Professional Documents
Culture Documents
For Conceptualization The Franck-Condon Principle
Uploaded by
Aitor Pastor0 ratings0% found this document useful (0 votes)
4 views1 pageOriginal Title
ed052p790
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
4 views1 pageFor Conceptualization The Franck-Condon Principle
Uploaded by
Aitor PastorCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 1
L. R.
Byers
University of Wisconsin A Teaching Aid for the Conceptualization
Madison, 53706
of the Franck-Condon Principle
In the last few years, photoelectron spectroscopy (PES)
has developed into a valuable tool for the experimental
chemist (1-3). One attribute of PES is the ease of under-
standing its elementary process—ionization. Although the
concept of ionization is introduced early, in an atomic con-
text, its natural extension to molecular systems is rarely
pursued. Ionization energies of monoatomic atoms are not
only difficult to obtain, but are also relatively uninteresting
in chemical discussions after being acquired. On the other
hand, the world of molecules viewed with the aid of PES is
beginning to show interesting and important chemical
trends heretofore obtained only with much greater effort
(4).
An informative example has been constructed in the ac-
companying illustration for the process
H2(i/' =
0) + hv —-
H2V =
0,1,2,..., 13) + e"
Even though this is the least complex molecular system, it
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.
was chosen specifically to demonstrate features which
modern quantum mechanics and its application in the field
Downloaded via UNIV DE ALICANTE on April 13, 2023 at 15:51:55 (UTC).
of electronic spectral analysis required many years to for-
mulate, e.g. the Franck-Condon principle (5).
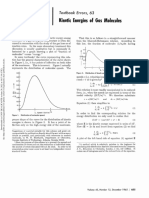
The appropriate energy curves (6) with their associated
vibrational energy levels, which are reproduced here, are
very accurately known from electronic band spectra and
are thus quantitative. Also drawn in for the v" = 0 level of
Juxtaposition of the potential energy curves of Sharp (6) for H2 (12g+) and
the ground state of Ha are the classical Franck-Condon H2+ (22g+) with the photoelectron spectrum of Siegbahn, et al. (8) for the
turning points and a qualitative picture (7) of the quan- ionization: H2 (12g+) — H2+ (22g+).
tum mechanical vibrational wavefunction (which is expect-
ed to be slightly skewed toward values of r > re due to the
asymmetry in the shape of the potential well). The inset parent. Numerical values for these differences may be
PES spectrum (8) for the ionization of H2 shows a plot of found in Siegbahn’s original spectrum (8).
signal intensity from the electron energy analyzer versus Other examples, which may be constructed for ioniza-
the binding energy, a quantity derived from the electrons’ tions of N2, NQ, and O2, are qualitatively similar, although
kinetic energy (9). generally fewer v' vibrational levels are observed (11, 12).
Qualitatively, a simultaneous comparison of the diagram It is hoped that this lucid illustration of a real molecular
with the spectrum reveals three interesting points. First, system may be usefully employed in the understanding of
according to the Franck-Condon principle, the 1/ = 2 v" **-
the points discussed herein.
=
0 transition might reasonably be expected to be the most
Literature Cited
intense, in that a vertical transition from the most probable
internuclear separation in the ground state of H2 would rise (1) Bock, H., and Mollere, P. D„ J. CHEM. EDUC.. 51,506 (1974).
(2) DeKock, R. L.. and Lloyd, D. R., Advan. Inorg. Chem. Radiochem., 16,65 (1974).
closest (shown by arrow) to this vibrationally excited level (3) Brundle, C. R., and Robin, M. B., in “Determination of Organic Structures by Phys-
in H>2+. This is indeed observed in the PES spectrum. Sec- ical Methods,” (Editors: Nachod. F. C., and Zuckerman, J. J.), Vol. 3, Academic
Press, New York, 1971, pp. 1-71.
ond, and further, the general shape of the envelope (shown (4) Baker, A. D., Accts. Chem. Res., 3, 17 (1970).
by dotted line) in the spectrum is easily seen to be analo- (5) Herzberg, G., "Spectra of Diatomic Molecules,” 2nd Ed., D. Van Nostrand Co.,
Princeton, 1950, pp. 194-196.
