Professional Documents
Culture Documents
CRISPR Poster
Uploaded by
Omar Garduño VidalOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CRISPR Poster
Uploaded by
Omar Garduño VidalCopyright:
Available Formats
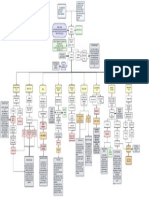
Advancing to
Non-Viral T Cell T Cell-Based Therapy Workflows: From Manufacturing to the Clinic
Immunotherapies
T Cell Activation Cancer cell
and Expansion death
Leukapheresis- PFN
White blood cells GzmB
are separated from Anti-CD3/
CD28
peripheral blood T Cell Engineering CAR-T TCR T
Cell Cell
CAR-T cells have radically changed cancer treatment. CAR
Viral Non-Viral
HDR
Template IFNγ
gene TNFα
T cells engineered to express novel Chimeric Antigen RNP
Complex
Receptors (CARs), can be armed with unique
tumor-killing properties. Conventionally, viral vectors
(e.g., lentivirus) have been used to deliver sequence T Cell Separation-
Magnetic nanobeads coated with
Viral vector Electroporation
T Cell Expansion-
payloads for CAR-T cell manufacturing. However, the antibodies are used to isolate T cells
CAR-T cells TCR T cells
Generate millions of
modified T cells and
random genome integration of virally introduced CARs MHC independent
tumor antigen recognition
MHC dependent
tumor antigen recognition
infusion into patient
that has received
presents oncogenic risks. CAR-T Cell TCR T Cell
lymphodepletion
chemotherapy
CRISPR/Cas9 systems are now being applied to precisely
modify T cell receptors (TCR) and re-write antigen
specificity. Initial CRISPR-based T cell engineering has
used adeno-associated virus (AAV) vectors to deliver DNA Conventional Untargeted Viral Viral Targeted CAR-scFv Non-Viral Targeted
payloads for targeted insertion via homology-directed CAR-T Cell Engineering T Cell Engineering TCR-T Cell Engineering
repair (HDR) into the TCR gene (i.e.,TRAC locus). Yet,
despite their high editing efficiency, a limited vector Self-inactivating-deletion in 3' LTR
prevents emergence of Transfection of Packaging
Promoter
Transfection of Packaging Cell line
(e.g., HEK293 cells expressing Recombinant TCR
anti-NY-ESO
E1a/E1b helper factors)
capacity and high manufacturing costs have restricted replication-competent recombinants Cell line ITR CAR-scFv ITR
cancer antigen
Knock-out
Endogenous
TCR
Targeting the TRAC locus for
insertion of recombinant TCR or
(e.g., HEK293 cells) β
viral engineering workflows.
α
Promoter γ
ε CAR constructs improves the
α
5' LTR CAR 3' LTR Viral δ
ε
δ
β
potency and reduces the
Replication REP CAP
ε
γ
exhaustion of engineered T cells
and Capsid ε
Currently, non-viral CRISPR/Cas9 editing provides an gag pol
Helper-Viral
Replication
E4 E2a VA
Viral
attractive alternative. In non-viral systems, Packaging
rev In contrast to TCR T cells, a CAR-scFv
ribonucleoprotein complexes (RNPs) of Cas9 protein and Different vectors for gag-pol, rev and
env sequences in third-generation
targeted to the TRAC locus imparts MHC
indpendent recognition of cell surface AAV Viral Insertion
Precise
NY-ESO Insertion
HDR
Lentiviral vectors enable producing γ ε ssDNA Recombinant
guide RNA targeting the TRAC locus are
ε δ
Viral env tumor antigens, similar to conventional Particles
Envelope replication-deficient virus particles or TCR (e.g., anti
CAR-T cells TRAC dsDNA NY-ESO)
co-electroporated into T-cells with a synthetic DNA
Transduction
payload encoding a CAR-scFv or recombinant TCR of Activated Targeted CAR
(scFv) gene
Transduction T Cell Ribonucleoprotein
(RNP) Complex
Un-targeted CAR
sequence. CAR-T Cells
gene insertion
T Cells Retroviral
Particles
CAR-scFv
TCR T cells
insertion RNP Complex
Targeting TRAC
locus
Electroporation
Non-viral editing approaches are continually evolving to
improve accuracy and efficiency. Most recently, synthetic
long single-stranded DNA (long ssDNA) payloads have
been safely and efficiently delivered to simplify cGMP T Benefits of Genome Editing with Benefits of Long ssDNA HDR
cell therapy manufacturing workflows.
