Professional Documents
Culture Documents
Crystal: Splitting
Uploaded by
Aitor PastorOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Crystal: Splitting
Uploaded by
Aitor PastorCopyright:
Available Formats
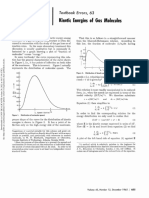
GUEST AUTHOR Textbook Errors, 58
J. J. Zuckerrnan
Cornell University
Ithaca, New York
Crystal Field Splitting Diagrams
It is now over a decade since the tlieo- regular octahedron about the metal ion, the charges on
retinal treatment of transition metal complexes which the ligands will repel an electron to a greater extent if it
goes by the general name Crystal Field or Ligand Field is in a dz2 or dX2-y2 orbital than if it is in a dxy, dxz, or
Theory came into general acceptance in inorganic chem- dyz orbital, since the former point toward the ligands
istry. {In current usage these terms are interchange- and the latter point between the axes of approach.
able, but more specifically the crystal field theory does (The ligands either possess a net negative charge, i.e.,
not consider the role played by the ligands further than are anions, or align themselves so that a negative re-
gion of charge is nearest the central atom. In electro-
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
to credit them with producing an electrostatic field,
while the ligand field theory includes molecular orbital static theory, the ligands can be considered as point
considerations. Our discussion relates to electrostatic charges or point dipoles.) Thus for the field-free ion or
crystal field theory only.) Its use as part of course atom in vacuo, the d-orbitals are energetically degener-
Downloaded via UNIV DE ALICANTE on June 26, 2022 at 16:44:35 (UTC).
work in inorganic chemistry is now well established, and ate, but their energies become differentiated in a con-
simplified explanations based upon the usual Crystal certed process as the energies of all the orbitals are
Field Splitting Diagram are about to filter down into raised with the approach of the ligands. In the case of
the freshman curriculum. This article is written in an octahedral symmetry the original degeneracy is de-
attempt to bring this much used diagram into better stroyed, and the energies are split into two groups with
agreement with the realities of the situation.1 the dzi and dxt-v-t orbitals assuming a higher energy and
Central to the idea of the crystal field approach is the dxy, dxz, and dyz orbitals at a lower energy. The
that the energies of the d-orbitals of a metal ion in a two higher energy orbitals are called dy according to
transition metal complex split in the presence of per- van Vleck, e„ according to Mulliken, and y-.-, in Bethe’s
turbation by the surrounding ligands. This effect in original notation. The three lower energy orbitals are
crystal lattices was discussed by Bethe in 1929.2 In a called dt by van Vleck, t2„ by Mulliken, and 75 by
field of any given geometry, certain of the d-orbitals find Bethe.3
themselves oriented more in the direction of the field of The fact that all the d-orbital energy levels are raised
the ligands than others, and the energies of these or- by the repulsion of the ligands is emphasized. In fact
bitals are raised by the electrostatic repulsions of the the differentiation of the d-orbital energy levels is a
ligands while the energies of the other d-orbitals are relatively small effect superimposed on the much
affected to a smaller extent. larger repulsive effect of the crystal field on the orbitals.
It is usual to illustrate the splitting of the five de- This repulsion of the negative electron cloud about the
generate d-orbitals of a transition metal ion for the case metal ion by the negative charge or dipole of the ligand
of three representative symmetries: octahedral, tetra- is in its turn small by comparison with the overall
hedral, and square planar. A single energy diagram is attraction of the positive metal ion for the ligands.
frequently used to show all three types of splittings with These repulsions are contained in the lattice energy of a
reference to the free ion or field-free ion as the five-fold crystal or the ligation energy of a metal complex. [For
degenerate ground state of the d-orbitals is usually example, in octahedral Ti (H20)e3+ the splitting of the
labeled. This standard Crystal Field or Ligand Field two sets of d-orbitals is of the order of 60 kcal/mole
Splitting Diagram must by now be familiar to both while the hydration energy of Ti3+ is approximately
students and teachers. 1000 kcal/mole.] The d-orbital energy level splitting
A new diagram, (p. 316), is recommended to replace is measured in terms of a parameter A, 10 Dq or Ei-E2,
the one now in use. Consider the case of the octahedral whose magnitude is assumed to be proportional to the
field first. If a complex is formed by bringing six strength of the crystal field: the stronger the field, the
identical ligands along x, y, and z axes so as to form a larger the value of A or Dq or E\-E2.