gous to the shape of the vibrational probability distribution (6) Redrawn from data of Sharp, T. E., Atomic Data. 2, 119 (1971).
over r in the v" 0 level of H2. It should be mentioned that
=
(7) Ref. (5), pp. 93-94.
the actual shape of this envelope is dependent upon transi- (8) Redrawn from illustration of Siegbahn, K., et al., "ESCA—Atomic, Molecular and
Solid State Structure Studied by Means of Electron Spectroscopy,” Almquist and
tion probabilities between the various pairs of states (10), Wiksells, Uppsala, 1967, p. 209.
but, the alteration in the envelope is qualitatively negligi- (9) Turner, D. W., Prnc. Roy. Soc., Ser. A., 307,15 (1968).
(10) Berkowitz, J., and Spohr, R., J. Electron Spectrosc.. 2, 143 (1973).
ble. Third, the regular decrease in the spacings of the v' vi- (11) Turner, D. W„ and May, D. P., J. Chem. Phys., 45, 471 (1966).
brational energy levels, due to anharmonicity, is readily ap- (12) Gilmore. F. R., J. Quant. Spectrosc. Radiat. Transfer, 5, 369 (1965).
+ + +
790 / Journal of Chemical Education
You might also like
- Bipolar ElectrodesDocument9 pagesBipolar ElectrodesLoga NathanNo ratings yet
- How Would You Integrate The Equations of Motion in Dissipative Particle Dynamics Simulations?Document15 pagesHow Would You Integrate The Equations of Motion in Dissipative Particle Dynamics Simulations?vellankividwatNo ratings yet
- Exact Analytical Form of Diatomic Molecular OrbitalsDocument7 pagesExact Analytical Form of Diatomic Molecular OrbitalsChen LiNo ratings yet
- Kinetics: Electrode ProcessesDocument8 pagesKinetics: Electrode ProcessesAitor PastorNo ratings yet
- Bestem K1Document19 pagesBestem K1Chuaks ChuaksNo ratings yet
- Journal - Interatomic and Intermolecular Coulombic DecayDocument75 pagesJournal - Interatomic and Intermolecular Coulombic DecayThảo HàNo ratings yet
- Amini, H., Lee, W. & Di Carlo, D. Inertial Microfluidic Physics. Lab Chip 14, 2739-2761 (2014)Document23 pagesAmini, H., Lee, W. & Di Carlo, D. Inertial Microfluidic Physics. Lab Chip 14, 2739-2761 (2014)문진식No ratings yet
- Phos With MLDocument11 pagesPhos With MLpinakiNo ratings yet
- Observation of Attractive and Repulsive Polarons in A Bose-Einstein CondensateDocument12 pagesObservation of Attractive and Repulsive Polarons in A Bose-Einstein Condensatequant007No ratings yet
- Schreier Et Al 2023 Trends in Electrocatalysis The Microenvironment Moves To Center StageDocument6 pagesSchreier Et Al 2023 Trends in Electrocatalysis The Microenvironment Moves To Center Stagepandiaraj1988No ratings yet
- Grupo 1Document7 pagesGrupo 1João VictorNo ratings yet
- A Comprehensive Review of Fluorescence Correlation SpectrosDocument21 pagesA Comprehensive Review of Fluorescence Correlation Spectrosthomas abramsNo ratings yet
- Explainable Machine Learning of The Underlying Physics of High-Energy Particle Collisions by Lai, Neill, Ploskon and RingerDocument6 pagesExplainable Machine Learning of The Underlying Physics of High-Energy Particle Collisions by Lai, Neill, Ploskon and Ringerkevinchu021195No ratings yet
- Establishing An Organic Chemistry Intuition For ElectrochemistryDocument11 pagesEstablishing An Organic Chemistry Intuition For ElectrochemistrymohdhafizmdaliNo ratings yet
- Perelstein 2011Document66 pagesPerelstein 2011good feelNo ratings yet
