Professional Documents
Culture Documents
Callister7e SM ch12 19
Uploaded by
Wellington AparecidoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Callister7e SM ch12 19
Uploaded by
Wellington AparecidoCopyright:
Available Formats
12-22
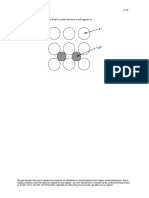
12.19 This problem asks that we compute the density of CaF2. A unit cell of the fluorite structure is
shown in Figure 12.5. It may be seen that there are four CaF2 units per unit cell (i.e., n' = 4 formula units/unit cell).
Assume that for each of the eight small cubes in the unit cell
2 r 2+ + 2 r −
a = Ca F
3
and, from Table 12.3
2 (0.100 nm) + 2 (0.133 nm)
a = = 0.269 nm = 2.69 x 10-8 cm
3
The volume of the unit cell is just
3
[
VC = (2a) 3 = (2)(2.69 x 10-3 cm) ] = 1.56 x 10−22 cm3
Thus, from Equation 12.1
n'Ź( ACa + 2 AF )
ρ =
VC N A
(4 formula units/unit cell) [40.08 g/mol + (2)(19.00 g/mol)]
=
(1.56 x 10-22 cm3/unit cell)( 6.023 x 1023 formula units/mol)
= 3.33 g/cm3
The measured density is 3.18 g/cm3.
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for testing or instructional purposes only to
students enrolled in courses for which the textbook has been adopted. Any other reproduction or translation of this work beyond that permitted
by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful.
You might also like
- Callister7e SM Ch12 22aDocument1 pageCallister7e SM Ch12 22aWellington AparecidoNo ratings yet
- Solutions Fundamentals of Semiconductor FabricationDocument85 pagesSolutions Fundamentals of Semiconductor Fabricationgarmsirian0% (2)
- Cap 2Document33 pagesCap 2Ricardo Rincon VegaNo ratings yet
- Problem #1: Session #16: Homework SolutionsDocument6 pagesProblem #1: Session #16: Homework SolutionsMelo ChingNo ratings yet
- Engineering M12 Solutions Chapter 03 MSEDocument5 pagesEngineering M12 Solutions Chapter 03 MSEmelbaz1No ratings yet
- Callister7e SM Ch12 21Document1 pageCallister7e SM Ch12 21Wellington AparecidoNo ratings yet
- Target: Jee (Main) Class - XII NO. 1 Hints & Solutions: Height of HCP Unit CellDocument5 pagesTarget: Jee (Main) Class - XII NO. 1 Hints & Solutions: Height of HCP Unit CellPrasann KatiyarNo ratings yet
- Callister7e SM Ch12 18Document1 pageCallister7e SM Ch12 18Wellington AparecidoNo ratings yet
- Problem #2b: Chromium Crystallizes With A Body-Centered Cubic Unit Cell. The Radius of ADocument8 pagesProblem #2b: Chromium Crystallizes With A Body-Centered Cubic Unit Cell. The Radius of ARadica AyuNo ratings yet
- Mseg302 Homework 2 - Chapters 3 and 4 - SolutionsDocument7 pagesMseg302 Homework 2 - Chapters 3 and 4 - SolutionsTyler Szarko100% (2)
- Microelectronic Circuit Design 5th Edition Jaeger Solutions ManualDocument15 pagesMicroelectronic Circuit Design 5th Edition Jaeger Solutions Manuala851945412No ratings yet
- Chapter 3 in Class Problems SolutionsDocument7 pagesChapter 3 in Class Problems Solutionsarnob vsd100% (2)
- Review Problems For Chapter3Document11 pagesReview Problems For Chapter3johandreher100% (1)
- ch03 HW KeyDocument7 pagesch03 HW KeyNasser SANo ratings yet
- Microelectronic Circuit Design 5th Edition Jaeger Blalock Solution ManualDocument21 pagesMicroelectronic Circuit Design 5th Edition Jaeger Blalock Solution Manualruth100% (24)
- Solution Manual For Microelectronic Circuit Design 5Th Edition Jaeger Blalock 0073529605 9780073529608 Full Chapter PDFDocument36 pagesSolution Manual For Microelectronic Circuit Design 5Th Edition Jaeger Blalock 0073529605 9780073529608 Full Chapter PDFbrenda.gibeau201100% (12)
- Cap 3Document12 pagesCap 3Milton OrtegaNo ratings yet
- Vibration Cat 2 MSDocument4 pagesVibration Cat 2 MSkalu kioNo ratings yet
- Cats 5Document8 pagesCats 5api-26456455No ratings yet
- PDFDocument6 pagesPDFAna Lorraine DalilisNo ratings yet
- Pset03 Edwin, Johana, Yanielka - AsdDocument6 pagesPset03 Edwin, Johana, Yanielka - AsdLa'Nana Joha FalconettNo ratings yet
- Ch3 ExtraDocument5 pagesCh3 ExtraAli RajabNo ratings yet
- Defect ProblemsDocument8 pagesDefect Problemsndreddy_pu100% (2)
- SM ch1 Mat Meyers 2Document37 pagesSM ch1 Mat Meyers 2infinity_azNo ratings yet
- Askeland Chap 4 SolutionDocument10 pagesAskeland Chap 4 SolutionDamita de Peña50% (2)
- ECE331 HW2 SolnDocument16 pagesECE331 HW2 SolnDean WinchesterNo ratings yet
- Chapter 4 SolutionsDocument3 pagesChapter 4 Solutionstysir sarhanNo ratings yet
- 12 Rotational and Vibrational Spectra: Answers To Discussion QuestionsDocument38 pages12 Rotational and Vibrational Spectra: Answers To Discussion QuestionsshaheerasadafNo ratings yet
