Professional Documents
Culture Documents
Ed066p853 2
Uploaded by
Aitor PastorOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ed066p853 2
Uploaded by
Aitor PastorCopyright:
Available Formats
overhead projector edited by
demon/tration/
Doris Kolb
Bradley University
Peoria, IL 61625
Change in Optical Rotation with Wavelength mixed with TCNE. On the other hand, benzene derivatives
E. Koubek and H. Quinn
containing an increasing number of methyl groups form
TCNE charge-transfer complexes increasingly of greater
U.S. Naval Academy color in terms of depth and wavelength shift. The formation
Annapolis, MD 21402
of the colored complexes can be illustrated by adding a few
Recently in this column Hambly1 described a demonstra- drops of colorless aromatic compound (e.g., methylbenzene)
tion involving a solution of sucrose contained in a 50-mL to a few milliliters of a colorless solution of TCNE (1% in
graduated cylinder. This is a simplified version of that dem- CH2CI2) in a small beaker on the stage of an overhead projec-
onstration. It is easier to prepare and to carry out, and the tor. Immediately upon mixing, the solution turns bright
visual image is much larger. The demonstration can be used yellow-orange. With mesitylene, 1,3,5-trimethylbenzene,
to illustrate the effect of an optically active material on the color produced is a much deeper orange-red. An easy
plane-polarized light, as well as to show that the light rota- way to demonstrate these colored charge-transfer complex-
tion is a function of the wavelength of the light used. es, graphically illustrating the correlation of color and reac-
Procedure: Place a polarizing film2 on an overhead projec- tivity, is to prepare a set of small French square bottles
tor. Set a small crystallizing dish containing about 1 in. of containing various aromatic compounds plus TCNE and to
Karo light corn syrup on top of the film, and then place compare the colors. To prepare the samples add 2.0 mL of
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
another piece of polarizing film on top of the dish. By rotat- TCNE solution (1% in CH2CI2) to a half-ounce French
Downloaded via UNIV DE ALICANTE on April 24, 2023 at 07:49:48 (UTC).
ing the top sheet of film, it can be shown that blue light is square bottle; then fill the bottle with an aromatic com-
transmitted first, and then other colors of the rainbow ap- pound, and screw on the cap. (Caution: Samples should be
pear as the film is rotated through larger angles. Not only is prepared in a well-ventilated hood, and gloves should be
this a colorful demonstration, but it also explains why mono- worn when handling TCNE.) For solid aromatic compounds
chromatic sodium D light is used in a polarimeter. (e.g., hexamethylbenzene) or for those compounds whose
absorption is too great to transmit light (e.g., methoxyben-
zene), CH2CI2 can be used to dilute the solution until it is
1
Hambly, G. F. J. Chem. Educ. 1988, 65, 623. transparent. Prepared in this manner, the capped French
2
Available from Edmund Scientific Co., Barrington, NJ 08007. square bottles can be laid side by side on the stage of an
overhead projector to allow the whole class to see the array of
colors associated with the various aromatic compounds. The
mixtures are usually stable for several months. An interest-
ing informative display can be made by using some of the
following benzene derivatives:
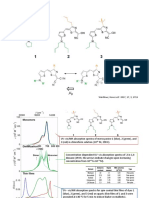
Aromatic Pi Cloud Availability: Formation of _Aromatic Color of Complex
Colored Charge-Transfer Complexes trifluoromethylbenzene none
benzonitrile none
Kenneth E. Kolb benzene light yellow
Bradley University methylbenzene yellow-orange
Peoria, IL 61625 1,3-dimethylbenzene orange
In organic lectures the comparative availability of the pi 1,3,5-trimethylbenzene orange-red
electron cloud of the benzene ring is correlated with the hexamethylbenzene (diluted) purple-violet
methoxybenzene (diluted) dark lavender
reactivity of various substituted benzenes. One way to dem-
onstrate the variance of pi electron availability in the ben- fluorobenzene lemon yellow
zene ring is to observe the color of the charge-transfer com-
chlorobenzene yellow
bromobenzene darker yellow
plex formed between an aromatic compound and tetracyan- iodobenzene red-orange
oethylene (TCNE). The absorptivity and color of the
resulting complex correlates well, in a qualitative manner, In the first group of compounds, which goes from deacti-
with the availability of pi electrons in the aromatic com- vated aromatic rings to very active ones, the color changes
pound and hence with its ease of electrophilic aromatic sub- progressively from colorless to deep lavender. In the second
stitution. Thus benzene compounds containing meta-direct- group the colors show that with bromobenzene and iodoben-
ing deactivating substituents, such as trifluoromethylben- zene the pi cloud is more available for complexation than
zene, form no pi complex with TCNE and give no color when with fluorobenzene or chlorobenzene.