The center of gravity of the d-orbital energies repre-
sents the energy the five d-orbitals would have in the
Suggestions of material suitable for this column, and guest
columns suitable for publication directly, should be sent with as presence of a spherically symmetric field. It is the
many details as possible, and particularly with references to weighted mean energy of the d-orbitais in the presence
modern textbooks, to Karol J. Mysels, Department of Chem- of the ligands. In another view, if we may break the
istry, University of Southern California, Los Angeles 7, California. concerted process of raising the energy and differenti-
! Since the
purpose of this column is to prevent the spread
and continuation of errors and not the evaluation of individual ating into two steps, then this is the energy to which the
texts, the source of errors discussed will not be cited. In order
to be presented, an error must occur in at. least two independent
recent standard books. 3
See footnote 7 in the Resource Paper I, “Ligand Field
2
Bethe, H., Ann. Physik. 3, 133 (1929). Theory,” by F, A. Cotton, this Journal 41, 466 (1964).
Volume 42, Number 6, June 1965 / 315
Table 1. The d-Orbital Energy Levels in Crystal Fields of Different Symmetries
C.N. Structure dx*.. y« dx. dx
1 ..." -3A4Dq 5A4Dq -3A4Dq 0.57 Dq 0.57 Dq
2 linear" -6.28 10.28 -6.28 1.14 1.14
3 trigonal1’ 5.46 -3.21 5.46 -3.86 -3.86
4 tetrahedral -2.67 -2.67 1.78 1.78 1.78
4 square planar6 12.28 -4.28 2.28 -5.14 -5.14
5 trigonal bipyramid' —0.82 7.07 -0.82 —2.72 —2.72
5 square pyramid' 9.14 0.86 —0.86 —4,57 —4.57
6 octahedron 6.00 6.00 -
4.00 -
4.00 -
4,00
7 pentagonal bypyrimid' 2.82 4.93 2.82 —5.28 —5.28
“
Bonds lie along z axis. ‘
Pyramid base in xy plane.
i
Bonds in the xy plane. Only electrostatic perturbations are considered.
five degenerate d-orbitals would be raised before split- that in a tetrahedral complex with the same charge per
ting took place. It is the zero of energy in the per- ligand and the same bond distances as in the octahedral
turbed system, and the algebraic sum of all energy one. d-Orbital energy levels in crystal fields of various
shifts from this level is zero. This is a simple statement symmetries can be calculated, and some results of single
of the "preservation of the center of gravity” rule from electron energies are listed in Table l,6 These values
quantum mechanics. The rule is quite general for all were used in constructing the figure.
splittings where the forces are purely electrostatic and A further note to Figure 1 concerns the positions of
where only the sets of levels being split is considered. the centers of gravity for each of the three types of
To take the octahedral case where the total splitting is fields commonly represented. Since the center of
Aoct, the upper two orbitals lie 3/=, Aoct above and the gravity of each of the splittings represents the condition
lower three lie Vs A„t below the energy the orbitals of the ion in the presence of a spherically symmetric
would have in a spherically symmetric field. Note field of the same strength (all other factors being held
that this zero level is not the free ion or field-free ion constant) as the actual field of the ligands, then it can
energy which is much lower. be seen that for identical ligands the field strength will
By analogous and by now familiar argument the be a simple function of the number of ligands, i.e., of
splittings for the tetrahedral and square planar cases the coordination number of the complex. Thus for
are worked out.4 In the presence of a tetrahedral field similar ligands the center of gravity of an octahedral
the orbitals most affected in the octahedral example are complex will lie at higher energy than that of a tetra-
least raised, and vice versa. Here the upper three hedral or square planar complex, and these last two
orbitals lie at 2/5 At8t above the center of gravity and the will have identical centers of gravity. This is shown in
lower two at 3/s Ate, below. Further, it can be shown the figure.
