Professional Documents
Culture Documents
Fecl So Take Transition Metal Orbital and Find The Oxidation Number To Know If Its Paramagnetic or Diamagnetic
Uploaded by
yara hazemOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fecl So Take Transition Metal Orbital and Find The Oxidation Number To Know If Its Paramagnetic or Diamagnetic
Uploaded by
yara hazemCopyright:
Available Formats
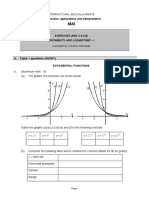
Fecl so take transition metal orbital and find the oxidation number to know if its paramagnetic or
diamagnetic.
- If theres an empty place in compound and an electron can go to in the orbital which makes
color
- Complex ions containing transition metals are usually coloured, whereas the
similar ions from non-transition metals aren't. That suggests that the partly filled d
orbitals must be involved in generating the colour in some way. Remember that
transition metals are defined as having partly filled d orbitals.
- Because theres no empty spaces for electrons to transfer into orbital d.
- two bonds
- Oxygen can form only two bonds because it requires two electrons to complete its octet
after which it will not have any more vacant orbitals left to accept more electrons and
form more bonds.
-
Read book and copy notes
You might also like
- 4.1 Ionic Bonding & Structure: Mrs. Page IB Chem. 2015-2016Document38 pages4.1 Ionic Bonding & Structure: Mrs. Page IB Chem. 2015-2016api-546066323No ratings yet
- Ionic Bonding Part 1 EdexcelDocument4 pagesIonic Bonding Part 1 EdexcelKevin The Chemistry Tutor100% (1)
- BondingDocument15 pagesBondingFrancis EssilfieNo ratings yet
- CH2 BondingDocument17 pagesCH2 BondingDoc CrocNo ratings yet
- Chemical BondingDocument69 pagesChemical BondingMenaga IlangkovanNo ratings yet
- Transition Metals Part 1 EdexcelDocument5 pagesTransition Metals Part 1 EdexcelKevin The Chemistry TutorNo ratings yet
- Chemistry Block-D: Oxidation State Colour MagnetismDocument21 pagesChemistry Block-D: Oxidation State Colour MagnetismNurhadi BNo ratings yet
- A Transition Metal Is An Element With A Partially InorganicDocument7 pagesA Transition Metal Is An Element With A Partially InorganicRashid KanetsaNo ratings yet
- The D-Block Elements-Transition ElementsDocument6 pagesThe D-Block Elements-Transition ElementsFabry OseNo ratings yet
- CH 3 HLDocument3 pagesCH 3 HLAshmita KumarNo ratings yet
- D and F Block Questions and AnswersDocument2 pagesD and F Block Questions and AnswersVishan PalNo ratings yet
- General Characteristics of Dblock ElementsDocument11 pagesGeneral Characteristics of Dblock Elementsssatechies62No ratings yet
- The Colours of Complex Metal Ions: Why Do We See Some Compounds As Being Coloured?Document13 pagesThe Colours of Complex Metal Ions: Why Do We See Some Compounds As Being Coloured?dharul khairNo ratings yet
- The Colours of Complex Metal IonsDocument27 pagesThe Colours of Complex Metal IonsCheu Hann Jong100% (2)
- Origin of Color in Complex IonsDocument5 pagesOrigin of Color in Complex IonsThya efeNo ratings yet
- A2 Chemistry 3.5.3 Transition MetalsDocument3 pagesA2 Chemistry 3.5.3 Transition MetalscharlotteNo ratings yet
- D and F Block Elements-1Document12 pagesD and F Block Elements-1ilias1973No ratings yet
- Reasioning Ques 12 2023Document55 pagesReasioning Ques 12 2023TANISHK YADAVNo ratings yet
- Chapter 5: Electricity and Chemistry: ConductivityDocument13 pagesChapter 5: Electricity and Chemistry: Conductivityapi-181176018No ratings yet