Volume 66 Number 10 October 1989 853
You might also like
- BOQ Fountain Water Body (1) .XLSXBDocument6 pagesBOQ Fountain Water Body (1) .XLSXBPrashant Singh Chauhan60% (5)
- Safety Officer Interview Questions and AnswersDocument48 pagesSafety Officer Interview Questions and AnswersRaza Muhammad Soomro60% (5)
- Preparación y Caracterización Del Mesetilen Tricarbonil Molibdeno PDFDocument2 pagesPreparación y Caracterización Del Mesetilen Tricarbonil Molibdeno PDFSofia BujosaNo ratings yet
- D Annenberg 1963Document11 pagesD Annenberg 1963Rick MortyNo ratings yet
- Nanoparticula Del CloroplastosDocument5 pagesNanoparticula Del CloroplastosBeetNo ratings yet
- Macromolecular Rapid Communications - 2017 - Koo - Distinct Interfacial Fluorescence in Oil in Water Emulsions Via ExcitonDocument5 pagesMacromolecular Rapid Communications - 2017 - Koo - Distinct Interfacial Fluorescence in Oil in Water Emulsions Via Excitonpurin phokhunNo ratings yet
- Crystals: Electro-Optical E Liquid Crystal Device by Micro-Encapsulation With A Pigment-Doped ShellDocument11 pagesCrystals: Electro-Optical E Liquid Crystal Device by Micro-Encapsulation With A Pigment-Doped ShellLibatique GeorgeNo ratings yet
- Acs CGD 7b00460Document5 pagesAcs CGD 7b00460Joakin BahamondesNo ratings yet
- Piantanida 2020Document10 pagesPiantanida 2020diana valentinovaNo ratings yet
- 2017 - Milind R. Shreykar - Stimuli-Responsive Luminescent Coumarin Thiazole Hybrid DyeDocument5 pages2017 - Milind R. Shreykar - Stimuli-Responsive Luminescent Coumarin Thiazole Hybrid DyeTomas Delgado MontielNo ratings yet
- Dwnload Full Clinical Chemistry Theory Analysis Correlation 5th Edition Kaplan Test Bank PDFDocument36 pagesDwnload Full Clinical Chemistry Theory Analysis Correlation 5th Edition Kaplan Test Bank PDFcharlesdrakejth100% (9)
- Fabrication and Morphological Control of Electrospun Ethyl Cellulose NanofibersDocument4 pagesFabrication and Morphological Control of Electrospun Ethyl Cellulose NanofibersMaría BorregoNo ratings yet
- Control de La Reologia Utlizando AsociativoDocument11 pagesControl de La Reologia Utlizando AsociativoLATINA DE PINTURASNo ratings yet
- Patente FenolDocument12 pagesPatente FenolNaydaNo ratings yet
- Effect of Thiophene-Based P-Spacers On N-ArylphenoDocument9 pagesEffect of Thiophene-Based P-Spacers On N-ArylphenoTomas Delgado MontielNo ratings yet
- Pigment Vs DyeDocument3 pagesPigment Vs DyeBoonyarit LurdgrienggraiyingNo ratings yet
- One-Pass Pigment Printing of Bright Colours On A Dark Textile BackgroundDocument4 pagesOne-Pass Pigment Printing of Bright Colours On A Dark Textile Backgroundfathi mustafaNo ratings yet
- A Method To Collect An Infrared Spectrum in Solution: Hai-Shui W, Fei L, and Hongju ZDocument2 pagesA Method To Collect An Infrared Spectrum in Solution: Hai-Shui W, Fei L, and Hongju ZAnurak OnnnoomNo ratings yet
- Ahmed Et Al 2006 Color Research Application PDFDocument5 pagesAhmed Et Al 2006 Color Research Application PDFBitcoin cash CollectorNo ratings yet
- FIX Rencana Pembelajaran MK Fiswanman-2018Document5 pagesFIX Rencana Pembelajaran MK Fiswanman-2018GaluhFahmiNo ratings yet
- Ncomms 7229Document8 pagesNcomms 7229Kmw18 ce013No ratings yet
- Popovich 1967Document6 pagesPopovich 1967Hussnain AliNo ratings yet
- Merocyanines.: Würthner, Nano Lett. 2017, 17, 3, 1719Document8 pagesMerocyanines.: Würthner, Nano Lett. 2017, 17, 3, 1719sidharth pandaNo ratings yet