- The Spallation Neutron Source: A Powerful Tool For Materials ResearchDocument4 pagesThe Spallation Neutron Source: A Powerful Tool For Materials Researchtestonly261No ratings yet
- Ed 3Document33 pagesEd 3quarksteam2023No ratings yet
- Polymers 15 01636Document20 pagesPolymers 15 01636fbn2377No ratings yet
- SE3-Equivariant Graph Neural Networks For Data-EffDocument15 pagesSE3-Equivariant Graph Neural Networks For Data-Effmohammed ezzatNo ratings yet
- Optomechanics With BECDocument7 pagesOptomechanics With BECلب اشقياءNo ratings yet
- 2021 - J. Phys. Chem. Lett. - BibhisanDocument7 pages2021 - J. Phys. Chem. Lett. - Bibhisanbibhisan royNo ratings yet
- From Fundamental Theories To Quantum Coherences in Electron TransferDocument15 pagesFrom Fundamental Theories To Quantum Coherences in Electron Transferces.trashcanNo ratings yet
- Mechanistic Modeling of Kinetics. Model Study: CompoundDocument7 pagesMechanistic Modeling of Kinetics. Model Study: CompoundMia PhanNo ratings yet
- Three-Body Entanglement in Particle DecaysDocument6 pagesThree-Body Entanglement in Particle Decaysqinghong caoNo ratings yet
- Toward An Accurate Strongly-Coupled Many-Body Theory Within The Equation of Motion FrameworkDocument24 pagesToward An Accurate Strongly-Coupled Many-Body Theory Within The Equation of Motion FrameworkGaston GBNo ratings yet
- Penci Keta LDocument5 pagesPenci Keta LvenuNo ratings yet
- Jacs 5b09519Document10 pagesJacs 5b09519Giggly HadidNo ratings yet
- In Situ Irradiated X Ray Photoelectron Spectroscopy Investigation OnDocument8 pagesIn Situ Irradiated X Ray Photoelectron Spectroscopy Investigation OnPrasaanth RanuNo ratings yet
- 2017 Sacrificial Electron Donor Reagents For Solar Fuel ProductionDocument13 pages2017 Sacrificial Electron Donor Reagents For Solar Fuel Productionvincent picardNo ratings yet
- Born Oppenheimer ApproximationDocument24 pagesBorn Oppenheimer Approximationarpanmanna.phychemNo ratings yet
- The Birth of Molecular Electronics: April 27, 2017 10:2 Molecular Electronics: 2nd Edition 9in X 6in b2892Document16 pagesThe Birth of Molecular Electronics: April 27, 2017 10:2 Molecular Electronics: 2nd Edition 9in X 6in b2892Satish Kumar KollaNo ratings yet
- Franck-Condon Factors and Their Use in Undergraduate Quantum MechanicsDocument7 pagesFranck-Condon Factors and Their Use in Undergraduate Quantum MechanicsAitor PastorNo ratings yet
- Murugan Et Al 2023 Computation of Neighborhood M Polynomial of Cycloparaphenylene and Its VariantsDocument10 pagesMurugan Et Al 2023 Computation of Neighborhood M Polynomial of Cycloparaphenylene and Its Variantsvidhya sivaNo ratings yet
- 1 s2.0 S0009261423005572 MainDocument11 pages1 s2.0 S0009261423005572 MainABDELKHALK ABOULOUARDNo ratings yet
- Mechanism For Damage To DNA by Low-Energy ElectronsDocument4 pagesMechanism For Damage To DNA by Low-Energy ElectronsRanjan SutradharNo ratings yet
- Berova 2007Document18 pagesBerova 2007Nayim SepayNo ratings yet
- Calculo Spectra ClorofilaDocument6 pagesCalculo Spectra ClorofilaGabriel Henrique BatistaNo ratings yet
- Kyoto Nara Hyogo: Background Images Are Drawn Based On o Cial Pictures Provided by RIKENDocument4 pagesKyoto Nara Hyogo: Background Images Are Drawn Based On o Cial Pictures Provided by RIKEN123 456No ratings yet
- An Ecosystem For Digital Reticular Chemistry: AccessDocument19 pagesAn Ecosystem For Digital Reticular Chemistry: AccessPICHE MME SecretaryNo ratings yet
