicist August Beer.
4. The unit that is less frequently used in Spectrometry.
a. Micrometer
b. 10-8 cm
c. Nanometer
d. 10-4 cm
Answer: B
Rationale: Angstrom (10-8 cm) is used less than Micrometer (10-4 cm) and Nanometer (10-7 cm).
5. Frequency is the number of complete cycles which pass a given point per second (cps). How many
Hertz is there in 1 cps?
a. 1
b. 10
c. 1/2
d. 2
Answer: A
Rationale: Frequency can also be expressed in Hertz, where 1 Hertz is 1 cps.
6. If the Wavelength is 495 nm, what is the color absorbed?
a. Green-Blue
b. Blue
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 5 Teacher’s Guide
c. Green
d. Blue-Green
Answer: D
Rationale: Blue-Green is the color absorbed if the Wavelength is 490-500 nm.
7. The length of a complete wave or cycle, from the peak of one wave to the peak of the next.
a. Frequency
b. Electromagnetic Spectrum
c. Wavelength
d. NOTA
Answer: C.
Rationale: Wavelength is the distance between identical points (adjacent crests) in the adjacent cycles of a
waveform signal propagated in space.
8. The following are applications of UV-Vis Spectrometry in Pharmaceutical Analysis, except:
a. Structure elucidation of organic compounds
b. Detection of purity
c. Determination of pKa values of some drugs
d. Determination of partition coefficients and solubilities of drugs
Answer: B.
Rationale: Detection of Impurities
9. Which of the following is paired correctly?
a. Fingerprint – 8-15 μm
b. Vis – 200 to 380 nm
c. UV – 380 to 780 nm
d. AOTA
Answer: A.
Rationale: Fingerprint – 8-15 μm, Vis – 380 to 780 nm, UV – 200 to 380 nm
10. Which of these electrons absorb UV radiation and undergoes transitions from ground state to an
excited state?
a. Π
b. N
c. Σ
d. AOTA
Answer: D
Rationale: Molecules have either π,n,σ electrons.
This document is the property of PHINMA EDUCATION
PHA 052: Pharmaceutical Analysis 2
Module # 5 Teacher’s Guide
D. LESSON WRAP-UP
Teacher directs the student to mark (encircle) their place in the work tracker which is simply a visual to help
students track how much work they have accomplished and how much work there is left to do. This tracker will
be part of the student activity sheet.
PERIOD 1 PERIOD 2 PERIOD 3
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
AL Strategy: MINUTE PAPER
After the instructor collects all papers, he/she will now summarize the topic. Towards the end of the class, ask
the students to bring out and write on a half sheet of paper written feedback to the following questions: Firstly,
what was the most meaningful or important thing they learned during the class. Secondly, the important
question remains unanswered. Make sure to position yourself at the door. Conversely, instruct the students to
file out towards the exit door and collect the “minute papers” as students depart from the room. Respond to
students’ feedback during the next class meeting or as soon as possible
Most meaningful or important thing they learned from this session: (Why did they find it meaningful or
important?)
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
Question which remains unanswered: (What will they do to find the answer?)
________________________________________________________________________________________
________________________________________________________________________________________
________________________________________________________________________________________
This document is the property of PHINMA EDUCATION
You might also like
- Application of Spectral Studies in Pharmaceutical Product development: (Basic Approach with Illustrated Examples) First Revised EditionFrom EverandApplication of Spectral Studies in Pharmaceutical Product development: (Basic Approach with Illustrated Examples) First Revised EditionNo ratings yet
- Report Ni RizaDocument8 pagesReport Ni RizaInga Budadoy NaudadongNo ratings yet
