Professional Documents
Culture Documents
Amennorhea
Uploaded by
kutra3000Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Amennorhea
Uploaded by
kutra3000Copyright:
Available Formats
CHAPTER 10
Amenorrhea
Abha Majumdar, Neeti Tiwari
INTRODUCTION
Amenorrhea is absence of menses in a young girl till a particular age or in a
woman of reproductive age for a definite period. This is categorized into two
types; primary and secondary amenorrhea. Primary amenorrhea is diagnosed
if a girl fails to menstruate by the age of 15 years in the presence of secondary
sexual characteristics or by the age of 13 years in absence of secondary sexual
characteristics.1 Secondary amenorrhea, on the other hand, is defined as
cessation of menses in otherwise regularly menstruating women for a length of
time equivalent to her three menstrual cycles or for 6 months.2
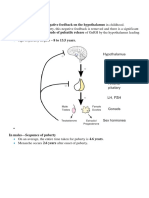
PHYSIOLOGY OF MENSTRUATION (FLOWCHART 1)
Normal menstrual flow requires a patent outflow tract between internal genital
organs and perineum, i.e. patency and continuity of uterine cavity with the
cervical canal and vaginal canal to the perineal area. To achieve menstruation
the uterine cavity needs to be lined with endometrium which must develop under
the influence of steroidal hormones secreted by the ovary. These steroidal
hormones are estrogen and progesterone; estrogen causes proliferation of the
endometrium and primes it to the effect of progesterone which in turn leads to
secretory changes within this estrogen-primed endometrium. Withdrawal of
progesterone secretion stops to support endometrial growth and brings about
shedding of this carefully designed endometrium, thus resulting in
menstruation.
The ovarian steroid production is orchestrated by the higher centers from the
brain comprising of the pituitary gland and the hypothalamus. The principal
pituitary hormones are follicle-stimulating hormone (FSH) and luteinizing
hormone (LH) from its anterior lobe, which influence the cyclical ovarian steroid
production. Hypothalamus regulates pituitary by secreting pulsatile
gonadotropin-releasing hormone (GnRH) which reaches pituitary via the portal
vessels of the pituitary stalk and thus a hypothalamic-pituitary-ovarian (HPO)
axis is established. Environmental factors like stress, excessive weight loss or
gain, and certain drugs can influence the menstrual pattern through the
hypothalamus and central nervous system. The ovarian hormones estrogen
and progesterone also provide feedback signals to anterior pituitary and
hypothalamus to control their secretions.
Flowchart 1: Hypothalamo-pituitary-ovarian axis.
(GnRH: gonadotropin-releasing hormone; FSH: follicle-stimulating hormone; LH:
luteinizing hormone)
CLASSIFICATION OF AMENORRHEA (ACCORDING TO ETIOLOGY
LIST OF CONDITIONS) (TABLE 1)
Menstruation is regulated by complex interaction of the hormonal signals and
disturbance at any level can result in amenorrhea. There are three important
areas involved in the causation of amenorrhea. Firstly, it could be the passage
to reveal menstruation, i.e. uterovaginal canal may be absent or obstructed;
secondly the endocrine organ responsible for menstruation, i.e. ovary itself, may
have failed and lastly there could be reduced or absent stimulus from anterior
pituitary or the hypothalamus to the ovary owing to dysfunction of HPO axis
resulting in amenorrhea.
Table 1: Classification of amenorrhea.