- 317 - Highly Efficient Light-Harvesting Ruthenium Sensitizer For Thin-Film Dye-Sensitized Solar CellsDocument7 pages317 - Highly Efficient Light-Harvesting Ruthenium Sensitizer For Thin-Film Dye-Sensitized Solar CellsAlberto RangelNo ratings yet
- Expt - UV-Vis Spectroscopy - ManualDocument5 pagesExpt - UV-Vis Spectroscopy - ManualSneha SNo ratings yet
- Solvatochromism HOMO LUMODocument2 pagesSolvatochromism HOMO LUMOzajitNo ratings yet
- Structure-Related Optical Properties of Luminescent Hetero-OpalsDocument7 pagesStructure-Related Optical Properties of Luminescent Hetero-OpalsJuanVillegasNo ratings yet
- Experiment 9Document6 pagesExperiment 9Muzammil Iqbal100% (1)
- Brief Notes: A Simple Metitod For Removing The Resin From Epoxy-Embedded TissueDocument2 pagesBrief Notes: A Simple Metitod For Removing The Resin From Epoxy-Embedded TissueVansala GanesanNo ratings yet
- Study The Effect of Thermally Stimulated Discharge Current On Polar Polysulfone and Multiwall Carbon Nanotube NanocompositeDocument4 pagesStudy The Effect of Thermally Stimulated Discharge Current On Polar Polysulfone and Multiwall Carbon Nanotube NanocompositeAime ChaudryNo ratings yet
- N719 Characterization of The Adsorption of Ru-Bpy Dyes On Mesoporous TiO2 Films WithDocument8 pagesN719 Characterization of The Adsorption of Ru-Bpy Dyes On Mesoporous TiO2 Films WithFrancescaSacchiNo ratings yet
- Micelar Effect, J y H DimersDocument10 pagesMicelar Effect, J y H DimersMario FloresNo ratings yet
- Chemcomm: CommunicationDocument4 pagesChemcomm: CommunicationSreedevi KrishnakumarNo ratings yet
- Ri2017-01 Ruco-Blanc enDocument8 pagesRi2017-01 Ruco-Blanc entitus riadi chandraNo ratings yet
- Synthesis of Flame Retardant Dye and Its Application On Silk FabricDocument16 pagesSynthesis of Flame Retardant Dye and Its Application On Silk FabricTJPRC PublicationsNo ratings yet
- Dispersion of Graphene in Ethanol Using A Simple Solvent Exchange Method PDFDocument4 pagesDispersion of Graphene in Ethanol Using A Simple Solvent Exchange Method PDFary.engenharia1244No ratings yet
- Foam Optimization Strategies Consumer Formulations: Formulation ScienceDocument6 pagesFoam Optimization Strategies Consumer Formulations: Formulation ScienceYurelii Chiguiils100% (1)
- Chemical Physics: Julien Preat, Denis Jacquemin, Catherine Michaux, Eric A. PerpèteDocument13 pagesChemical Physics: Julien Preat, Denis Jacquemin, Catherine Michaux, Eric A. PerpèteNgọc Tân PhạmNo ratings yet
- J Saa 2020 119309Document9 pagesJ Saa 2020 119309Anand SolomonNo ratings yet
- Solution Grown Pbs Nanoparticle Films: Rakesh K. Joshi, Aloke Kanjilal, H.K. SehgalDocument5 pagesSolution Grown Pbs Nanoparticle Films: Rakesh K. Joshi, Aloke Kanjilal, H.K. SehgalSubbaraju GvNo ratings yet
- Effects of Ethanol On Optimizing Porous Films of Dye-Sensitizedef101546aDocument5 pagesEffects of Ethanol On Optimizing Porous Films of Dye-Sensitizedef101546aمصطفى محمودNo ratings yet
- Jana 03Document5 pagesJana 03Dr. Amit Kumar JanaNo ratings yet
- 2020 - Zhijie Xu - Theoretical Study of T Shaped Phenothiazine-CarbazoleDocument10 pages2020 - Zhijie Xu - Theoretical Study of T Shaped Phenothiazine-CarbazoleTomas Delgado MontielNo ratings yet
- Nitration of Benzene: Adia, Sophia Cassandra, ADocument3 pagesNitration of Benzene: Adia, Sophia Cassandra, AS AdiaNo ratings yet