- Uv Spectroscopy 5Document11 pagesUv Spectroscopy 5Emmanuella OffiongNo ratings yet
- METU Chem. Eng. Dept. Ch.E. 410 Chem. Eng. Lab. II: Experiment 2.4 Uv-Visible Spectrophotometry (Uv)Document5 pagesMETU Chem. Eng. Dept. Ch.E. 410 Chem. Eng. Lab. II: Experiment 2.4 Uv-Visible Spectrophotometry (Uv)newtonNo ratings yet
- SpectrophotometryDocument22 pagesSpectrophotometryaziskfNo ratings yet
- MFSN 802 PresentationDocument27 pagesMFSN 802 PresentationGibsonNo ratings yet
- Ei 6501 Analytical Instruments Unit-I Colorimetry and SpectrophotometryDocument24 pagesEi 6501 Analytical Instruments Unit-I Colorimetry and SpectrophotometryBarani DharanNo ratings yet
- Uv SPDocument17 pagesUv SPछेरबहादुर लेउवाNo ratings yet
- Uv Visible SpectrosDocument28 pagesUv Visible Spectrosjoshishravan3003No ratings yet
- Uv Vis & FtirDocument15 pagesUv Vis & FtirVannessa Shallomy100% (2)
- What Is UV SpectrosDocument20 pagesWhat Is UV SpectrosSimranNo ratings yet
- UV Spectroscopy - Principle, Instrumentation, Applications - Instrumentation - Microbe NotesDocument5 pagesUV Spectroscopy - Principle, Instrumentation, Applications - Instrumentation - Microbe NotesIJAJ-PHARMA TUTOR100% (1)
- Tugas B Ingg Ke 2Document6 pagesTugas B Ingg Ke 2Wirda AffiyantiNo ratings yet
- Color Wavelength NM Violet Blue Cyan Green Yellow Orange RedDocument4 pagesColor Wavelength NM Violet Blue Cyan Green Yellow Orange RedJhenard John Lansangan BeltranNo ratings yet
- Spectrophotometry and ColorimetryDocument5 pagesSpectrophotometry and ColorimetryHarish.UNo ratings yet
- Analytical Chemistry Basic and SpectrophotometerDocument11 pagesAnalytical Chemistry Basic and SpectrophotometerVirender BhattiNo ratings yet
- UV SpectrosDocument4 pagesUV SpectrosCarlton GrantNo ratings yet
- Ultra Violet - Visible SpectrosDocument13 pagesUltra Violet - Visible SpectrosSherin SunnyNo ratings yet
- Anachem Group 3 SpectrosDocument15 pagesAnachem Group 3 SpectrosApufwplggl JomlbjhfNo ratings yet
- UVSpectrosDocument22 pagesUVSpectrosAbu Tareq SarkerNo ratings yet
- Q3 - W4 - Research II - SPECTROSCOPIC FTIR UV V2Document9 pagesQ3 - W4 - Research II - SPECTROSCOPIC FTIR UV V210N - B10 Maligaya, Timothy Andre C.No ratings yet
- A StudyDocument6 pagesA StudySunanda VashishatNo ratings yet
- SpectrophotometryDocument7 pagesSpectrophotometrySantanah Daxene DayloNo ratings yet
- Absorption Spectroscopy: Sanjay M. Nilapwar, Maria Nardelli, Hans V. Westerhoff, and Malkhey VermaDocument17 pagesAbsorption Spectroscopy: Sanjay M. Nilapwar, Maria Nardelli, Hans V. Westerhoff, and Malkhey VermaAbdo MohdyNo ratings yet
- Literature Review of Uv SpectrosDocument6 pagesLiterature Review of Uv Spectroseeyjzkwgf100% (1)
- Deldio Bsp2e Mod1 Ex1-2Document12 pagesDeldio Bsp2e Mod1 Ex1-2Veronica DeldioNo ratings yet
- Development and Optimization of Uv-Vis Spectroscopy - A ReviewDocument11 pagesDevelopment and Optimization of Uv-Vis Spectroscopy - A ReviewJafar MohammadNo ratings yet
- InstrumentationDocument4 pagesInstrumentationRainneTayNo ratings yet
- Practical Lab - Finals PDFDocument8 pagesPractical Lab - Finals PDFSarmad HussainNo ratings yet
- Uv Visible RadiationDocument6 pagesUv Visible RadiationNimra LiaqatNo ratings yet
- Uv-Visible Spectroscopy TheoryDocument8 pagesUv-Visible Spectroscopy TheoryHamdan afzalNo ratings yet
- UV-Vis Spectroscopy Uses & ApplicationsDocument5 pagesUV-Vis Spectroscopy Uses & Applicationskurrupp k.pNo ratings yet
- UV Visible SpectrosDocument12 pagesUV Visible SpectrosAbdullah Bin TariqNo ratings yet
- Ultraviolet-Visible SpectroscopyDocument12 pagesUltraviolet-Visible SpectroscopySoumya Ranjan SahooNo ratings yet
- Environmental Engineering Spectrophotometry Part 1Document4 pagesEnvironmental Engineering Spectrophotometry Part 1Sherald AgustinNo ratings yet