Ribonucleoprotein (RNP) Complex Templates for T Cell Engineering
References RNP complex stability supports high editing
Improved Efficiency
RNP
▪ Eyquem, J. et al. (2017). Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature Publishing
Group. https://doi.org/10.1038/nature21405 functionality
▪ Irving, M. et al. (2021). Choosing the Right Tool for Genetic Engineering: Clinical Lessons from Chimeric Antigen Receptor-T
Cells. Human Gene Therapy. https://www.liebertpub.com/doi/10.1089/hum.2021.173
▪ Transient nuclease activity decreases
Reduced Off-Targets
Ivica, N. A., & Young, C. M. (2021). Tracking the car-t revolution: Analysis of clinical trials of car-t and tcr-t therapies for the
treatment of cancer (1997–2020). Healthcare (Switzerland). https://doi.org/10.3390/HEALTHCARE9081062/S1
▪ Kalidasan, V. et al. (2021). A guide in lentiviral vector production for hard-to-transfect cells, using cardiac-derived c-kit expressing unspecific editing and toxicity CTS
cells as a model system. Scientific Reports. https://doi.org/10.1038/s41598-021-98657-7
▪ Mansilla-Soto, J. et al. (2022). HLA-independent T cell receptors for targeting tumors with low antigen density. Nature Medicine.
https://doi.org/10.1038/s41591-021-01621-1 RNPs eliminate risk of genome dsDNA VS. ssDNA Under GMP-suitable conditions
▪
▪
Milone, M. C., & O’Doherty, U. (2018). Clinical use of lentiviral vectors. Leukemia. https://doi.org/10.1038/s41375-018-0106-0
Moretti, A. et al. (2022). The Past, Present, and Future of Non-Viral CAR T Cells. Frontiers in Immunology.
No DNA Interference insertional events
ssDNA HDR templates designed
with Cas9 target sequences (CTS)
https://doi.org/10.3389/FIMMU.2022.867013/BIBTEX
▪ Morgan, R. A., & Boyerinas, B. (2016). Genetic Modification of T Cells. Biomedicines. support high knock-in efficiencies of
46-62%, yielding millions of
https://doi.org/10.3390/BIOMEDICINES4020009
Transient CRISPR machinery limits risk of
▪ Nguyen, D. N. et al. (2020). Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing
No Immunogenicity non-virally modified T cells
▪
efficiency. Nature Biotechnology. doi:10.1038/s41587-019-0325-6.
Roth, T. L. et al. (2018). Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. adverse immunological reactions
doi:10.1038/s41586-018-0326-5.
▪ Shy, B. R. et al. (2022). High-yield genome engineering in primary cells using a hybrid ssDNA repair template and
RNPs are immediately available for editing Best for long Lower cytotoxicity
Rapid Gene Editing
small-molecule cocktails. Nature. https://doi.org/10.1038/s41587-022-01418-8
▪ Wu, L. et al. (2020). Signaling from T cell receptors (TCRs) and chimeric antigen receptors (CARs) on T cells. Cellular & Molecular Increased insertion efficiency
Immunology. https://doi.org/10.1038/s41423-020-0470-3 without the lag of transcription and translation insertions
(5-10 kb) Improved insertion accuracy
▪ Zhao, Q. et al. (2021). Engineered TCR-T Cell Immunotherapy in Anticancer Precision Medicine: Pros and Cons. Frontiers.