Anatomic or end organ defects
• Mullerian agenesis
• Complete androgen insensitivity syndrome
• Endometrial hypoplasia or aplasia
• Cervical agenesis
• Transverse vaginal septum
• Imperforate hymen
• Intrauterine synechiae (Asherman's syndrome)
Primary ovarian failure
• Abnormal karyotype:
– 45, XO Turner's or mosaic Turner's syndrome (gonadal agenesis)
– 46XY AIS and Swyer syndrome (gonadal dysgenesis)
• Normal karyotype:
– 46XX pure gonadal agenesis
• Metabolic disorder: Galactosemia
• Enzymatic deficiency (17α-hydroxylase, 17,20-lyase, aromatase)
• Premature ovarian failure—Injury or idiopathic or resistant ovary
• Fragile X syndrome
Pituitary causes
• Tumors—prolactinoma and pituitary adenoma
• Hyperprolactinemia—Idiopathic and drug induced
• Necrosis—Sheehan syndrome or pan hypopituitarism
• Space occupying lesions—empty sella and arterial aneurysm
• Mutation of FSH or LH receptor
• Autoimmune disease
Hypothalamic causes
• Dysfunctional—stress, exercise, and nutrition related
• Isolated gonadotropin deficiency—Kallmann's syndrome and hypogonadotropic hypogonadism
• Infections (tuberculosis, syphilis, and encephalitis)
• Inflammatory or infiltrative—sarcoidosis and hemochromatosis
• Chronic debilitating diseases
• Tumors—craniopharyngioma and hamartoma
Other endocrine gland disorders
• Adrenal—adult onset adrenal hyperplasia and Cushing syndrome
• Thyroid—hypothyroidism and hyperthyroidism
• Ovarian tumors—granulosa—theca cell tumor, Brenner tumor, cystic teratomas
• Multifactorial—polycystic ovary syndrome
(AIS: androgen insensitivity syndrome; FSH: follicle-stimulating hormone; LH: luteinizing
hormone)
In women presenting with secondary amenorrhea 66% are either due to
hypothalamic-pituitary causes or due to polycystic ovarian syndrome (PCOS)
which is multifactorial in origin with dysfunction at all the three levels of the HPO
axis. Hyperprolactinemia and ovarian failure are further responsible for 13% and
12% of cases of secondary amenorrhea, respectively. In the remaining cases
who present as secondary amenorrhea, end organ failure is responsible for 7%
of cases whereas 2% are due to hyperandrogenic states like ovarian tumor or
nonclassic congenital adrenal hyperplasia (CAH).3
In cases presenting with primary amenorrhea, gonadal agenesis and
dysgenesis leading to primary ovarian failure alone accounts for 40% of cases.
Out of the remaining cases of primary amenorrhea, 22% of girls have normal
secondary sex characters such as adequate breast development but have end
organ pathologies. In this group, mullerian agenesis is seen in 10% of cases,
androgen insensitivity syndrome (AIS) in 9%, and other obstructive pathologies
in 3%. Hypothalamo-pituitary causes are responsible for approximately 20% of
cases of primary amenorrhea. The rest 18% of girls have constitutional delay in
attaining menarche.3
EVALUATION OF AMENORRHEA
In many instances, diagnosis can be clinched on the basis of detailed history
and physical examination only. Hence, it is important to keep in mind the
common causes while evaluating the patient for the first time.
HISTORY AND EXAMINATION
Primary Amenorrhea
The history of pubertal development is important in terms of breasts, axillary
and pubic hair growth, absence of which suggests primary ovarian or pituitary
failure. If the patient is remarkably short as compared to other family members,
Turner's syndrome should be considered. It is pertinent to seek history of
significant stress or change in diet, exercise, and weight to rule out
hypothalamo-pituitary causes. History of severe headaches, fatigue, and visual
field defects also point toward hypothalamo-pituitary tumors. If secondary sex
characters are well developed along with symptoms and signs of hirsutism or
virilization one needs to consider PCOS, CAH, androgen secreting ovarian or
adrenal tumors as one of the underlying causes.
Presence of Y chromosome, absent uterus or obstruction of menstrual flow
generally presents with adequate development of secondary sexual
characteristics. It is worthwhile to take detailed drug history as many drugs like
antidepressants, antipsychotics lead to hyperprolactinemia which can also
result in primary amenorrhea. Constitutional delay is a diagnosis of exclusion
and is generally accompanied by family history of delayed puberty.
Examination of a patient of primary amenorrhea should always be done in
presence of mother or guardian especially if she is a minor. Evaluation of
pubertal changes, i.e. height, weight, and growth charting, tanner staging of
breasts and pubic hair is important in a girl with primary amenorrhea (Fig. 1).