- Spectro Photo Me TryDocument8 pagesSpectro Photo Me Trysushil4056No ratings yet
- Ass 1 SDFGHJK DFGHJK DFGHJK Ertyuiop SDFGHJKLDocument10 pagesAss 1 SDFGHJK DFGHJK DFGHJK Ertyuiop SDFGHJKLriniz92No ratings yet
- Introduction To SpectrosDocument24 pagesIntroduction To SpectrosPIRZADA TALHA ISMAIL100% (1)
- Spectrophotometric Measurements Techniques For Fermentation ProcessDocument16 pagesSpectrophotometric Measurements Techniques For Fermentation ProcessSamjith ThomasNo ratings yet
- Spectrophotometry Guided Questions 1 PDFDocument1 pageSpectrophotometry Guided Questions 1 PDFLuci FernNo ratings yet
- UV Visible SpectrosDocument6 pagesUV Visible SpectrosNagarakshita V KNo ratings yet
- Spectrophotometer-Principle, Instrumentation, Applications: What Is A Spectrophotometer?Document2 pagesSpectrophotometer-Principle, Instrumentation, Applications: What Is A Spectrophotometer?Talhas ProductionNo ratings yet
- UV SpectrosDocument7 pagesUV Spectrosbszool006No ratings yet
- PH 7.4 Pharmaceutical Analysis-Iii (Theory) : Unit - IDocument25 pagesPH 7.4 Pharmaceutical Analysis-Iii (Theory) : Unit - Ikari gowdaNo ratings yet
- BOE201 Principle Techniques of Basic Spectrophotometer AAS (1) LEE CHI YIEN 151877Document14 pagesBOE201 Principle Techniques of Basic Spectrophotometer AAS (1) LEE CHI YIEN 151877Chiyien LeeNo ratings yet
- Introduction To Molecular Spectroscopy: By: M.Z.IqbalDocument24 pagesIntroduction To Molecular Spectroscopy: By: M.Z.IqbalMuhammad TausifNo ratings yet
- Group 4 Ultraviolet Visible SpectrosDocument6 pagesGroup 4 Ultraviolet Visible SpectrosJane Frances JabricaNo ratings yet
- Module-6 Unit-4 UV-Vis Spectroscopy SpectrosDocument11 pagesModule-6 Unit-4 UV-Vis Spectroscopy SpectrosManikandan KKNo ratings yet
- Activity - ColorimetricDocument3 pagesActivity - ColorimetricbernantebellerryNo ratings yet
- Lec Module 1 Pharm AnalysisDocument20 pagesLec Module 1 Pharm AnalysisJonas BorjNo ratings yet
- Ultraviolet and Visible (UV-Vis) Absorption Spectroscopy: A Ecl /I)Document17 pagesUltraviolet and Visible (UV-Vis) Absorption Spectroscopy: A Ecl /I)Des MamNo ratings yet
- Principles and Application of Spectroscopic Techniques: Chapter ThreeDocument113 pagesPrinciples and Application of Spectroscopic Techniques: Chapter ThreeKetsela YirdawNo ratings yet
- Spektro (1,2)Document16 pagesSpektro (1,2)Asnita HfsaniNo ratings yet
- Full Report Uv-Vis and FtirDocument25 pagesFull Report Uv-Vis and FtirAmirHakimRusli100% (6)
- Course Code: PHA 305 UV-Visible SpectrophotometryDocument38 pagesCourse Code: PHA 305 UV-Visible SpectrophotometryMahadi Hasan KhanNo ratings yet
- COMSATS University Islamabad, Abbottabad Campus Department of BiotechnologyDocument5 pagesCOMSATS University Islamabad, Abbottabad Campus Department of BiotechnologyHamas QadeerNo ratings yet
- Principle: Visible and Ultraviolet (Uv) SpectrosDocument10 pagesPrinciple: Visible and Ultraviolet (Uv) SpectrosKalyanNo ratings yet
- Ultraviolet-Visible Spectroscopy (Uv-Vis) - FinalDocument15 pagesUltraviolet-Visible Spectroscopy (Uv-Vis) - FinalSwati V NairNo ratings yet
- Uv-Vis Spectroscopy ThesisDocument4 pagesUv-Vis Spectroscopy ThesisCustomCollegePaperUK100% (2)
- Pha052 TG 9Document14 pagesPha052 TG 9Alcea InguilloNo ratings yet
- Pha052 TG 8Document12 pagesPha052 TG 8Alcea InguilloNo ratings yet
- Pha052 TG 7Document7 pagesPha052 TG 7Alcea InguilloNo ratings yet
- Pha052 TG 1Document7 pagesPha052 TG 1Alcea InguilloNo ratings yet
- HPLCDocument2 pagesHPLCHarish KalaiyarvanNo ratings yet
- Identification of Organic and Inorganic Compounds by SpectrosDocument79 pagesIdentification of Organic and Inorganic Compounds by SpectrosAin SkNo ratings yet
- UV-Visible Assignment - ChemContent PDFDocument5 pagesUV-Visible Assignment - ChemContent PDFmohammedabubakrNo ratings yet