https://doi.org/10.3389/fimmu.2021.658753
You might also like
- Systematic Approaches To Phylogeny)Document26 pagesSystematic Approaches To Phylogeny)Mhi IsmailNo ratings yet
- Introduction To General Botany PDFDocument14 pagesIntroduction To General Botany PDFnathaniel100% (4)
- The Ten Best Tools To Boost Your Immune SystemDocument347 pagesThe Ten Best Tools To Boost Your Immune SystemmamedeiaNo ratings yet
- Modul Dermatovenerologi 2 - DR DeaDocument21 pagesModul Dermatovenerologi 2 - DR DeaDendy AgusNo ratings yet
- Automation in Clinical PathologyDocument52 pagesAutomation in Clinical Pathologybhanupriya kakarala100% (1)
- Key Presentations From The 2022 ASCO Annual Meeting: Breast Cancer IssueDocument42 pagesKey Presentations From The 2022 ASCO Annual Meeting: Breast Cancer IssueVirgen de CandelariaNo ratings yet
- Guide Gi CancerDocument1 pageGuide Gi Cancerapi-446887131No ratings yet
- Haisle Moon - NBS 2021 PosterDocument1 pageHaisle Moon - NBS 2021 PosterCBR UBCNo ratings yet
- 4D OvaryDocument108 pages4D OvaryrubenNo ratings yet
- Introduction To BMS-clinical Immunology-BbDocument46 pagesIntroduction To BMS-clinical Immunology-BbBiology BảoNo ratings yet
- 095 Neurology Physiology Descending Tracts Corticospinal TractDocument1 page095 Neurology Physiology Descending Tracts Corticospinal TractxcqzprnfkrNo ratings yet
- Abcam Adhesion and MethastasisDocument1 pageAbcam Adhesion and MethastasisJosé Jiménez VillegasNo ratings yet
- Red and White Simple Modern Blood Donation PosterDocument1 pageRed and White Simple Modern Blood Donation PosterNochNo ratings yet
- FinalsDocument14 pagesFinalsZarina AvesNo ratings yet
- Patho 1aDocument3 pagesPatho 1aaaaalliah2No ratings yet
- Adobe Scan 18 Jun 2023Document8 pagesAdobe Scan 18 Jun 2023vishalyadav5656No ratings yet
- Queen's Medical Centre Corridor Plan NHS: E FloorDocument1 pageQueen's Medical Centre Corridor Plan NHS: E Floorgoogle manNo ratings yet
- Snapshot Cancer Immunotherapy 1681565886Document2 pagesSnapshot Cancer Immunotherapy 1681565886samanehr1No ratings yet
- Types of Muscles and Their MovementsDocument2 pagesTypes of Muscles and Their Movementsfunny video all indiaNo ratings yet
- Cell Division, Cell Cycle and Cell TransportDocument3 pagesCell Division, Cell Cycle and Cell Transportandrea.baceovaNo ratings yet
- HematologyDocument5 pagesHematologyJoshua AtienzaNo ratings yet
- FiebreDocument1 pageFiebreJENNIFER DIANA MORENO PRECIADONo ratings yet
- Proliferation Lymphatic: Leading ?Document6 pagesProliferation Lymphatic: Leading ?flzhathrhNo ratings yet
- 2chemical MediatorDocument1 page2chemical MediatorUu UuNo ratings yet
- Nipt TRF MedgenomeDocument4 pagesNipt TRF MedgenomepathbiomedxNo ratings yet
- Pi Is 0168827817319955Document3 pagesPi Is 0168827817319955kookyinNo ratings yet
- Tumor Microenvironment and The Immune SystemDocument1 pageTumor Microenvironment and The Immune Systemnurul hidayahNo ratings yet
- Pharma 1bDocument8 pagesPharma 1baaaalliah2No ratings yet
- EndocrineDocument19 pagesEndocrineIsabel Bibat DavidNo ratings yet
- Cases NotesDocument5 pagesCases NotessofiakeziacabaroNo ratings yet