- Effect of Solution Concentration On The Electrospray/Electrospinning Transition and On The Crystalline Phase of PVDFDocument6 pagesEffect of Solution Concentration On The Electrospray/Electrospinning Transition and On The Crystalline Phase of PVDFRana Sabouni TabariNo ratings yet
- 5 - Cortés-Burgos (2021) - Effects of PbS-NPs Doping On The Photovoltaic Performance of Natural Dye-Sensitized TiO2 PhotoelectrodesDocument9 pages5 - Cortés-Burgos (2021) - Effects of PbS-NPs Doping On The Photovoltaic Performance of Natural Dye-Sensitized TiO2 Photoelectrodesmaria cortesNo ratings yet
- Surface plasmon optical characterization of lipid monolayers at 5 μm lateral resolutionDocument5 pagesSurface plasmon optical characterization of lipid monolayers at 5 μm lateral resolutionKaren Régules MedelNo ratings yet
- Robust Chromatic Adaptation Based Color CorrectionDocument15 pagesRobust Chromatic Adaptation Based Color CorrectionKaung KaungNo ratings yet
- Krishnamurthy 2006Document5 pagesKrishnamurthy 2006501237198002No ratings yet
- Dyeing Effects of Bifunctional Reactive Dyes On Knitted Cotton FabricsDocument4 pagesDyeing Effects of Bifunctional Reactive Dyes On Knitted Cotton Fabricsmahbub690No ratings yet
- Experiment#2FR-3MBIO2 8Document8 pagesExperiment#2FR-3MBIO2 8Charisse PondocNo ratings yet
- 2018 May TZ2 Paper 3 HL Chemistry MarkschemeDocument10 pages2018 May TZ2 Paper 3 HL Chemistry MarkschememounishadmNo ratings yet
- Hexane and WaterDocument4 pagesHexane and WaterFrancis Adu-marfoNo ratings yet
- Organometal Perovskite Light Absorbers Toward A 20% Efficiency Low-Cost Solid-State Mesoscopic Solar Cell (Article)Document7 pagesOrganometal Perovskite Light Absorbers Toward A 20% Efficiency Low-Cost Solid-State Mesoscopic Solar Cell (Article)yascheNo ratings yet
- Measuring Zeta Potential in Concentrated Industrial SlurriesDocument14 pagesMeasuring Zeta Potential in Concentrated Industrial SlurriesKharisma Rizky PangestuNo ratings yet
- Study On Calculation of Diffusion Coefficient For Sublimation Diffusion of Disperse Dye Using Fourier SeriesDocument10 pagesStudy On Calculation of Diffusion Coefficient For Sublimation Diffusion of Disperse Dye Using Fourier SeriesMd SolaimanNo ratings yet
- "K-Epsilon (K-) Turbulence Model": Gabriel F.R Lopes Ph.D. Nelson Moraga Benavides January 21-2019Document5 pages"K-Epsilon (K-) Turbulence Model": Gabriel F.R Lopes Ph.D. Nelson Moraga Benavides January 21-2019Diego AlejandroNo ratings yet
- Separation of Benzene and Deuterated Benzenes by Reversed-Phase and Recycle Liquid Chromatography Using Monolithic Capillary ColumnsDocument6 pagesSeparation of Benzene and Deuterated Benzenes by Reversed-Phase and Recycle Liquid Chromatography Using Monolithic Capillary Columnsראול אפונטהNo ratings yet
- Ki PHD ThesisDocument4 pagesKi PHD Thesismarialackarlington100% (2)
- Photosynthesis: Simple Demonstration: A of Hill ReactionDocument1 pagePhotosynthesis: Simple Demonstration: A of Hill ReactionSneha PatilNo ratings yet
- Importante ElMzioui2019 Article ATheoreticalInvestigationOfTheDocument11 pagesImportante ElMzioui2019 Article ATheoreticalInvestigationOfTheTomas Delgado MontielNo ratings yet
- Nanomaterials: Biomedical, Environmental, and Engineering ApplicationsFrom EverandNanomaterials: Biomedical, Environmental, and Engineering ApplicationsSuvardhan KanchiNo ratings yet
- Low-Noise Simplex Optimization Experiment: FutilityDocument2 pagesLow-Noise Simplex Optimization Experiment: FutilityAitor PastorNo ratings yet