- Characterization of Proteins Using Ion Exchange Chromatography and Gel Filtration ChromatographyDocument4 pagesCharacterization of Proteins Using Ion Exchange Chromatography and Gel Filtration ChromatographyEricka GalangNo ratings yet
- GNPS Classic Molecular Networking Overview: Enter Authors Here University of California - San DiegoDocument9 pagesGNPS Classic Molecular Networking Overview: Enter Authors Here University of California - San DiegoskaractNo ratings yet
- Titroline 7500 KF FlyerDocument1 pageTitroline 7500 KF FlyerkanosriNo ratings yet
- Mass SpectrometryDocument49 pagesMass SpectrometryUbaid ShabirNo ratings yet
- EDX and WDX Spectroscopy ComparisonDocument28 pagesEDX and WDX Spectroscopy Comparisonarun aryaNo ratings yet
- Beer'S Law Laboratory Experiment/ SimulationDocument5 pagesBeer'S Law Laboratory Experiment/ SimulationCelyn MillanoNo ratings yet
- Bruker - WikipediaDocument4 pagesBruker - WikipediaPY WangNo ratings yet
- Xiao CHM4130L Lab Manual 2013-1Document35 pagesXiao CHM4130L Lab Manual 2013-1visa1032No ratings yet
- Atomic Absorption Spectroscopy Determination of IronDocument8 pagesAtomic Absorption Spectroscopy Determination of IronShirley Cheong67% (6)
- Unit - 2 MCQ'sDocument5 pagesUnit - 2 MCQ'srishavr2001No ratings yet
- FL52337 Ftir Spectrometer Selection GuideDocument1 pageFL52337 Ftir Spectrometer Selection GuideBenitoNo ratings yet
- IR Spectroscopy Basics - Part 1Document17 pagesIR Spectroscopy Basics - Part 1Dan GuerreroNo ratings yet
- Excedrin Lab Write UpDocument7 pagesExcedrin Lab Write UpPatrick SmithNo ratings yet
- Mossbauer SpectrosDocument15 pagesMossbauer SpectrosSiddharth TanguturiNo ratings yet
- Spectrophotometric Determination of Iron Using 1,10-PhenanthrolineDocument9 pagesSpectrophotometric Determination of Iron Using 1,10-Phenanthrolinedawin_mornaNo ratings yet
- Nmr-Nuclear Overhauser Enhancement (NOE)Document2 pagesNmr-Nuclear Overhauser Enhancement (NOE)sajialex1No ratings yet
- Tanabe Sugano DiagramsDocument7 pagesTanabe Sugano DiagramsDonnie Vailoces ReyesNo ratings yet
- Origins of Atomic SpectraDocument2 pagesOrigins of Atomic SpectraSean CollinsNo ratings yet
- Biochemistry Assignment 1Document14 pagesBiochemistry Assignment 1Ananya DwivediNo ratings yet
- Background Correction in AASDocument1 pageBackground Correction in AASSean CollinsNo ratings yet
- Khulna University of Engineering &: TechnologyDocument4 pagesKhulna University of Engineering &: TechnologyASHIR HASAN NIBIRNo ratings yet
- Determination of Copper by AASDocument18 pagesDetermination of Copper by AASscarmathor9092% (50)
- NexION 300 BrochureDocument12 pagesNexION 300 BrochureJhamal SalazarNo ratings yet
- Mass Spectrometry: An IntroductionDocument21 pagesMass Spectrometry: An IntroductionSatriani 0557No ratings yet
- MSCCH-17/18/19 CHE-504 IR Spectroscopy LectureDocument22 pagesMSCCH-17/18/19 CHE-504 IR Spectroscopy Lectureabdelfattah oufNo ratings yet
- Nhan, TT.Document4 pagesNhan, TT.fernandaNo ratings yet
- Omed0104 2022 MayDocument4 pagesOmed0104 2022 MayIffa NooramNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Meltdown: Nuclear disaster and the human cost of going criticalFrom EverandMeltdown: Nuclear disaster and the human cost of going criticalRating: 5 out of 5 stars5/5 (5)
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastFrom EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo ratings yet
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Gas-Liquid And Liquid-Liquid SeparatorsFrom EverandGas-Liquid And Liquid-Liquid SeparatorsRating: 3.5 out of 5 stars3.5/5 (3)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeRating: 4 out of 5 stars4/5 (9)
- Stuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldFrom EverandStuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldRating: 4 out of 5 stars4/5 (289)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet