Professional Documents
Culture Documents
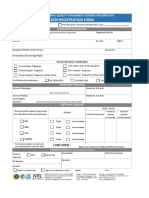
CCG - Sanger Sequencing Mlpa Request Form
Uploaded by
api-644140941Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CCG - Sanger Sequencing Mlpa Request Form
Uploaded by
api-644140941Copyright:
Available Formats
Accreditation No.
20401
Accredited for compliance with NPAAC Standards and ISO 15189
Sanger Sequencing / MLPA Request Form Test Requested: ☐ Sanger Sequencing ☐ MLPA
PATIENT DETAILS
MRN: Phone/ Mobile:
Surname: Address:
Given Name: DOB: Address cont.:
Gender: ☐ Female ☐ Male ☐ Unknown Email:
REQUESTING DOCTOR
Name: Provider Number:
Address: ☐ Email to:
Phone/ Mobile: ☐ Hard copy:
Signature:
COPY REPORT TO
Doctor: ☐ Email copy to:
Phone/ Mobile: ☐ Hard copy:
SPECIMEN INFORMATION (Collector / Sender to complete)
Print Name: Signature: Date/Time of Collection:
EDTA Whole Blood Number of tubes collected: Collect: 1 x 5-10ml EDTA (adults) or 1 x 2-5ml EDTA (children)
Extracted DNA (50-100ng/µl, total volume ≥50µl) Concentration: Elution Buffer: Total Volume:
Other sample types (i.e. buccal swab, saliva), details:
For ACT Pathology Collection Centres:
- Register test as “CCG Test”. Refer to Kestral ALT-9 for more information.
For All Other Collection Centres:
- Send samples to: Canberra Clinical Genomics, The Australian National University, Hugh Ennor Building, 117 Garran Rd ACTON 2601
REASON FOR TEST
☐ Confirmation of previous finding ☐ Diagnostic ☐ Predictive Test* ☐ Family studies
Additional information:
* Sample from the known affected individual is required as a positive control. Laboratory recommends 2 blood samples collected at different times.
DNA VARIANT DETAILS
No. Gene Chr : Coordinates (indicate GRch37/ GRch38)
1
2
PATIENT CONSENT
I understand:
• My / my child’s DNA will be tested for the gene(s) associated with my / my child’s condition. Only the variants listed above will be
analysed and interpreted
• Test results may have implications for the health care of my blood relatives
• My / my child’s de-identified results may be used to help the counselling and testing of other family members
• Testing will not currently affect the ability to obtain health insurance but may affect applications for some types of risk-rated insurances
such as life and income protection insurance
• Testing is voluntary and I can withdraw or cancel testing at any stage
• My/ my child’s DNA sample will be stored in accordance with national diagnostic laboratory guidelines
I consent to the testing described above. The test has been explained to me by a health professional and I have had the opportunity to ask
questions and I am satisfied with the explanations.
Patient / Parent / Guardian Name Patient / Parent/ Guardian Signature Date
Health Professional Name Health Professional Signature Date
Deliver samples to: Canberra Clinical Genomics, The Australian National University, Hugh Ennor Building, 117 Garran Rd ACTON 2601
Laboratory hours: 8:30am – 4:30pm. Laboratory: (02) 6125 7756. Office: (02) 5124 5630. Email: CCG@act.gov.au COR41 v4.0
You might also like
- One Step Med: General Medical Information Record Keeping ManualFrom EverandOne Step Med: General Medical Information Record Keeping ManualNo ratings yet
- IMPORTANT: The Original of This Form Is To Be Kept by The Seafarer. A Copy Must Be Kept by The ClinicDocument2 pagesIMPORTANT: The Original of This Form Is To Be Kept by The Seafarer. A Copy Must Be Kept by The ClinicTigreal MLNo ratings yet
- Httpsen - Bndrgene.med - Sauploads75417541248labm Qms Doc X 002 Informed Consnet Form 1 PDFDocument2 pagesHttpsen - Bndrgene.med - Sauploads75417541248labm Qms Doc X 002 Informed Consnet Form 1 PDFz.a.alsa3dNo ratings yet
- Kardex Vac TemplateDocument2 pagesKardex Vac TemplateEllyza EvangelistaNo ratings yet
- Government of Telangana Covid Interim Test Report Centre For DNA Fingerprinting and Diagnostics (CDFD)Document2 pagesGovernment of Telangana Covid Interim Test Report Centre For DNA Fingerprinting and Diagnostics (CDFD)RameshAithagoinaNo ratings yet
- Health & Family Welfare Department: Government of West BengalDocument2 pagesHealth & Family Welfare Department: Government of West BengalSyed's Way PoolNo ratings yet
- Health & Family Welfare Department: Government of West BengalDocument2 pagesHealth & Family Welfare Department: Government of West BengalAnkit JhaNo ratings yet
- Biochemistry Test Name Results Biological Reference Interval Units Specimen Test MethodDocument6 pagesBiochemistry Test Name Results Biological Reference Interval Units Specimen Test Methodsanjit biswasNo ratings yet
- Aperiomics Order Form: Xplore-PATHODocument2 pagesAperiomics Order Form: Xplore-PATHOSasu AlexandruNo ratings yet
- RT PCR Test ReportDocument2 pagesRT PCR Test ReportMoumita MandalNo ratings yet
- Health & Family Welfare Department: Government of West BengalDocument2 pagesHealth & Family Welfare Department: Government of West BengalBiswajit GhoshNo ratings yet
- Aithsh Gia Eidikes Syn8hkes E3etashs University of MichiganDocument1 pageAithsh Gia Eidikes Syn8hkes E3etashs University of MichiganMaria AndromidaNo ratings yet
- Clinical Microbiology Laboratory Test RequisitionDocument1 pageClinical Microbiology Laboratory Test RequisitionRhodjane Dela Cruz0% (1)
- Health & Family Welfare Department: Government of West BengalDocument2 pagesHealth & Family Welfare Department: Government of West BengalAnkit JhaNo ratings yet
- HIM 200 Health RecordDocument32 pagesHIM 200 Health RecordMaryamNo ratings yet
- Drug Test Request: Accession No.: OR#: Code No.Document6 pagesDrug Test Request: Accession No.: OR#: Code No.OptimaCare MalabonNo ratings yet
- CoC & CCF Forms PDFDocument6 pagesCoC & CCF Forms PDFRitz Bautista BalanayNo ratings yet
- Huntington Disease - HTT GeneDocument2 pagesHuntington Disease - HTT GeneAnupriya ParthibanNo ratings yet
- NEQAS Registration Form 2020-SerologyDocument2 pagesNEQAS Registration Form 2020-SerologyMark Jefferson Sanchez Merto100% (5)
- Laboratory Report: Patient: Ordering PhysicianDocument1 pageLaboratory Report: Patient: Ordering PhysicianJake MorganNo ratings yet
- Lab. Clinico Teresita: AntigenDocument1 pageLab. Clinico Teresita: AntigenRaul MelendezNo ratings yet
- 2034 PDFDocument2 pages2034 PDFJagyanseni BeheraNo ratings yet
- TRN2044095 7939696 Clinical ReportDocument10 pagesTRN2044095 7939696 Clinical ReportWARNET SANTONo ratings yet
- Lab Report - 040767-2122 - 1Document1 pageLab Report - 040767-2122 - 1Muhammad ImranNo ratings yet
- Sample Id: 18344783 Icmr Specimen Referral Form For Covid-19 (Sars-Cov2)Document2 pagesSample Id: 18344783 Icmr Specimen Referral Form For Covid-19 (Sars-Cov2)Asupalli SirishaNo ratings yet
- TRN2044041 7939695 Clinical ReportDocument10 pagesTRN2044041 7939695 Clinical ReportWARNET SANTONo ratings yet
- RTPCR Format - NegativeDocument3 pagesRTPCR Format - NegativeAtul BangalNo ratings yet
- Health & Family Welfare Department: Government of West BengalDocument2 pagesHealth & Family Welfare Department: Government of West BengalSounak JasuNo ratings yet
- Report 125f75b4Document2 pagesReport 125f75b4drupscNo ratings yet
- Sze To Wing LeeDocument1 pageSze To Wing LeeHY Hong YiNo ratings yet
- Thal - DNA Analysis Request Consent Form - Version 4.1 Issued 03 Jan 2023 With BI ConsentDocument2 pagesThal - DNA Analysis Request Consent Form - Version 4.1 Issued 03 Jan 2023 With BI ConsentZalina ZamirayyanNo ratings yet
- Xiulan Zhang - Sars - Cov - 2 - RT - PCR - Best - DiagnosticsDocument2 pagesXiulan Zhang - Sars - Cov - 2 - RT - PCR - Best - DiagnosticsOlan PrinceNo ratings yet
- Covid19 3Document2 pagesCovid19 3Bahirkhand SchoolNo ratings yet
- Covid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedDocument2 pagesCovid-19 (Sars-Cov-2 Rna RT-PCR) : Result: Not Detected Remark: Individual Specimens Reference Range: Not DetectedRonni PriceNo ratings yet
- Laboratory Services: Patient Name Lab No Uhid Sample Date Age/Gender Receiving Date Referred by Report Date Report StatusDocument26 pagesLaboratory Services: Patient Name Lab No Uhid Sample Date Age/Gender Receiving Date Referred by Report Date Report StatusTamzid Rabby TanmoyNo ratings yet
- Pathology ReportDocument1 pagePathology ReportAmirah AqilahNo ratings yet
- Please Refer To Our Website For Full List of Specialist ServicesDocument1 pagePlease Refer To Our Website For Full List of Specialist ServicesawdchiNo ratings yet
- Your Test Result: ICMR Registration Number: COREG001Document3 pagesYour Test Result: ICMR Registration Number: COREG001BCom HonsNo ratings yet
- Melba Sub PacketDocument14 pagesMelba Sub Packetpkpraj29No ratings yet
- RT-PCR Test Report: Name of The Covid19 Testing Centre: VRDL, Zoram Medical College, Falkawn, Mizoram, IndiaDocument1 pageRT-PCR Test Report: Name of The Covid19 Testing Centre: VRDL, Zoram Medical College, Falkawn, Mizoram, IndiaFelaNo ratings yet
- Neha Sharma - 31862159 - 9 12 2018 - 18 9 PDFDocument2 pagesNeha Sharma - 31862159 - 9 12 2018 - 18 9 PDFAnonymous ClNzkEwgVJNo ratings yet
- Icmr 33930643Document2 pagesIcmr 33930643saicharan reddyNo ratings yet
- Test Requisition Form: Promotion/Contract CodeDocument5 pagesTest Requisition Form: Promotion/Contract CodeTiberiu DomuncuNo ratings yet
- Test Report: Method: Qualitative Real Time PCR by Quantstudio 5 (ABI) Thermo Scientific, USA Icmr Reg No: PacapalabvgDocument2 pagesTest Report: Method: Qualitative Real Time PCR by Quantstudio 5 (ABI) Thermo Scientific, USA Icmr Reg No: PacapalabvgDevendra SinghNo ratings yet
- Registration FormDocument1 pageRegistration Formمدینة سرجيكل وہاڑیNo ratings yet
- Peripheral Blood - N: MR M Edukondalu Kphb9502Document1 pagePeripheral Blood - N: MR M Edukondalu Kphb9502Edukondalu MorlaNo ratings yet
- Peripheral Blood - N: MR M Edukondalu Kphb9502Document1 pagePeripheral Blood - N: MR M Edukondalu Kphb9502Edukondalu MorlaNo ratings yet
- Laboratory Report /:: Dis. At:::::: 31 Years 0122201673 Male Mr. Shakti Singh GaurDocument2 pagesLaboratory Report /:: Dis. At:::::: 31 Years 0122201673 Male Mr. Shakti Singh GaurShakti singh gaur100% (1)
- Microbiology Report: UrineDocument1 pageMicrobiology Report: UrineWaqas SaleemNo ratings yet
- RAJESH BHOSALE ReportDocument1 pageRAJESH BHOSALE ReportPADMANABANNo ratings yet
- FRM 01326 Request For HLA HPA Compatible Platelets V11Document4 pagesFRM 01326 Request For HLA HPA Compatible Platelets V11baghdad labNo ratings yet
- Hasil Pemeriksaan Laboratorium: Laboratory Test ResultDocument1 pageHasil Pemeriksaan Laboratorium: Laboratory Test ResultsandraNo ratings yet
- Report 2Document2 pagesReport 2vipultrivedi9049No ratings yet
- CGH202106011819 Lab-2021-0279065 Laboratory Covid-Pcr-TestDocument2 pagesCGH202106011819 Lab-2021-0279065 Laboratory Covid-Pcr-TestAaron David SubaNo ratings yet
- Test Report: Orf 1ab Negative N Gene Negative Internal Control Pass 2019-Ncov NegativeDocument1 pageTest Report: Orf 1ab Negative N Gene Negative Internal Control Pass 2019-Ncov NegativeNilotpal RaiNo ratings yet
- New Health Screening Medical FormDocument4 pagesNew Health Screening Medical Formsalmankhan.484875No ratings yet
- Sample Id: 20799863 Icmr Specimen Referral Form For Covid-19 (Sars-Cov2)Document2 pagesSample Id: 20799863 Icmr Specimen Referral Form For Covid-19 (Sars-Cov2)Revanth ReddyNo ratings yet
- Blood Test (2) - 2Document1 pageBlood Test (2) - 2Dhruv JainNo ratings yet
- Case HistoryDocument6 pagesCase Historypradeepgade1No ratings yet
- Us 7XH3 3xuw5Document2 pagesUs 7XH3 3xuw5Hood RobertsNo ratings yet