We carefully need to look for signs of hirsutism, acne, striae, and acanthosis
nigricans. A patient of Turner's syndrome can present with typical stigmata on
examination, i.e. short height, webbed neck, low hair line, shield chest, widely
spaced nipples or on the other hand may have an absolutely normal physical
appearance. A gentle pelvic examination is required to establish patency of
outflow tract, i.e. hymenal opening, depth of vagina, presence and size of cervix
and uterus. 15% of patients with primary amenorrhea have blind or absent
vagina. Clitoromegaly may be found in patients with virilizing disorders such as
CAH.
Secondary Amenorrhea
History of significant change in diet and exercise, loss of weight, acute stress
or chronic illness suggest functional hypothalamic amenorrhea. On the other
hand severe headaches, visual disturbances, polyuria, and polydipsia can be
due to hypothalamic or pituitary tumors.
Fig. 1: Tanner stages of pubertal development in female.
Source: Reprinted with permission from Roede MJ, van Wieringen JC. Growth
diagrams 1980: Netherlands third nationwide survey. Tijdschr Soc Gezondheidsz.
1985;63:1-34.
History of severe obstetric hemorrhage followed by failure of lactation and
secondary amenorrhea implies the possibility of Sheehan's syndrome. If there
is a history of repeated curettage done after delivery or abortion or a surgical
procedure such as myomectomy or even uterine artery embolization done for
conservative management of fibroids preceding amenorrhea, Asherman's
syndrome is a strong possibility.4 Asherman's syndrome with destruction of
endometrial lining can also occur following genital Koch's or long standing
endometritis. Significant weight gain with acne and hirsutism is a feature of
PCOS whereas sudden onset virilization with deepening of voice indicates
adrenal or androgen secreting ovarian tumor. Symptoms of estrogen deficiency,
e.g. hot flashes, vaginal dryness, and decreased libido along with secondary
amenorrhea, could be present due to premature ovarian failure (POF). History
of milk discharge from breast makes it pertinent to obtain a drug intake history
as mentioned before.
While examining a patient with amenorrhea apart from making a note of
weight and body mass index one must also look for signs of hirsutism, acne,
acanthosis nigricans, and striae in these women. Any thyroid enlargement or
discharge from breasts should be noted. Pelvic examination is done to look for
presence of vaginal dryness, size of the uterus, and adnexal masses.
INVESTIGATIONS FOR PRIMARY AND SECONDARY AMENORRHEA
• Rule out pregnancy
• Pelvic ultrasound: 2D pelvic ultrasound is done to look for presence and size
of uterus, endometrial thickness, ovarian volume, antral follicle count, and to
rule out adnexal masses. Serum prolactin and thyroid profile
• Hormonal tests: Serum FSH, LH, estradiol (E2), and anti-Müllerian hormone
(AMH).
– Low serum FSH, LH, and E2 levels with normal or low AMH indicate
hypogonadotropic hypogonadism and one must look for hypothalamic and
pituitary causes in these women.
– Normal FSH and E2 with normal or high LH and AMH are generally
associated with PCOS and all three hormones in normal range are mostly
associated with obstructive amenorrhea.
– High serum levels of FSH and LH with low serum E2 imply ovarian failure.
• Serum prolactin higher than the normal limit on two occasions without any
history of drug intake which may elevate prolactin levels, may be considered
as a cause for oligomenorrhea or amenorrhea.
• Thyroid profile which includes estimation of T3, T4, and thyroid-stimulating
hormone (TSH) should be done in women with amenorrhea as 40% of
patients with hypothyroidism can have hyperprolactinemia. Low serum level of
T4 in primary hypothyroidism leads to increased secretion of thyrotropin
releasing hormone (TRH).This results in increased stimulation of both
thyrotropes and lactotropes thereby increasing both TSH and prolactin.5 This
can manifest as galactorrhea and/or as amenorrhea.