that, all else being equal (cation, ligands, cation-ligand The actual magnitude of the splitting (we have called
distance, etc.), the total tetrahedral splitting, A™,., is A in the figure) is proportional in the first instance to
4/9 of Aoct, so that the magnitude of the crystal field the strength of the crystal field generated by tire ligands
splitting in an octahedral complex will bo over twice which depends upon the charge or dipole moment, as
well as size, polarizability and ability to form Tr-bonds.6
Splitting diagrams for the linear, trigonal, trigonal bipyrami-
4
In addition, the following generalizations can be made:
dal, and cubic fields have been recently given by A. L. Com- (1) Auct is approximately 45% larger when 4d orbitals
panion and M. A. Komarynsky, in this Journal 41, 257
are used and 75% larger for 5d (assuming metal of
(1964).
identical valence in the same periodic group).
d;3 dn-y3 (2) Aoct is 40-80% larger for the trivalent. ion than
the divalent ion of the same metal.
(3) AMt varies roughly between 20-40 kcal/mole for
most divalent hexacoordinated 3d complexes.
(4) The common ligands may be arranged in a se-
quence of regular increase of A0ct values for
their complexes with any given metal ion.
This dependence of A„,t values on the identity
of the ligands is known as the spectrochem-
ical series.
fi
Basolo, F., and Pearson, It., “Mechanisms
of Inorganic Reactions,” John Wiley & Sons, New
York, 1958, p. 55.
6
“It seems noteworthy, although not a point to
be stressed unduly, that by taking into account, both
types of crystal field parameters—shifts in the cen-
ter of gravity for d-orbitals as well as splittings of
plonor their energies—the diagram in the figure de-
nation
scribes qualitatively the results of even the most
sophisticated molecular orbital studies of the energy
levels for the d-like orbitals of transition metal
complex ions and molecules.” (Private communi-
Free Ion cation, Dr. G. L, Goodman, 1964.)
316 / Journal of Chemical Education
A note on the limitations in the use of Crystal Field through the configurations d1 to d9 are quite drastic,
Splitting Diagrams is in order in conclusion. First, involving not only changes in the relative magnitudes of
the differentiation of the d-orbital energy levels, as the splittings, but the appearance of additional split-
important as it may be for explaining differences be- tings and even reversals hi the energies of the d-orbitals
tween various complexes makes up only a small frac- as well. The situation is further complicated by dis-
tion (5 -10%) of the total binding energy of the com- tortions from perfect octahedral or other geometry.
plex. A much more serious limitation comes about be- These distortions are quite common and produce addi-
cause the d-orbital energy levels for various crystal tional splittings. Thus to represent correctly the situa-
fields are readily obtained only for the case of a single tion in each of the wide range of possible electronic and
d-eleetron, and diagrams such as Figure 1 are strictly geometric configurations in transition metal complexes
applicable, therefore, only to the one electron case. would require a family of Crystal Field Splitting Dia-
Systems with more than one d-electron are complicated grams. Any one diagram which attempts to serve all
by electron-electron interactions. It turns out that the situations must be used with care.
diagrams can be used equally well for the d’ and d6
cases (for reasons which we will not go into), and also to
Acknowledgment
approximate the d* and d9 cases. The diagrams for
configurations d2 and d1 and d3 and ds are much more The author thanks Professor R. C. Fay of this uni-
complex. The changes in the diagrams as one goes versity for helpful discussions.