- Transition ElementsDocument8 pagesTransition ElementsSaksham AroraNo ratings yet
- Chemistry Block-D: Complex Formation Coordination NumberDocument23 pagesChemistry Block-D: Complex Formation Coordination NumberNurhadi BNo ratings yet
- Origin of Color in Complex Ions - Chemistry LibreTextsDocument5 pagesOrigin of Color in Complex Ions - Chemistry LibreTextsSaurabh ThapaNo ratings yet
- Chemistry Project 21Document12 pagesChemistry Project 21Onyekachukwu Akaekpuchionwa OkonkwoNo ratings yet
- Inorganic Chemistry Transition Metals: D-Block ElementsDocument15 pagesInorganic Chemistry Transition Metals: D-Block ElementsDineshNo ratings yet
- Chemical Bonding Reading MaterialDocument6 pagesChemical Bonding Reading MaterialJohann Carlo C. AldecoaNo ratings yet
- First Row D Block ElementsDocument4 pagesFirst Row D Block ElementsShivam KumarNo ratings yet
- Transition Metals and Their ChemistryDocument15 pagesTransition Metals and Their ChemistryMer CyNo ratings yet
- D and F BlockDocument28 pagesD and F Blockchetankapri4No ratings yet
- 3,4,5 Chapter Chemistry XyzDocument136 pages3,4,5 Chapter Chemistry XyzizhanfilzaNo ratings yet
- Chemistry Chapter 4,5Document122 pagesChemistry Chapter 4,5omer anwarNo ratings yet
- 12 Chemistry Imp The Dandf Block Elements MixDocument14 pages12 Chemistry Imp The Dandf Block Elements MixPrinceNo ratings yet
- Transition Metal Chemistry: Study Pack: 17Document35 pagesTransition Metal Chemistry: Study Pack: 17ytshortsfromopus65No ratings yet
- Electronic Configuration: Variable Oxidation StatesDocument6 pagesElectronic Configuration: Variable Oxidation StatesSonu SahilNo ratings yet
- OrgChem NotesDocument33 pagesOrgChem NotesJoses CalindasNo ratings yet
- Transition ElementsDocument15 pagesTransition ElementsNikoli MajorNo ratings yet
- Transition Elements-Ii: StructureDocument14 pagesTransition Elements-Ii: Structurekaladhar reddyNo ratings yet
- Class 12 CH 8 D and F Block ElementsDocument5 pagesClass 12 CH 8 D and F Block ElementsKumar Pratik50% (2)
- Transition MetalsDocument88 pagesTransition MetalsRamazan AshirkhanNo ratings yet
- F334 - The Steel StoryDocument11 pagesF334 - The Steel StoryBecky TenneyNo ratings yet
- Chemistry Unit 5 Part 2Document80 pagesChemistry Unit 5 Part 2Will AndyNo ratings yet
- ElectrolysisDocument10 pagesElectrolysisFaithNo ratings yet
- Transition MetalsDocument20 pagesTransition Metalsdulalsushant3No ratings yet
- General Chemistry 1 NotesDocument12 pagesGeneral Chemistry 1 NotesAwesomeAA CommandsNo ratings yet
- Notes - Structure of AtomDocument8 pagesNotes - Structure of AtomHardikNo ratings yet
- 1.trans. Metals..introDocument20 pages1.trans. Metals..introPAVITRA A/P THEVINDRAN MoeNo ratings yet
- Secondary 2 - Chemistry - Lesson 3Document19 pagesSecondary 2 - Chemistry - Lesson 3Michelle PannieNo ratings yet
- Science-Grade-9-Handout-2-Ion Formation and LEDSDocument10 pagesScience-Grade-9-Handout-2-Ion Formation and LEDSClinton YmbongNo ratings yet
- Structure of AtomDocument14 pagesStructure of AtomSiddh PatelNo ratings yet
- 4 Structure OftheatomDocument15 pages4 Structure OftheatomVaibhav V VeenajNo ratings yet
- Inorganic Chemistry Lesson 10 CHEMICAL BONDING PDFDocument41 pagesInorganic Chemistry Lesson 10 CHEMICAL BONDING PDFKayra Myke VelascoNo ratings yet
- Lesson 2 Electron and Lewis StructureDocument19 pagesLesson 2 Electron and Lewis StructurezzzzzNo ratings yet