- For Conceptualization The Franck-Condon PrincipleDocument1 pageFor Conceptualization The Franck-Condon PrincipleAitor PastorNo ratings yet
- Acs Jchemed 7b00647Document5 pagesAcs Jchemed 7b00647Aitor PastorNo ratings yet
- 6 CHM 5710 Using Character TablesDocument35 pages6 CHM 5710 Using Character TablesAitor PastorNo ratings yet
- Spectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsDocument10 pagesSpectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsAitor PastorNo ratings yet
- Instrumental: Simplex OptimizationDocument4 pagesInstrumental: Simplex OptimizationAitor PastorNo ratings yet
- Numerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationDocument8 pagesNumerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationAitor PastorNo ratings yet
- Franck-Condon Factors and Their Use in Undergraduate Quantum MechanicsDocument7 pagesFranck-Condon Factors and Their Use in Undergraduate Quantum MechanicsAitor PastorNo ratings yet
- Of Chemistry: Calculation Factors UndergraduateDocument3 pagesOf Chemistry: Calculation Factors UndergraduateAitor PastorNo ratings yet
- Electronic Tetrahedral Complexes: Nickel (LL)Document2 pagesElectronic Tetrahedral Complexes: Nickel (LL)Aitor PastorNo ratings yet
- Active: of Optically ComplexDocument2 pagesActive: of Optically ComplexAitor PastorNo ratings yet
- Textbook Errors, 62 Difference Between and Liquids and SolidsDocument2 pagesTextbook Errors, 62 Difference Between and Liquids and SolidsAitor PastorNo ratings yet
- Air-Sensitive: Argon Techniques Manipulation ofDocument1 pageAir-Sensitive: Argon Techniques Manipulation ofAitor PastorNo ratings yet
- Resource: Papers-IIIDocument17 pagesResource: Papers-IIIAitor PastorNo ratings yet
- Lattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheDocument7 pagesLattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheAitor PastorNo ratings yet
- Coordination: Complexes CobaltDocument3 pagesCoordination: Complexes CobaltAitor PastorNo ratings yet
- Oxidation and Reduction Reactions in Organic ChemistryDocument4 pagesOxidation and Reduction Reactions in Organic ChemistryKhubaib BaryaalNo ratings yet
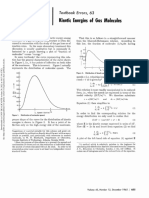
- Textbook Errors, 63 Kinetic Molecules: EnergiesDocument2 pagesTextbook Errors, 63 Kinetic Molecules: EnergiesAitor PastorNo ratings yet
- Crystal: SplittingDocument3 pagesCrystal: SplittingAitor PastorNo ratings yet
- Ed 400583 QDocument4 pagesEd 400583 QAitor PastorNo ratings yet
- Ab 036Document6 pagesAb 036Aitor PastorNo ratings yet
- Half-Wave Potential: SourceDocument1 pageHalf-Wave Potential: SourceAitor PastorNo ratings yet
- Acs Jchemed 5b00170Document5 pagesAcs Jchemed 5b00170Aitor PastorNo ratings yet
- Ed5b00170 Si 001Document27 pagesEd5b00170 Si 001Aitor PastorNo ratings yet
- Acssuschemeng 2c00095Document10 pagesAcssuschemeng 2c00095Aitor PastorNo ratings yet
- Error Titrations Mixtures: MinimumDocument4 pagesError Titrations Mixtures: MinimumAitor PastorNo ratings yet
- TheBaldwinRules RevisedandExtendedDocument29 pagesTheBaldwinRules RevisedandExtendedAitor PastorNo ratings yet
- Styer 2000Document7 pagesStyer 2000Aitor PastorNo ratings yet
- Claisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroDocument64 pagesClaisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroAitor PastorNo ratings yet