Volume 42, Number 6, June 1965 j 317
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Practical 22.1 Iron Wool Redox TitrationDocument6 pagesPractical 22.1 Iron Wool Redox TitrationDanielle CarterNo ratings yet
- Chemistry 1A03 Introductory Chemistry I: Unit 3 Atomic Structure & TheoryDocument57 pagesChemistry 1A03 Introductory Chemistry I: Unit 3 Atomic Structure & TheoryRob SmithNo ratings yet
- Fundamentals of MasstransferandkineticshydrogenationDocument14 pagesFundamentals of MasstransferandkineticshydrogenationRamandhaPrasetyaAdibrataNo ratings yet
- Low-Noise Simplex Optimization Experiment: FutilityDocument2 pagesLow-Noise Simplex Optimization Experiment: FutilityAitor PastorNo ratings yet
- Numerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationDocument8 pagesNumerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationAitor PastorNo ratings yet
- Acs Jchemed 7b00647Document5 pagesAcs Jchemed 7b00647Aitor PastorNo ratings yet
- 6 CHM 5710 Using Character TablesDocument35 pages6 CHM 5710 Using Character TablesAitor PastorNo ratings yet
- Ed066p853 2Document1 pageEd066p853 2Aitor PastorNo ratings yet
- Instrumental: Simplex OptimizationDocument4 pagesInstrumental: Simplex OptimizationAitor PastorNo ratings yet
- Of Chemistry: Calculation Factors UndergraduateDocument3 pagesOf Chemistry: Calculation Factors UndergraduateAitor PastorNo ratings yet
- For Conceptualization The Franck-Condon PrincipleDocument1 pageFor Conceptualization The Franck-Condon PrincipleAitor PastorNo ratings yet
- Spectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsDocument10 pagesSpectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsAitor PastorNo ratings yet
- Coordination: Complexes CobaltDocument3 pagesCoordination: Complexes CobaltAitor PastorNo ratings yet
- Air-Sensitive: Argon Techniques Manipulation ofDocument1 pageAir-Sensitive: Argon Techniques Manipulation ofAitor PastorNo ratings yet
- Textbook Errors, 62 Difference Between and Liquids and SolidsDocument2 pagesTextbook Errors, 62 Difference Between and Liquids and SolidsAitor PastorNo ratings yet
- Electronic Tetrahedral Complexes: Nickel (LL)Document2 pagesElectronic Tetrahedral Complexes: Nickel (LL)Aitor PastorNo ratings yet
- Oxidation and Reduction Reactions in Organic ChemistryDocument4 pagesOxidation and Reduction Reactions in Organic ChemistryKhubaib BaryaalNo ratings yet
- Resource: Papers-IIIDocument17 pagesResource: Papers-IIIAitor PastorNo ratings yet
- Franck-Condon Factors and Their Use in Undergraduate Quantum MechanicsDocument7 pagesFranck-Condon Factors and Their Use in Undergraduate Quantum MechanicsAitor PastorNo ratings yet
- Active: of Optically ComplexDocument2 pagesActive: of Optically ComplexAitor PastorNo ratings yet
- Textbook Errors, 63 Kinetic Molecules: EnergiesDocument2 pagesTextbook Errors, 63 Kinetic Molecules: EnergiesAitor PastorNo ratings yet
- Lattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheDocument7 pagesLattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheAitor PastorNo ratings yet
- Ed 400583 QDocument4 pagesEd 400583 QAitor PastorNo ratings yet
- Ab 036Document6 pagesAb 036Aitor PastorNo ratings yet
- Half-Wave Potential: SourceDocument1 pageHalf-Wave Potential: SourceAitor PastorNo ratings yet
- Acs Jchemed 5b00170Document5 pagesAcs Jchemed 5b00170Aitor PastorNo ratings yet
- Ed5b00170 Si 001Document27 pagesEd5b00170 Si 001Aitor PastorNo ratings yet