- Media 823904 SMXXDocument26 pagesMedia 823904 SMXXДемьян МатченкоNo ratings yet
- The Researcher's Guide To The Hallmarks of Cancer Research TargetsDocument7 pagesThe Researcher's Guide To The Hallmarks of Cancer Research TargetsNey de MolinaNo ratings yet
- Micro 22 RATIO by Clerky StubuDocument13 pagesMicro 22 RATIO by Clerky StubuJohn RamosNo ratings yet
- Winchester Anesthesia Ebook PDFDocument59 pagesWinchester Anesthesia Ebook PDFAdi Nugroho Melyana100% (1)
- Interpreting PDX Dot BlotsDocument2 pagesInterpreting PDX Dot BlotsSpy CameraNo ratings yet
- Cushing's SyndromeDocument1 pageCushing's SyndromeAmber BlodduweddNo ratings yet
- MulteeDocument1 pageMulteeGavin MuhammadNo ratings yet
- Stahl Wire Rope HoistsDocument16 pagesStahl Wire Rope HoistsagungNo ratings yet
- Senng - Ch/6Mo) 76Nmlhlmalle8I: MenfalareeDocument5 pagesSenng - Ch/6Mo) 76Nmlhlmalle8I: MenfalareeGachibag IdNo ratings yet
- Oral Sur 3 FinalDocument20 pagesOral Sur 3 Finalnapat kidsanakaraketNo ratings yet
- Molecular Biology Workflow Solutions BrochureDocument62 pagesMolecular Biology Workflow Solutions BrochureJoséMaríaMoralesMuñozNo ratings yet
- Nervous System Mind MapDocument1 pageNervous System Mind MapayishahNo ratings yet
- Connective TissueDocument35 pagesConnective Tissues vNo ratings yet
- INFOGRAFIA 1 EnglishDocument1 pageINFOGRAFIA 1 EnglishCRISTIAN GIOVANNY AGUIRRE LEONNo ratings yet
- 8.b.hemato - SplinaDocument10 pages8.b.hemato - SplinaAna MîndrilăNo ratings yet
- Modern Treatment Family Health Optima Insurance PlanDocument1 pageModern Treatment Family Health Optima Insurance PlanBhanu PrakashNo ratings yet
- Updated NCCN Guidelines For Cervical Cancer.22Document2 pagesUpdated NCCN Guidelines For Cervical Cancer.22Hari NugrohoNo ratings yet
- Planning Units Aquia Harbour 2017 PDFDocument1 pagePlanning Units Aquia Harbour 2017 PDFIrene Marie HollerbackNo ratings yet
- Final Ideal Clinic Framework - Version 17 On 3 Aug 2017Document92 pagesFinal Ideal Clinic Framework - Version 17 On 3 Aug 2017Finger TladiNo ratings yet
- BiologyDocument1 pageBiologyPhimsiya ThongyooNo ratings yet
- FTP - Ahlzzaty: AtiyahDocument10 pagesFTP - Ahlzzaty: Atiyahaisyah afiahNo ratings yet
- IGCSE Biology Summary Notes For Topics Listed in DescriptionDocument1 pageIGCSE Biology Summary Notes For Topics Listed in DescriptionskyeNo ratings yet
- Nephron Map PDFDocument1 pageNephron Map PDFDan TadeoNo ratings yet
- Hypertensive RetinopathyDocument4 pagesHypertensive RetinopathyNikithaNo ratings yet
- Procalcitonin: Bacterial Infections and SepsisDocument14 pagesProcalcitonin: Bacterial Infections and SepsisAhmed GaberNo ratings yet
- Hepatitis BDocument6 pagesHepatitis BCarmen MargoNo ratings yet
- Or Rudimentary Minute Spine: Schistosoma Japonicum Oncomelania QuadrasiDocument1 pageOr Rudimentary Minute Spine: Schistosoma Japonicum Oncomelania QuadrasiMaikka IlaganNo ratings yet
- Neoplasm PDFDocument1 pageNeoplasm PDFZul Azim AnuarNo ratings yet
- PathologyDocument64 pagesPathologyyeshwanth sree pranavNo ratings yet