• Progestin challenge test (Flowchart 2): This test is a simple way to assess
ovarian function. If a patient gets withdrawal bleeding after progestin treatment
it implies normal circulating estrogen levels and a patent outflow tract. For
conducting this test either tablet medroxyprogesterone acetate (MPA) is given
in doses of 10 mg once a day or sustained release micronized progesterone
300 mg orally once a day for 5 days or a single dose of 200 mg injectable
progesterone in oil is given, to initiate a withdrawal bleed which occurs usually
within 7 days (range of 5–10 days) after conclusion of progestin treatment.5 If
very little bleeding or spotting occurs it generally indicates marginal levels of
circulating estrogen. Endometrial thickness on ultrasound correlates well with
estrogen levels as well as with the progestin challenge test; endometrial
thickness of 6 mm or more predicts withdrawal bleeding with 95% accuracy. It
is important to note that there is a subset of patients who may not have
withdrawal bleeding after progestin treatment despite adequate circulating
estrogen levels and endometrial thickness; this is due to decidualization of
endometrium in response to high androgen levels as sometimes seen in
PCOS women with hyperandrogenemia.6
• Magnetic resonance imaging (MRI) of head: In patients of hypogonadotropic
hypogonadism where there is suspicion of hypothalamic or pituitary masses,
MRI of head is mandatory.
• Chromosomal analysis: Karyotype helps to clinch the diagnosis in patients of
primary amenorrhea with chromosomal abnormalities; deletion of one X
chromosome is associated with Turner's syndrome, (46, XO) or presence of Y
chromosome in a female phenotype is either due to AIS also called testicular
feminization syndrome, or due to Swyer syndrome. Chromosomal analysis is
also desirable in patients of secondary amenorrhea with POF to rule out
mosaic variants of Turner's syndrome.
Flowchart 2: Progestin challenge test.
(FSH: follicle-stimulating hormone; MRI: magnetic resonance imaging; PCOS:
polycystic ovary syndrome; POF: premature ovarian failure)
EVALUATION OF SPECIFIC CONDITIONS IN AMENORRHEA
• Primary amenorrhea with blind vagina (Flowchart 3): Presence of cyclical pain
may suggest the possibility of imperforate hymen or transverse vaginal
septum which can be diagnosed by local vaginal examination as well as
confirmed by ultrasound examination by seeing collected blood in the vagina.
If a girl with blind vagina is asymptomatic and has scant pubic hair consider
the possibility of AIS, whereas if she has normal secondary sex characteristics
the diagnosis of Mullerian agenesis is to be considered also known as Mayer-
Rokitansky-Küster-Hauser (MRKH) syndrome. Both AIS and MRKH syndrome
are characterized by congenital absence of uterus and vagina. A karyotype
will help to finally differentiate between the two as it will be normal 46XX in
Mullerian agenesis while in AIS it will be 46XY.
• Secondary amenorrhea with hyperandrogenism: Women presenting with
absence of menses with clinical symptoms and signs of raised circulating
androgen levels, e.g. acne, hirsutism, male pattern baldness and in severe
cases signs of virilization which include features of clitoromegaly, can be
attributed to one of the following probable causes:
– Ovarian: PCOS and ovarian neoplasm
– Adrenal: Late onset CAH, Cushing's syndrome, and adrenal neoplasm.
To identify the underlying cause one needs to assess serum testosterone,
serum dehydroepiandrosterone (DHEA-S), and serum 17α-
hydroxyprogesterone and then interprets the results to arrive on a diagnosis
according to Table 2.
• Secondary amenorrhea with POF: The diagnosis of POF is made if a
persistently high serum FSH and with low E2 is seen in women with secondary
amenorrhea. History of surgical intervention on ovaries, e.g. cystectomy or
ovarian irradiation or chemotherapy should be looked for in such cases. POF
can be encountered also in chronic smokers, obese women, carriers of
hepatitis B, C or HIV, or those who have had chronic pelvic inflammatory
diseases, and genial tuberculosis. Family history of premature menopause
should be sought for. In absence of these, it is important to identify the
underlying pathology for which following tests are recommended:
Flowchart 3: Evaluation of blind or absent vagina.
Table 2: Evaluation of secondary amenorrhea and hyperandrogenism.
Hormone Level Indication
Testosterone ≤200 ng/dL PCOS
>200 ng/dL Evaluate for adrenal or ovarian tumor
DHEA-S ≤700 µg/dL PCOS
≤700 µg/dL Evaluate for adrenal or ovarian tumor
17α-hydroxyprogesterone >200 ng/dL Consider ACTH stimulation test to diagnose
CAH
(PCOS: polycystic ovarian syndrome; DHEAS: dehydropianandrosterone sulfate; ACTH:
adrenocorticotropic hormone; CAH: congenital adrenal hyperplasia)
– Karyotype: Turner mosaics can have normal cycles for few years before they
get amenorrheic.
