Professional Documents
Culture Documents
CertiFicate 3
CertiFicate 3
Uploaded by
G & M Soft Technologies Pvt Ltd0 ratings0% found this document useful (0 votes)
31 views6 pagesOriginal Title
CertiFicate-3
Copyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
0 ratings0% found this document useful (0 votes)
31 views6 pagesCertiFicate 3
CertiFicate 3
Uploaded by
G & M Soft Technologies Pvt LtdCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You are on page 1of 6
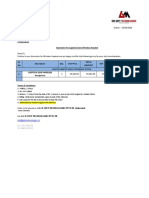
TELANGANA STATE POLLUTION CONTROL BOARD
Teflon Offi, Door. No 7-164, Sabas a= 508 2
No: 08462-297774, e-mail go-nsbsocb@telagana oovin
‘Webste: wm spcb 99 gov
(issued under Rule 10 of the Bio-Medical Waste Management Rules, 2016)
Order No.11561 PCBIRONZB/BMWM2022 -¢)) Date: 26.10.2022,
4. Wis. Anvita Hospital, Opp: Saraswathi Temple, Banswada, Kamareddy
District is hereby granted an authorisation for Generation, Segregation,
Colleton & Storage of Bio-Medical Waste
2, Mis. Anvita Hospital, Opp: Saraswathi Temple, Banswada, Kamareddy
District hereby authorized for handling of biomedical waste as per the capacity
siven below;
() Number of beds of HCF: 9 Beds.
(i) Quantity of Biomedical waste handled, treated or disposed:
[Type of Waste Category | Quantity permed forHandling
Yellow 0.5 Kg/Day
Red 0.4 Kg / Day
White Translucent) 0.1K Day
Blue =
3. This authorisation shall be in force for a period upto 30.09.2027.
4. This authorisation is subject to the conaitions stated below and to such other
conditions as may be specified in the rules for the time being in force under the
Environment (Protection) Act, 1986
(
Nizamabad, EAMRIROMEN TL ENGIEER
eee TS. Pollution Control Board
“CLEAN AND GREEN” TELANGANA STATE
Terms and conditions of the authorizations:
1, The HCF shall provide CC Camera at waste storage area and pick up point of CBMMWTE
vehicle and the recorded data shall be stored for @ period of,
3 months and available for inspection by the Board Officials
2. The authorisation shall comply withthe provisions ofthe Environmen: (Protection) Act,
1986 and the rules made there under.
3. The authorisation or Its renewal shall be produced for inspection at the request of an
officer authorised by the Telangana State Polltion Control Board
4. The person authorized shall not rent, lend, sel transfer or otherwise transport the
biomedical wastes without obtaining prior permission of the Telangara State Pollution
Control Board
5. Any unauthorised change in personnel, equipment or working conditions as mentioned
In the application by the person authorised shall consttute a breach ofthis authorisation
6, tis the duty ofthe authorised person to take prior permission of the Telangana State
Pollution Contol Board to cose down the faclity and such other terms and conditions,
that may be stipulated by the Telangana State Pollution Control Board,
7. Any other conditions for compliance 8s per the Guidelines issued by tre MoEF & CC or
‘CPCB from time to time.
8. The Bio Madical Waste shall be disposed for treatment after disinfecion and
segregation tothe following Common Bio-Medical Waste Treatment Fait.
Mis. Sri Medicare Services.
Padkal (V), Jakranpally (M),
Nizamabad District.
‘SPECIAL CONDITIONS
1. All the provisions of the Bio-Medical Waste Management Rules, 2016 must be complied
with
2, The HCF shall take all necessary steps to ensure that bio-medical waste is handled
without any adverse effect to human health and the environment and in accordance with
Bio-Medical Waste Management Rules, 2016.