- General Working Principles of CH NH PBX Perovskite Solar CellsDocument6 pagesGeneral Working Principles of CH NH PBX Perovskite Solar CellsShameer KhanNo ratings yet
- Unanswered Questions in The Electroweak Theory: FurtherDocument53 pagesUnanswered Questions in The Electroweak Theory: Furthercifarha venantNo ratings yet
- A Revolution in Optical ManipulationDocument7 pagesA Revolution in Optical ManipulationAyan BanerjeeNo ratings yet
- Impact of Inherent Periodic Structure On Effective PDFDocument24 pagesImpact of Inherent Periodic Structure On Effective PDFskynet621No ratings yet
- Blin 2013Document12 pagesBlin 2013Facundo HerreraNo ratings yet
- Kahn Molecular MagnetismDocument200 pagesKahn Molecular MagnetismAna Cerdeira100% (3)
- Rsos.191204 LCTEMDocument24 pagesRsos.191204 LCTEMRamesh SoniNo ratings yet
- Marthan - F. Albert Cotto Teoria Del Campo LigandoDocument12 pagesMarthan - F. Albert Cotto Teoria Del Campo LigandoJuan Diego CarrilloNo ratings yet
- Jacobs.2023.Theory and Simulation of Multiphase Coexistence in Biomolecular MixturesDocument17 pagesJacobs.2023.Theory and Simulation of Multiphase Coexistence in Biomolecular Mixturessourav gangulyNo ratings yet
- Informing Geometric Deep Learning With Electronic Interactions To Accelerate Quantum ChemistryDocument12 pagesInforming Geometric Deep Learning With Electronic Interactions To Accelerate Quantum ChemistryMirok ZhangNo ratings yet
- 2012 - Sanchez de Armas - Phys Chem Chem PhysDocument9 pages2012 - Sanchez de Armas - Phys Chem Chem PhysTomas Delgado MontielNo ratings yet
- Perspectives On A New Age of Materials For Petroleum: AccessDocument2 pagesPerspectives On A New Age of Materials For Petroleum: AccessDanielaNo ratings yet
- Perspective: Concepts in Bio-Molecular Spectroscopy: Vibrational Case Studies On MetalloenzymesDocument16 pagesPerspective: Concepts in Bio-Molecular Spectroscopy: Vibrational Case Studies On Metalloenzymesmustafa alasadyNo ratings yet
- High Electric Field On Water Microdroplets Catalyzes SpontaneousDocument7 pagesHigh Electric Field On Water Microdroplets Catalyzes Spontaneoushiter1126zdmNo ratings yet
- Jacs.6b11263 Methanol ProductionDocument8 pagesJacs.6b11263 Methanol ProductionLuz Idalia Ibarra RodriguezNo ratings yet
- 2012 - Anselmi-Filippo - Phys Chem Chem Phys 15963 - Interplay dye-TiO2Document12 pages2012 - Anselmi-Filippo - Phys Chem Chem Phys 15963 - Interplay dye-TiO2Tomas Delgado MontielNo ratings yet
- Wang Et Al 2019 Quantitative Adjustment To The Molecular Energy Parameter in The Lake Thomas Theory of Polymer FractureDocument6 pagesWang Et Al 2019 Quantitative Adjustment To The Molecular Energy Parameter in The Lake Thomas Theory of Polymer FracturelizixicNo ratings yet
- Linkers in COFDocument10 pagesLinkers in COFhridita purbaNo ratings yet
- Kehoe 2013Document8 pagesKehoe 2013Menjamin SalasNo ratings yet
- DT 1203.5072v1Document12 pagesDT 1203.5072v1gaminster6935No ratings yet
- X-ray Photoelectron Spectroscopy: An introduction to Principles and PracticesFrom EverandX-ray Photoelectron Spectroscopy: An introduction to Principles and PracticesNo ratings yet
- Low-Noise Simplex Optimization Experiment: FutilityDocument2 pagesLow-Noise Simplex Optimization Experiment: FutilityAitor PastorNo ratings yet
- Numerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationDocument8 pagesNumerical Evaluation of Energy Levels and Wave Functions For Hindered Internal RotationAitor PastorNo ratings yet
- Acs Jchemed 7b00647Document5 pagesAcs Jchemed 7b00647Aitor PastorNo ratings yet
- 6 CHM 5710 Using Character TablesDocument35 pages6 CHM 5710 Using Character TablesAitor PastorNo ratings yet
- Ed066p853 2Document1 pageEd066p853 2Aitor PastorNo ratings yet
- Instrumental: Simplex OptimizationDocument4 pagesInstrumental: Simplex OptimizationAitor PastorNo ratings yet
- Of Chemistry: Calculation Factors UndergraduateDocument3 pagesOf Chemistry: Calculation Factors UndergraduateAitor PastorNo ratings yet
- Franck-Condon Factors and Their Use in Undergraduate Quantum MechanicsDocument7 pagesFranck-Condon Factors and Their Use in Undergraduate Quantum MechanicsAitor PastorNo ratings yet
- Spectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsDocument10 pagesSpectroscopy,: Laser-Induced Fluorescence Dynamics, DiagnosticsAitor PastorNo ratings yet
- Electronic Tetrahedral Complexes: Nickel (LL)Document2 pagesElectronic Tetrahedral Complexes: Nickel (LL)Aitor PastorNo ratings yet
- Active: of Optically ComplexDocument2 pagesActive: of Optically ComplexAitor PastorNo ratings yet
- Textbook Errors, 62 Difference Between and Liquids and SolidsDocument2 pagesTextbook Errors, 62 Difference Between and Liquids and SolidsAitor PastorNo ratings yet
- Air-Sensitive: Argon Techniques Manipulation ofDocument1 pageAir-Sensitive: Argon Techniques Manipulation ofAitor PastorNo ratings yet
- Resource: Papers-IIIDocument17 pagesResource: Papers-IIIAitor PastorNo ratings yet
- Lattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheDocument7 pagesLattice Prediction Kapustinskii Equations and Born-Haber: Energy and Chemical The TheAitor PastorNo ratings yet
- Coordination: Complexes CobaltDocument3 pagesCoordination: Complexes CobaltAitor PastorNo ratings yet
- Oxidation and Reduction Reactions in Organic ChemistryDocument4 pagesOxidation and Reduction Reactions in Organic ChemistryKhubaib BaryaalNo ratings yet
- Textbook Errors, 63 Kinetic Molecules: EnergiesDocument2 pagesTextbook Errors, 63 Kinetic Molecules: EnergiesAitor PastorNo ratings yet
- Crystal: SplittingDocument3 pagesCrystal: SplittingAitor PastorNo ratings yet
- Ed 400583 QDocument4 pagesEd 400583 QAitor PastorNo ratings yet
- Ab 036Document6 pagesAb 036Aitor PastorNo ratings yet
- Half-Wave Potential: SourceDocument1 pageHalf-Wave Potential: SourceAitor PastorNo ratings yet
- Acs Jchemed 5b00170Document5 pagesAcs Jchemed 5b00170Aitor PastorNo ratings yet
- Ed5b00170 Si 001Document27 pagesEd5b00170 Si 001Aitor PastorNo ratings yet
- Acssuschemeng 2c00095Document10 pagesAcssuschemeng 2c00095Aitor PastorNo ratings yet
- Error Titrations Mixtures: MinimumDocument4 pagesError Titrations Mixtures: MinimumAitor PastorNo ratings yet
- TheBaldwinRules RevisedandExtendedDocument29 pagesTheBaldwinRules RevisedandExtendedAitor PastorNo ratings yet
- Styer 2000Document7 pagesStyer 2000Aitor PastorNo ratings yet
- Claisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroDocument64 pagesClaisen Rearrangement Over The Past Nine Decades: Ana M. Martı N CastroAitor PastorNo ratings yet
- Chemistry Worksheet Summer AssignmentDocument10 pagesChemistry Worksheet Summer AssignmentJohn Joseph CambaNo ratings yet
- SMKCH Sem1 Trial 2014Document9 pagesSMKCH Sem1 Trial 2014Norbert LimNo ratings yet