- Steel Sheet, Zinc-Coated (Galvanized) or Zinc-Iron Alloy-Coated (Galvannealed) by The Hot-Dip ProcessDocument13 pagesSteel Sheet, Zinc-Coated (Galvanized) or Zinc-Iron Alloy-Coated (Galvannealed) by The Hot-Dip Processraulpalma93No ratings yet
- APEG Polyester Fabric Belt EPDocument1 pageAPEG Polyester Fabric Belt EPmuhammad IhsanNo ratings yet
- Valorizacion Del Desecho de Aguas Residuales Del Prosamiento de AceitunasDocument11 pagesValorizacion Del Desecho de Aguas Residuales Del Prosamiento de AceitunasJalcamNo ratings yet
- GC Application: Alcohols On ZB-WAX 30x.25x.25Document2 pagesGC Application: Alcohols On ZB-WAX 30x.25x.25qncargbNo ratings yet
- CertificateDocument28 pagesCertificateKhaldi KaisNo ratings yet
- Iso 19229 2019Document10 pagesIso 19229 2019laythNo ratings yet
- EP0257845A2Document56 pagesEP0257845A2Jen RealNo ratings yet
- High Performance ButterflyDocument19 pagesHigh Performance ButterflyEmily NguyenNo ratings yet
- Alfa Laval Hvo Pre Treatment Webinar Final 003Document27 pagesAlfa Laval Hvo Pre Treatment Webinar Final 003Lim Chee SiangNo ratings yet
- Recruitment and Selection Bharathi CementsDocument73 pagesRecruitment and Selection Bharathi Cementskum124267% (3)
- (5403) Sheet Surface Chemistry Theory eDocument24 pages(5403) Sheet Surface Chemistry Theory eboom rangNo ratings yet
- LIT1307 Test StandsDocument4 pagesLIT1307 Test StandsFabio Peres de LimaNo ratings yet
- 95 8576 9.2 - X3302 PDFDocument37 pages95 8576 9.2 - X3302 PDFSalik SiddiquiNo ratings yet
- 2009 H2 Chemistry Paper 2 (MCQ) + AnsDocument11 pages2009 H2 Chemistry Paper 2 (MCQ) + AnsIliyana IliNo ratings yet
- Colloid Chemistry and Phase Rule 4022146-1: (Essentials of Physical Chemistry - Arun Bahl & B.S. Bahl) Chapter 19 and 22Document51 pagesColloid Chemistry and Phase Rule 4022146-1: (Essentials of Physical Chemistry - Arun Bahl & B.S. Bahl) Chapter 19 and 22Razan khalidNo ratings yet
- Rare Earth Elements in CoalDocument4 pagesRare Earth Elements in CoalPepitoNo ratings yet
- Quintessence of The PhilosophersDocument32 pagesQuintessence of The PhilosopherstravellerfellowNo ratings yet
- Ionic EquilibriumDocument4 pagesIonic EquilibriumFu HongNo ratings yet
- Physical Pharmacy - 3rd Sem - Unit 2aDocument15 pagesPhysical Pharmacy - 3rd Sem - Unit 2aVishant Sirvi100% (1)
- Is 6262 1971Document19 pagesIs 6262 1971just_4_u_dear_in9549No ratings yet
- Prelab No. 1 - Determination-of-Noise-Level-Using-Sound-Level-Meter-Final - Group No. 7Document2 pagesPrelab No. 1 - Determination-of-Noise-Level-Using-Sound-Level-Meter-Final - Group No. 7louryNo ratings yet
- 2007 - Huber Et Al. - Synergies Between Bio and Oil Refineries For The Production of Fuels From Biomass PDFDocument18 pages2007 - Huber Et Al. - Synergies Between Bio and Oil Refineries For The Production of Fuels From Biomass PDFMayank KumarNo ratings yet
- Metabolic Acidosis & AlkalosisDocument3 pagesMetabolic Acidosis & AlkalosisJared MabulayNo ratings yet
- Matrices, Retainers, Wedge Placement. Separation of The Teeth. The Protective Role of Liners and BasesDocument42 pagesMatrices, Retainers, Wedge Placement. Separation of The Teeth. The Protective Role of Liners and BasesFatimah DewiNo ratings yet
- HR 12390 WDocument2 pagesHR 12390 Wapi-170472102No ratings yet
- BatteriesDocument29 pagesBatteriesgihan5dhananjaya5katNo ratings yet
- Topic 12 - Acid-Base Equilibria MCQsDocument11 pagesTopic 12 - Acid-Base Equilibria MCQsmegaordinaryday0% (1)
- ConcreteDocument11 pagesConcretesiddhi gangwalNo ratings yet