3. The HCF shall make a provision within the premises fora safe, vetlcted and secured
location for storage of segregated biomedical waste in colored bags or containers in the
‘manner as specified in Schedule | of the BMWM Rules, 2016. It shal be ensured that
there shall be no secondary handling, piferage of recyclables or inadvertent scattering
‘oF epilage by animals and the bio-medical waste from such place or gremises shall be
directy transported in the manner as prescribed in these rules to the common bio-
‘medical waste treatment facility or for the appropriate treatment and disposal, as the
‘case may be, in the manner as prescribed in Schedule | of the Bio-Medical Waste
Management Rules, 2016,
4. The HOF shal pretreat the laboratory waste, microbiological waste, bood samples and
blood bags through disinfection or sterlisation on-site in the manner as prescribed by
the World Health Organisation (WHO) or National AlDs Control Organisation (NACO)
{guidelines and then sent to the common biosnedical waste treatment facility for final
slisposal
5, The HGF shall phase out use of chionated plastic bags, cloves and slood bags within
two years from the date of notieation of the Bio-Medical Waste Management Rules,
2016
6. The HOF shall dispose of solid waste other than bio-medical waste in accordance with
the provisions of respective waste management rules made under the ‘levantlavs and
‘amends from time fo time,
7. The HCF shall not o give treated bio-medical waste with municipal sold waste.
8. Tho HGF shall establish a Bar- Code System for bags or containers containing bio-
‘medical waste to be sent out ofthe premises or place for any purpose within one year
from the date ofthe notification of te Bio Madieal Waste Management Rules, 2016
‘9, The HOF shall ensure segregaton of liquid chemical waste at source and ensure pre-
{teatment or neutralisation prior to mixing wth other fluent generated from health care
facies,
10.The HCF shall ensure treatment and disposal of quid waste in accordance with the
‘Water (Prevention and Control of Politon) Act. 1974 (6 of 1874).
11. The HOF shall maintain and update on day to day basis the bio-medical waste
‘management register and csplay the monthly record on its website according tothe bio-
radical waste generated In terms of category and colour coding as spectied in
‘Schedule | of the Bio-Medical Waste Management Rules, 206.
12:The HCF shal inform to TSPCB immediatly in case the operator of facility does not
collect he blo-macieal waste within the intended time or as par the agreed time,
18:The HOF shal establish 2 system to reviw and monitor the activites related to bio.
medical waste management by forming a new committee and the Commitee shall meet
‘once in every six month and tte record of te minutes of the naeting of this
‘committee shall be submited along withthe annual report to the prescribed author
14.1 the responsibilty of the occupier of the HF that the only segregated bio-medical
‘waste as par the Schedule —| of the EMW Management Rules, 2016 shal be handed
‘vor to common bio-medical waste treatment faciiy for treatment, processing and final
‘isposal
16ilt shall be ensured that no untreated bio-medical waste shall be mixed with other
‘wastes,
16.The bio-medical waste shall be segregated into containers or bags at the point of
‘generation In accordance with Schedule | of the BMW Management waste treatment
Rule, 2016 prior tis storage, transportation, treatment and disposal
{11The containers or bags refered to in sub-rule (2) shall be labelec as spectied in
Schedule IV of the BMI Management Rules, 2016. The bar cading and global
positioning system shall be added by the Occupier and Common bi>-Medical Waste
{reatment facity in one year time.
18.Unteated human anatomical waste, animal anatomical waste, soled waste and
biotechnology waste shall not be kept stored beyond a period of 48 rours. Provided
that in case for any reaton it becomes necessary to store such wasle beyond such a
Period, the occupier shall ake appropriate measures to ensure thatthe waste does not
‘dversely affect human health and the envconment and inform the prescribed authority
(TSPCB) along withthe reasons for doing so.
19.Dead Fetus below the viabilty period (as per the Metical Termination of Pregnancy Act
1971, amended from time to time) can be considered as human anatomical waste. Such
waste should be handed over tothe operator of common bio-medical waste treatment
‘and dsposal facility In yellow bag with a copy of the official Medical Termination of
Pregnancy cerificate fom the Obstetrician or the Medical Superintencent of hospital or
healthcare estabishment
20.Cytotoxic drug vials shall not be handed over to unauthorized person under any
‘Gteumstances. These shall be sent back to the manufactures for necessary disposal at
2 single point. As'a second option, these may be sent for incineration at common bio-
tacical waste Weatment and disposal faciily or TSDFS or plasma pyrsis 1S at
temperature>12006
21.Residual or ciscarded chemical wastes, used or discarded disinfectants and chemical
Sludge can be disposed at hazardous waste treatment, storage and dsposal fait. In
‘such case, the waste should be sent to hazardous waste treatment, storage and
tisporal facity through operator af common bio-medical waste eatrent and disposal
faciity ony
22.0n-site pretreatment of laboratory waste, microbiological waste, bicod samples and
blood bags should be disinfected or steriized as per the Guidelines of World Health
Organization or National AIDS Control Organization and then given to the common bio-
rmacical waste treatment and disposal faci.