- Acssuschemeng 2c00095Document10 pagesAcssuschemeng 2c00095Aitor PastorNo ratings yet
- Error Titrations Mixtures: MinimumDocument4 pagesError Titrations Mixtures: MinimumAitor PastorNo ratings yet
- TheBaldwinRules RevisedandExtendedDocument29 pagesTheBaldwinRules RevisedandExtendedAitor PastorNo ratings yet
- Styer 2000Document7 pagesStyer 2000Aitor PastorNo ratings yet
- Claisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroDocument64 pagesClaisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroAitor PastorNo ratings yet
- Fundamentals of Space-Vector TheoryDocument16 pagesFundamentals of Space-Vector Theoryni60No ratings yet
- 01261132Document32 pages01261132namithdevadigaNo ratings yet
- Chapter5 RDocument37 pagesChapter5 Rsusovan56No ratings yet
- Thermal Properties of Ab MaterialsDocument17 pagesThermal Properties of Ab MaterialsFrences Lois DURANNo ratings yet
- Stress-Strain Behavior of Concrete Confinad Overlapping Hoops at Low and High Strain RatesDocument1 pageStress-Strain Behavior of Concrete Confinad Overlapping Hoops at Low and High Strain RatesRonal J Clavijo RNo ratings yet
- CIE 1931 Color SpaceDocument15 pagesCIE 1931 Color SpaceMoji PiyapongNo ratings yet
- European Standard Norme Européenne Europäische NormDocument17 pagesEuropean Standard Norme Européenne Europäische NormTyler KirbyNo ratings yet
- Fem Analysis of Buckling: Jerzy Pamin E-Mail: Jpamin@L5.Pk - Edu.PlDocument40 pagesFem Analysis of Buckling: Jerzy Pamin E-Mail: Jpamin@L5.Pk - Edu.PlEmanuel FelisbertoNo ratings yet
- Numerical Analysis and Simulation of PlasticityDocument317 pagesNumerical Analysis and Simulation of Plasticitysrikanthrajaram18100% (1)
- Chemical Reactions: Reactants ProductsDocument16 pagesChemical Reactions: Reactants ProductsRSLNo ratings yet
- Gyromagnetic Ratio WikiDocument4 pagesGyromagnetic Ratio Wikibugoff7000% (1)
- Diode RectifiersDocument64 pagesDiode RectifiersAmineNo ratings yet
- Fan Reverse EngineeringDocument14 pagesFan Reverse Engineeringapi-332478778No ratings yet
- Physics 2019 1st TermDocument3 pagesPhysics 2019 1st TermsulayajannyNo ratings yet
- DSP-1 (Intro) (S)Document77 pagesDSP-1 (Intro) (S)karthik0433No ratings yet
- Peperiksaan Percubaan SPM 2004Document29 pagesPeperiksaan Percubaan SPM 2004Carolyn Chang Boon ChuiNo ratings yet
- AE264 Spring2014 HW1Document3 pagesAE264 Spring2014 HW1Serdar BilgeNo ratings yet
- Chapter 11 Three Dimensional Geometry: Exercise 11.1Document55 pagesChapter 11 Three Dimensional Geometry: Exercise 11.113 13No ratings yet
- Operations On Matrices - Linear AlgebraDocument35 pagesOperations On Matrices - Linear AlgebradocsdownforfreeNo ratings yet
- United States Patent: ShkondinDocument16 pagesUnited States Patent: ShkondinAngel DiosdadoNo ratings yet
- 1 - Units and Measurements, Errors and Dimensional AnalysisDocument18 pages1 - Units and Measurements, Errors and Dimensional AnalysisSivakumar Sarma100% (1)
- Airplane Propel L 00 Corp GoogDocument424 pagesAirplane Propel L 00 Corp Googviorelcroitoru100% (1)
- NumericalsDocument4 pagesNumericalsvs9458No ratings yet
- Periodic Table ReviewerDocument3 pagesPeriodic Table RevieweranonymousNo ratings yet
- 6325 05 PDFDocument33 pages6325 05 PDFMatias Montenegro MancillaNo ratings yet
- Experiment No:7, Verification of Coulomb's Law Using Coulomb BalanceDocument5 pagesExperiment No:7, Verification of Coulomb's Law Using Coulomb BalanceDebdoot GhoshNo ratings yet