- Transition Metal ChemistryDocument36 pagesTransition Metal ChemistryRojo JohnNo ratings yet
- Allotropy and Intermolecular ForcesDocument8 pagesAllotropy and Intermolecular ForcesD SNo ratings yet
- D & F Block Short NotesDocument4 pagesD & F Block Short NotesAlokNo ratings yet
- Untitled DocumentDocument2 pagesUntitled DocumentIrish BansalNo ratings yet
- Transition MetalsDocument64 pagesTransition MetalsMoxzJr VianzNo ratings yet
- Chemistry NotesDocument21 pagesChemistry NotesIzzy' IssariyaNo ratings yet
- Basic Concept Transition MetalsDocument18 pagesBasic Concept Transition MetalsHasnain Mohammad HanifNo ratings yet
- D and F Block ElementsDocument88 pagesD and F Block ElementsMayank ThakurNo ratings yet
- 4 3Document2 pages4 3yara hazemNo ratings yet
- Argumentative EssayDocument1 pageArgumentative Essayyara hazemNo ratings yet
- Argumentative Essay WritingDocument3 pagesArgumentative Essay Writingyara hazemNo ratings yet
- Our Business PlanDocument1 pageOur Business Planyara hazemNo ratings yet
- Ansoff MatrixDocument1 pageAnsoff Matrixyara hazemNo ratings yet
- Catalog Pipe SupportsDocument28 pagesCatalog Pipe Supportsyara hazemNo ratings yet
- MYP4-UOW3-CAT1 - Impact of Henry Ford 1Document8 pagesMYP4-UOW3-CAT1 - Impact of Henry Ford 1yara hazemNo ratings yet
- Mind Map TemplateDocument1 pageMind Map Templateyara hazemNo ratings yet
- Arguemantitive Essay Paragraph 4 ExampleDocument1 pageArguemantitive Essay Paragraph 4 Exampleyara hazemNo ratings yet
- Angle and Theme ExampleDocument1 pageAngle and Theme Exampleyara hazemNo ratings yet
- My NotesDocument1 pageMy Notesyara hazemNo ratings yet
- HUM-Y4-CAT1 - Ingenious Inventors and InnovatoDocument13 pagesHUM-Y4-CAT1 - Ingenious Inventors and Innovatoyara hazemNo ratings yet
- MYP4-UOW3-CAT2 - Impact On Our Lives 1Document9 pagesMYP4-UOW3-CAT2 - Impact On Our Lives 1yara hazemNo ratings yet
- (Mai 2.17) Sinusoidal ModelDocument10 pages(Mai 2.17) Sinusoidal Modelyara hazemNo ratings yet
- CoffeeDocument2 pagesCoffeeyara hazemNo ratings yet
- Unit 1 AnswersDocument13 pagesUnit 1 Answersyara hazemNo ratings yet
- Global CommonsDocument1 pageGlobal Commonsyara hazemNo ratings yet
- (Mai 2.13-2.14) Polynomial ModelsDocument20 pages(Mai 2.13-2.14) Polynomial Modelsyara hazemNo ratings yet
- (Mai 2.15-2.16) Power and Exponential ModelsDocument18 pages(Mai 2.15-2.16) Power and Exponential Modelsyara hazemNo ratings yet
- (Mai 2.18) Voronoi DiagramDocument8 pages(Mai 2.18) Voronoi Diagramyara hazemNo ratings yet
- (Mai 2.5-2.6) Exponents and Logarithms-IDocument8 pages(Mai 2.5-2.6) Exponents and Logarithms-Iyara hazemNo ratings yet
- How Is Current Knowledge Shaped by Its Historical DevelopmentDocument1 pageHow Is Current Knowledge Shaped by Its Historical Developmentyara hazemNo ratings yet
- CAS Sample PortfolioDocument58 pagesCAS Sample Portfolioyara hazemNo ratings yet
- Year 11 End of Year ExamDocument4 pagesYear 11 End of Year Examyara hazemNo ratings yet
- Business TestDocument2 pagesBusiness Testyara hazemNo ratings yet
- الأدب والقضايا العالميةDocument14 pagesالأدب والقضايا العالميةyara hazemNo ratings yet
- EasyJet Assessment 3.1 12a My AnswersDocument3 pagesEasyJet Assessment 3.1 12a My Answersyara hazemNo ratings yet
- Topic 1A. Number and Algebra NotesDocument41 pagesTopic 1A. Number and Algebra Notesyara hazemNo ratings yet
- SL ChecklistDocument5 pagesSL Checklistyara hazemNo ratings yet
- (Mai 1.2) ExponentsDocument6 pages(Mai 1.2) Exponentsyara hazemNo ratings yet