23.Syringes should be either mutlated or needias should be cut and or stored in tamper
too, leak proof and puncture proof containers for sharps storage.
24.The HOF shall maintain records related tothe generation, collection, reception, storage
‘wansportaio, treatment, disposal or anyother form of handing of bio-medical waste.
25.The HOF shall submit an annual report tothe prescribed authority (TSPCB) in Form —
IV, on ar before the 30" June of every year for the period from January to December of
the preceding year.
26.The HCF shall make avalable the annual report on is website and al the healthcare
facies shall make own website within two years from the date of notfeation ofthe Bio-
Medical Waste Management Rules, 2016.
27.1n case of any change in the bio-medical waste generation, handing, teatment and
isposal for which authorization was earler granted, the occupier of operator of HCF
shall inmate to the prescribed authority about the change or vatition nthe acviy and
shall submit 2 ffesh applicaton in Form Il for modification of the condiions of
Authorization,
28.In case of any major accident at HCF facility or anyother site while handing biomedical
waste, the authorised person shall inimate immediately to the prescribed authority
‘about such accident and forward a report within twenty-four hours inating regarding
the remedial steps taken in Form
29.The HCF shall ensure occupational safety ofall its health care woikers and others
involved in handling of biomedical waste by providing approptiate and adequate
personal protective equipments.
30.The occupier of the HOF or an operator of a common bio-medical waste treatment
facity shall be lable forall he damages caused tothe environment or the public due to
improper handing of bio-medical wastes. The occupier or operator of common bio-
‘medical waste treatment facity shall be lable for action under section 6 and section 18,
(ofthe Act. In ease of any velaton,
31.The HCF shall adopt the folowing treatment and disposal methods as described in the
‘Bio-Medical Waste Management ules, 2016,
|. Chemical treatment using atleast 10% Sodium Hypochiotte hang 30% residual
Chlorine for twenty minutes or any other equivalent chemical reagent that should
demonstrate Logi reduction efficiency for microorganisms as given in Schedule-
i
|i. Mutlation or shredding must be to an extent to prevent unauthorized reuse
32.The HCF shall provide taining to al is health care workers and ones, involved in
handling of bio matical wasta at tha time of Induction and thereafter al least once every
year and the deta of taining programmes conducted, number of personne! trained
{nd number of personnel not undergone any training shall be provided in the Annual
Roped.
38:The fact shal immunise all its health care workers and others, involved in hancling of
bio-medical waste for protection against diseases including Hepatitis Band Tetanus that
are likely to be transmitted by handing of biomedical waste, in the manner 9s
prescribed inthe Nationa Immunisation Policy or the guidlines of tha Ministry of Health
‘and Family Welfare issued ffom time to time.
'34.The facility shall conduct health check up atthe time of induction and at least once in a
‘year for alts healthcare workers and athers involved in handling of bo- medical waste
{and maintain the records forthe same.
ENVIRONMENTAL ENGINEER
—7efetéevIRONMENTAL ENGINEER
To, TS. Pollution Soatrol Board
Mis, Anvita Hospital, Regional Office, Nizamabad.
‘Opp: Saraswathi Temple,
Banswada, Kamareddy District.
NB:
‘This authorization shall be exited inthe above premises and should be produced from
time to time atthe request of the Inspecting Officer.