- Original PDF Fundamentals of General Organic and Biological Chemistry 8th Edition PDFDocument41 pagesOriginal PDF Fundamentals of General Organic and Biological Chemistry 8th Edition PDFgwen.garcia161100% (35)
- VMD TutorialDocument22 pagesVMD TutorialdennyNo ratings yet
- 4.2 (180 Marks) : MarkschemeDocument67 pages4.2 (180 Marks) : MarkschemeSemwezi EnockNo ratings yet
- Test Bank For Organic Chemistry 8th Edition McmurryDocument6 pagesTest Bank For Organic Chemistry 8th Edition McmurryOsvaldo Laite100% (29)
- Lecture - 3 - Properties of Pure SubstancesDocument91 pagesLecture - 3 - Properties of Pure SubstancesAbrar Hussain AwanNo ratings yet
- Checkpoint ChemistryDocument18 pagesCheckpoint ChemistryNgoc Quang NguyenNo ratings yet
- 3Document63 pages3api-3744800100% (3)
- IChO2010 PPDocument71 pagesIChO2010 PPSaranphongNo ratings yet
- Covalent Compounds Quiz 1Document3 pagesCovalent Compounds Quiz 1Rania AbdellatifNo ratings yet
- IVF - Molecular Filtration For Life - ENDocument8 pagesIVF - Molecular Filtration For Life - ENSergei KurpishNo ratings yet
- Mal1033: Groundwater HydrologyDocument63 pagesMal1033: Groundwater Hydrologyomed muhammadNo ratings yet
- Aeronomy of The Middle Atmosphere - 2005Document651 pagesAeronomy of The Middle Atmosphere - 2005DaviMouraNo ratings yet
- BITSAT 2015 BrochureDocument24 pagesBITSAT 2015 BrochureMota ChashmaNo ratings yet
- Sample Questions - Chapter 15Document8 pagesSample Questions - Chapter 15Rasel IslamNo ratings yet
- Chemical Bonds: Modular SystemDocument66 pagesChemical Bonds: Modular SystemKerimberdiNo ratings yet
- WCH11 01 Pef 20200123Document7 pagesWCH11 01 Pef 20200123Karim OwnNo ratings yet
- 3400.water Science Fair Projects. Using Ice Cubes, Super Soakers, and Other Wet Stuff by Madeline P. GoodsteinDocument129 pages3400.water Science Fair Projects. Using Ice Cubes, Super Soakers, and Other Wet Stuff by Madeline P. GoodsteinElizabeth FernandezNo ratings yet
- EDU303 Practice 1Document4 pagesEDU303 Practice 1Xia AlliaNo ratings yet
- Neet Test Schedule (2022-2023) SessionDocument19 pagesNeet Test Schedule (2022-2023) SessionFxhTDhNo ratings yet
- Chapter 9 Miscelaneous Glaze CalculationDocument20 pagesChapter 9 Miscelaneous Glaze CalculationPHAKVISETH PEMNo ratings yet
- PG Physics 2010 2011Document32 pagesPG Physics 2010 2011Priyankar SinghNo ratings yet
- Recitation 7 Handout TeacherDocument2 pagesRecitation 7 Handout TeacherEric MetzgerNo ratings yet
- Chemistry 1: Modified Strategic Intervention MaterialsDocument20 pagesChemistry 1: Modified Strategic Intervention MaterialsOrlando SamonteNo ratings yet
- Some Basic Concepts Lecture - 2 13 MayDocument53 pagesSome Basic Concepts Lecture - 2 13 MayAnkit PatelNo ratings yet
- GCFY - Chapter - 6 - Covalent BondingDocument2 pagesGCFY - Chapter - 6 - Covalent BondingNaim RahmanNo ratings yet
- Ricardo Obregon Martinez - Macromolecules - Dehydration Synthesis Gizmo - 13867984Document6 pagesRicardo Obregon Martinez - Macromolecules - Dehydration Synthesis Gizmo - 13867984Ricardo ObregonNo ratings yet
- Yearly Plan Science Form 4Document49 pagesYearly Plan Science Form 4Vikneswaran Gunahlan NeshNo ratings yet
- Introduction To Physiology, Chemical Composition of The BodyDocument42 pagesIntroduction To Physiology, Chemical Composition of The Bodymrskhan jalalNo ratings yet