- (D. Reheul, B. de Cauwer, M. Cougnon, J. AperDocument363 pages(D. Reheul, B. de Cauwer, M. Cougnon, J. AperEQo AttamiriNo ratings yet
- Dna, Genes and GenomesDocument4 pagesDna, Genes and GenomesRobert Martin PuertaNo ratings yet
- Genome MappingDocument22 pagesGenome MappingVarshika SinghNo ratings yet
- Molecular Biophysics and BiochemistryDocument3 pagesMolecular Biophysics and BiochemistryukrikeNo ratings yet
- Modularity and Domain Speci CityDocument10 pagesModularity and Domain Speci CityThales SilvaNo ratings yet
- Chapter Merinoetal 2018Document23 pagesChapter Merinoetal 2018Led TassoNo ratings yet
- I. Introduction To Anatomical Terms and Organization of The Human BodyDocument94 pagesI. Introduction To Anatomical Terms and Organization of The Human BodyAradani Ongchi Frena100% (1)
- GD060 - Gender - Biological TheoryDocument2 pagesGD060 - Gender - Biological TheoryoasisNo ratings yet
- New Species of The Genus Curvularia. Fungal Keratitis 2020Document14 pagesNew Species of The Genus Curvularia. Fungal Keratitis 2020Anonymous argZ1ZNo ratings yet
- Sargam SoodDocument1 pageSargam SoodMayank JunejaNo ratings yet
- 1 - Histology Lecture - Structure of Cell Membrane and Membranous OrganellesDocument40 pages1 - Histology Lecture - Structure of Cell Membrane and Membranous OrganellesAMIRA HELAYELNo ratings yet
- Dotter ManualDocument17 pagesDotter ManualVlad - Stefan BogdanNo ratings yet
- Bekish-VY - Multiple Choice Questions Book On Medical Biology - 2008Document78 pagesBekish-VY - Multiple Choice Questions Book On Medical Biology - 2008nishaninishaNo ratings yet
- EOL Exemplo 01Document7 pagesEOL Exemplo 01Amanda BarbioNo ratings yet
- Molecular MarkersDocument42 pagesMolecular Markerssaroj Burlakoti100% (1)
- Lesson 3 - Prokaryotic Cell and Eukaryotic CellDocument34 pagesLesson 3 - Prokaryotic Cell and Eukaryotic CellVillanueva, Liv Harlet A.No ratings yet
- West Bengal Board Class 10 Life Science 2016 Question PaperDocument6 pagesWest Bengal Board Class 10 Life Science 2016 Question PaperSarfaraz AhmadNo ratings yet
- More Than Meets The Eye (Nature429)Document2 pagesMore Than Meets The Eye (Nature429)mxolisilenox196No ratings yet
- Chap. 6 - 8 MaternalDocument16 pagesChap. 6 - 8 MaternalKaryll RomeroNo ratings yet
- Chapter 6Document22 pagesChapter 6Marlop CasicasNo ratings yet
- Nuevo Coronavirus en MurcielagosDocument22 pagesNuevo Coronavirus en MurcielagosCarlos Vinicio Chiluisa GuachoNo ratings yet
- NCG Guidelines 2020: Paediatric Hematolymphoid and Solid TumoursDocument46 pagesNCG Guidelines 2020: Paediatric Hematolymphoid and Solid TumoursSudipto BhattacharyaNo ratings yet
- Prokaryotic Vs Eukaryotic Cells NotesDocument4 pagesProkaryotic Vs Eukaryotic Cells NotesMischi Jeda ElumbaNo ratings yet
- Microbiota Composition in The Lower Respiratory Tract Is Associated With Severity in Patients With Acute Respiratory Distress by InfluenzaDocument11 pagesMicrobiota Composition in The Lower Respiratory Tract Is Associated With Severity in Patients With Acute Respiratory Distress by InfluenzaEdgar VázquezNo ratings yet
- The Lyran Expansion, The Orion Wars, and The Creation of The AnnunakiDocument4 pagesThe Lyran Expansion, The Orion Wars, and The Creation of The AnnunakiMarius Quarouble 1954 Remember?100% (4)
- Yarram Park Bull Sale Catalogue 2024Document44 pagesYarram Park Bull Sale Catalogue 2024GenR8WarrnamboolNo ratings yet
- Discussion of Agri KnowledgeDocument24 pagesDiscussion of Agri KnowledgerahulNo ratings yet
- Bio Informatics: Assignment No 03Document3 pagesBio Informatics: Assignment No 03rafiullahNo ratings yet
- Biology Investigatory ProjectDocument7 pagesBiology Investigatory ProjectMadhusmita DasNo ratings yet
- Sat Practice Test 1 Answers - CDocument17 pagesSat Practice Test 1 Answers - CAhmed EzzatNo ratings yet