You might also like
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (822)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (401)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (897)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (540)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5813)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (348)
- All Generator List-2 PDFDocument26 pagesAll Generator List-2 PDFG & M Soft Technologies Pvt Ltd100% (1)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1092)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (844)
- Quote For UNSW Global India PVT Ltd. Logitech VC April 2021Document1 pageQuote For UNSW Global India PVT Ltd. Logitech VC April 2021G & M Soft Technologies Pvt LtdNo ratings yet
- Quote For Potru IT Solutions Pvt. Ltd. Logitech Wireless Headset Sep 2020Document1 pageQuote For Potru IT Solutions Pvt. Ltd. Logitech Wireless Headset Sep 2020G & M Soft Technologies Pvt LtdNo ratings yet
- M.A Automation LUMI ProductsDocument1 pageM.A Automation LUMI ProductsG & M Soft Technologies Pvt LtdNo ratings yet
- Kothaguda 6000SFT Lights & WorkingDocument2 pagesKothaguda 6000SFT Lights & WorkingG & M Soft Technologies Pvt LtdNo ratings yet
- Laminate and Venner - ApprovedDocument1 pageLaminate and Venner - ApprovedG & M Soft Technologies Pvt LtdNo ratings yet
- MAXHUB Wireless Dongles PDFDocument2 pagesMAXHUB Wireless Dongles PDFG & M Soft Technologies Pvt LtdNo ratings yet
- Estimate 1 CCTVDocument2 pagesEstimate 1 CCTVG & M Soft Technologies Pvt LtdNo ratings yet
- Jio Buy Grocery Online at Best Prices Pan IndiaDocument2 pagesJio Buy Grocery Online at Best Prices Pan IndiaG & M Soft Technologies Pvt LtdNo ratings yet
- Quote For Volta Impex PVT LTD Logitech VC April 2021Document1 pageQuote For Volta Impex PVT LTD Logitech VC April 2021G & M Soft Technologies Pvt LtdNo ratings yet
- Quote For Nexgen Techtronics PVT Ltd. Logitech VC April 2021Document1 pageQuote For Nexgen Techtronics PVT Ltd. Logitech VC April 2021G & M Soft Technologies Pvt LtdNo ratings yet
- Quote For Zestiot Hyderabad Logitech VC November 2021Document2 pagesQuote For Zestiot Hyderabad Logitech VC November 2021G & M Soft Technologies Pvt LtdNo ratings yet
- Jio Order ViewDocument2 pagesJio Order ViewG & M Soft Technologies Pvt LtdNo ratings yet
- Jio Buy Grocery Online at Best Prices Pan IndiaDocument2 pagesJio Buy Grocery Online at Best Prices Pan IndiaG & M Soft Technologies Pvt LtdNo ratings yet
- CertiFicate 4Document6 pagesCertiFicate 4G & M Soft Technologies Pvt LtdNo ratings yet
- Logitech Meetup Expansion Mic.Document1 pageLogitech Meetup Expansion Mic.G & M Soft Technologies Pvt LtdNo ratings yet
- Quote For Suven Logitech VC Sep 2020Document1 pageQuote For Suven Logitech VC Sep 2020G & M Soft Technologies Pvt LtdNo ratings yet
- Quote For Waterways Shipyard PVT LTD Maxhub Interactive Display Feb 2022Document2 pagesQuote For Waterways Shipyard PVT LTD Maxhub Interactive Display Feb 2022G & M Soft Technologies Pvt LtdNo ratings yet
- 2223 03352Document4 pages2223 03352G & M Soft Technologies Pvt LtdNo ratings yet
- Samsung Business TV Brochure SWA 210413 WEBDocument8 pagesSamsung Business TV Brochure SWA 210413 WEBG & M Soft Technologies Pvt LtdNo ratings yet
- HP MOQ Modern BPC Aug'21 Pricelist - FTPDocument10 pagesHP MOQ Modern BPC Aug'21 Pricelist - FTPG & M Soft Technologies Pvt LtdNo ratings yet
- Endpoint Security Advanced DatasheetDocument2 pagesEndpoint Security Advanced DatasheetG & M Soft Technologies Pvt LtdNo ratings yet
- CertiFicate 6Document1 pageCertiFicate 6G & M Soft Technologies Pvt LtdNo ratings yet
- CertiFicate 5Document1 pageCertiFicate 5G & M Soft Technologies Pvt LtdNo ratings yet
- Select DatasheetDocument4 pagesSelect DatasheetG & M Soft Technologies Pvt LtdNo ratings yet
- 2223 03063Document2 pages2223 03063G & M Soft Technologies Pvt LtdNo ratings yet
- Blackwire 8225 Ds enDocument2 pagesBlackwire 8225 Ds enG & M Soft Technologies Pvt LtdNo ratings yet
- H570e Datasheet enDocument2 pagesH570e Datasheet enG & M Soft Technologies Pvt LtdNo ratings yet
- H650e DatasheetDocument2 pagesH650e DatasheetG & M Soft Technologies Pvt LtdNo ratings yet
- HP MOQ Traditional BPC Aug'21 Pricelist - FTPDocument6 pagesHP MOQ Traditional BPC Aug'21 Pricelist - FTPG & M Soft Technologies Pvt LtdNo ratings yet