- Ln-4 Idle Mind SolutionsDocument22 pagesLn-4 Idle Mind Solutionssanjay975No ratings yet
- Soild State Electronic Devices Chapter 1 SolDocument10 pagesSoild State Electronic Devices Chapter 1 Soljh010708No ratings yet
- HW1 (1) SolutionDocument6 pagesHW1 (1) SolutionRahmat WidodoNo ratings yet
- Dwnload Full Microelectronic Circuit Design 5th Edition Jaeger Solutions Manual PDFDocument36 pagesDwnload Full Microelectronic Circuit Design 5th Edition Jaeger Solutions Manual PDFpardonstopping.q54x100% (7)
- CIVN4002 Test MemoDocument8 pagesCIVN4002 Test MemoLesego MatojaneNo ratings yet
- Problem 1: EE324 HW2 Solution Fall 2012Document15 pagesProblem 1: EE324 HW2 Solution Fall 2012hdshfhNo ratings yet
- 3.091 Introduction To Solid State ChemistryDocument11 pages3.091 Introduction To Solid State ChemistryDrew JenkinsNo ratings yet
- Microelectronic Circuit Design 5th Edition Jaeger Solutions ManualDocument17 pagesMicroelectronic Circuit Design 5th Edition Jaeger Solutions Manualjulieshawwzgcieqfxa100% (14)
- Model Solution 1Document6 pagesModel Solution 1Rts SNo ratings yet
- Vacancy Problems SolutionDocument12 pagesVacancy Problems Solutionutsho dasNo ratings yet
- Solution Manual For Civil Engineering Materials 1st Edition Sivakugan Gnanendran Tuladhar Kannan ISBN 1305386647 9781305386648Document36 pagesSolution Manual For Civil Engineering Materials 1st Edition Sivakugan Gnanendran Tuladhar Kannan ISBN 1305386647 9781305386648stevenbrownpxckmdwsor100% (24)
- Laser Report For PresentationDocument5 pagesLaser Report For PresentationNaved Sadat YaminNo ratings yet
- Body-Centered Cubic ProblemsDocument8 pagesBody-Centered Cubic ProblemsKoh Jiun AnNo ratings yet
- Microelectronic Circuit Design 5th Edition Jaeger Solutions ManualDocument36 pagesMicroelectronic Circuit Design 5th Edition Jaeger Solutions Manualstibinesimousc6bgx97% (29)
- Design of Columns2 PDFDocument3 pagesDesign of Columns2 PDFPamela Joanne Falo AndradeNo ratings yet
- Physical Metallurgy (Miller Indices)Document17 pagesPhysical Metallurgy (Miller Indices)Majid Ullah Sajid MahmoodNo ratings yet
- Chapter 1 Solutions (Global Edition) : Prob. 1.1Document10 pagesChapter 1 Solutions (Global Edition) : Prob. 1.1靑山なつきNo ratings yet
- Updating - MTO I - Unit 2 ProblemsDocument3 pagesUpdating - MTO I - Unit 2 ProblemsmaheshNo ratings yet
- Solution 2Document4 pagesSolution 2Nishita KardaNo ratings yet
- Hw5soln 2006Document7 pagesHw5soln 2006DeepikaNo ratings yet
- Chapter 13Document15 pagesChapter 13anormal08No ratings yet
- Packing Efficiency of DiamondDocument3 pagesPacking Efficiency of Diamondsunny_hal0% (1)
- CE 412 - Lec 2B DDM Design ExampleDocument39 pagesCE 412 - Lec 2B DDM Design ExampleMuh UmaNo ratings yet
- MIT3 091SCF09 hw18 Sol PDFDocument6 pagesMIT3 091SCF09 hw18 Sol PDFNitinSrivastavaNo ratings yet
- Engi 4032FB03 (Midtermanswers)Document6 pagesEngi 4032FB03 (Midtermanswers)tekellamerZ aka tekellamerNo ratings yet
- Solutions For Sample Questions Used in LecturesDocument45 pagesSolutions For Sample Questions Used in LecturesAli Hussain100% (1)
- Design of JoistDocument26 pagesDesign of Joistallen2912No ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Hyrdoacoustic Ocean Exploration: Theories and Experimental ApplicationFrom EverandHyrdoacoustic Ocean Exploration: Theories and Experimental ApplicationNo ratings yet
- 3D Modeling of Nonlinear Wave Phenomena on Shallow Water SurfacesFrom Everand3D Modeling of Nonlinear Wave Phenomena on Shallow Water SurfacesNo ratings yet
- Callister7e SM Ch12 18Document1 pageCallister7e SM Ch12 18Wellington AparecidoNo ratings yet
- Callister7e SM Ch12 17Document1 pageCallister7e SM Ch12 17Wellington AparecidoNo ratings yet
- Callister7e SM Ch12 25aDocument1 pageCallister7e SM Ch12 25aWellington AparecidoNo ratings yet
- Callister7e SM ch12 16Document1 pageCallister7e SM ch12 16Wellington AparecidoNo ratings yet
- Callister7e SM ch12 24Document1 pageCallister7e SM ch12 24Wellington AparecidoNo ratings yet
- Callister7e SM Ch12 25bDocument1 pageCallister7e SM Ch12 25bWellington AparecidoNo ratings yet
- Callister7e SM Ch12 26Document1 pageCallister7e SM Ch12 26Wellington AparecidoNo ratings yet
- Callister7e SM ch12 23Document1 pageCallister7e SM ch12 23Wellington AparecidoNo ratings yet
- Callister7e SM Ch12 20Document1 pageCallister7e SM Ch12 20Wellington AparecidoNo ratings yet
- Callister7e SM Ch12 21Document1 pageCallister7e SM Ch12 21Wellington AparecidoNo ratings yet