0% found this document useful (0 votes)
336 views16 pagesAppendix E.3 CE-I03B-017 Software Validation Report 1.0
Uploaded by
A Wahid KemalCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
0% found this document useful (0 votes)
336 views16 pagesAppendix E.3 CE-I03B-017 Software Validation Report 1.0
Uploaded by
A Wahid KemalCopyright
© © All Rights Reserved
We take content rights seriously. If you suspect this is your content, claim it here.
Available Formats
Download as PDF, TXT or read online on Scribd
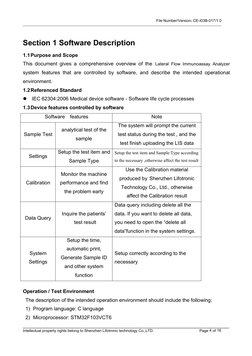
- Section 1 Software Description
- Section 2 Software Requirements Specification
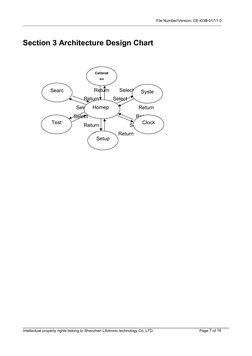
- Section 3 Architecture Design Chart
- Section 4 Software Development Environment Description
- Section 5 Verification and Validation Documentation
- Section 6 Software Risk Evaluation
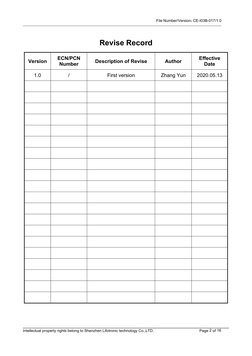
- Section 7 Revision Level History