- CAR T Cells in Cancer TherapyDocument1 pageCAR T Cells in Cancer Therapyhoangphuong08101992No ratings yet
- Repression and Activation of Promoter-Bound RNA Polymerase Activity by Gal RepressorDocument8 pagesRepression and Activation of Promoter-Bound RNA Polymerase Activity by Gal Repressoraxva1663No ratings yet
- 90 Days Countdown Neet Biology. Cb1198675309Document2 pages90 Days Countdown Neet Biology. Cb1198675309Jyöt SîlvērNo ratings yet
- Transcription and Translation NarrativeDocument3 pagesTranscription and Translation NarrativeBridget Anne BenitezNo ratings yet
- Reproductive CloningDocument12 pagesReproductive CloningAnh TranNo ratings yet
- LIFE CYCLE AND PATHOGENECITY OF Plasmodium Vivax and Entamoeba HistolyticaDocument7 pagesLIFE CYCLE AND PATHOGENECITY OF Plasmodium Vivax and Entamoeba Histolyticaarifsheikh4025No ratings yet
- Avian Physiology Lecture 003Document36 pagesAvian Physiology Lecture 003Susai Mari NixonNo ratings yet
- Experiment 1 and 2 Plant Tissue CultureDocument7 pagesExperiment 1 and 2 Plant Tissue CultureCliff Lim100% (9)
- Biology Answers PDFDocument30 pagesBiology Answers PDFأسيل الشلةNo ratings yet
- Factors That Influence Biogeochemical CyclesDocument1 pageFactors That Influence Biogeochemical CyclesLara Gatbonton100% (3)
- Free Radical Scavenging Activity of Marine SpongesDocument8 pagesFree Radical Scavenging Activity of Marine SpongeshadifaNo ratings yet
- Agricultural ScienceDocument266 pagesAgricultural ScienceAnonymous XXENB3eONo ratings yet
- Els q2 Las Week 3 1Document11 pagesEls q2 Las Week 3 1SeanNo ratings yet
- Ebooks On Science Direct ListDocument51 pagesEbooks On Science Direct ListYun-Kai ChengNo ratings yet
- Liu2017 EhpDocument6 pagesLiu2017 EhpmokengNo ratings yet
- Studiu Genetic Cardoș RodenwaldDocument5 pagesStudiu Genetic Cardoș RodenwaldMarius SpinuNo ratings yet
- Foundation Timetable 2017Document13 pagesFoundation Timetable 2017Wahida Amalin Ab RazakNo ratings yet
- Activity 1.5 (Jan Ray)Document4 pagesActivity 1.5 (Jan Ray)JAN RAY CUISON VISPERASNo ratings yet
- Science Answer KeyDocument6 pagesScience Answer KeyAngel MarieNo ratings yet
- Molecule of The Week - WaterDocument1 pageMolecule of The Week - WaterCraftychemistNo ratings yet
- Rubella Virus Infection, The Congenital Rubella Syndrome, and The Link To AutismDocument28 pagesRubella Virus Infection, The Congenital Rubella Syndrome, and The Link To AutismM Azzam SNo ratings yet
- Grade 9 WHLP and PA Week 3 & 4 ScienceDocument12 pagesGrade 9 WHLP and PA Week 3 & 4 ScienceSylvs Enong100% (1)
- The Importance of Interactions Between Organisms and Their EnvironmentDocument2 pagesThe Importance of Interactions Between Organisms and Their EnvironmentrobinsonbryNo ratings yet
- Mid Term Exams Revision of Science Class - 4: Q1.Answer The Following QuestionsDocument2 pagesMid Term Exams Revision of Science Class - 4: Q1.Answer The Following QuestionsPratibha JhaNo ratings yet
- Life ProcessDocument8 pagesLife ProcessKumar AbhishantNo ratings yet
- Chapter 12 Guided Notes-1Document7 pagesChapter 12 Guided Notes-1MasonNo ratings yet
- PTERIDOPHYTADocument16 pagesPTERIDOPHYTAshriya shuklaNo ratings yet