– FMR 1 gene mutation: 10–20% of carriers of Fragile X syndrome have
POF.7 Screening for Fragile X syndrome should always be considered in
women with POF with family history of premature menopause.
– Screening for autoimmune disorders: Premature ovarian failure may be
associated with autoimmune disorders like Addison's disease, autoimmune
thyroid disease, and type 1 diabetes. All patients with POF should have oral
glucose tolerance test, thyroid profile, and anti-thyroid peroxidase antibodies.
If symptoms warrant, Addison's disease should be ruled out with adrenal
antibody titer (titer < 1:10 is normal) or adrenocorticotropic hormone
stimulation test.8
Galactosemia is a rare inherited autosomal recessive disorder associated
with POF. The deficiency of galactose-1-uridyl transferase leads to
accumulation of toxic metabolites which can lead to reduced initial oocyte pool,
increased follicular atresia during maturation, and perhaps reduced maturation
of primordial follicles. These girls usually have impaired growth and
development and can present as primary amenorrhea, secondary amenorrhea
or oligomenorrhea.9
Hypogonadotropic hypogonadism: Both primary and secondary amenorrhea
can be a manifestation of hypogonadotropic hypogonadism which is
diagnosed by low serum FSH, LH, and estradiol levels. It is pertinent to look
for an underlying cause in order to correct and treat or reverse the disorder
effectively. Following conditions should be looked for:
– Severe weight loss: Significant weight loss either due to severe exercise,
extreme nutritional deficiency or restriction, anorexia nervosa, bulimia or
wasting due to chronic illnesses can lead to abnormal pattern of GnRH
secretion causing disruption of the HPO axis and hence amenorrhea. This
response of higher centers in the brain is considered vital which aim to
suppress reproductive function as a protective reaction to stressful situations
in the body.10
– Hyperprolactinemia: High serum prolactin levels may lead to
hypogonadotropic hypogonadism which is one of the common causes of
secondary amenorrhea. If it affects a girl before menarche it can also present
as delayed puberty with primary amenorrhea. A woman with increasing levels
of prolactin usually progresses from luteal phase defect to anovulation,
amenorrhea and finally frank features of hypoestrogenism.11 Only one-third of
patients with hyperprolactinemia will present with galactorrhea. If serum
prolactin levels are more than 100 ng/mL, an MRI brain is recommended to
rule out prolactinoma or other adenomas of the pituitary gland.
– Sheehan's syndrome: History of severe hemorrhage associated with
cardiovascular collapse following delivery, with failure of lactation to occur and
secondary amenorrhea, suggests postpartum pituitary gland necrosis.
Immense hypertrophy and hyperplasia of lactotrophs during pregnancy leads
to enlargement of anterior pituitary without corresponding increase in blood
supply, hence hypovolemic shock due to acute blood loss quite often may
cause ischemic necrosis of the gland and deficient secretion of gonadotropins
eventually.12
– Kallmann's syndrome: This condition is characterized by congenital deficiency
in the pulsatile release of GnRH which is typically associated with a loss of
sense of smell also known as anosmia. Inheritance can be sex-linked or
autosomal dominant or recessive therefore obtaining a family history is
important.13
– Idiopathic hypogonadotropic hypogonadism (IHH): IHH is characterized by
decreased secretion of gonadotropins and thus of sex steroids with intact
uterus resulting in amenorrhea with poorly developed secondary sex
characters. The pathogenesis is failure of GnRH neurons to develop or
differentiate from hypothalamus for which many genetic mutations have been
studied. It is different from Kallmann's syndrome by the fact that patients with
IHH have intact sense of smell.14
MANAGEMENT OF AMENORRHEA
Amenorrhea if left untreated may lead to many complications. On one side it
could be associated with poorly developed secondary sex characteristics along
with other hypoestrogenic symptoms such as hot flashes, vaginal dryness, and
high risk for osteoporosis owing to deficient mineralization of bones, stroke, and
heart disease and on the other side it may also be associated with unopposed
estrogen exposure leading to endometrial hyperplasia and sometimes also to
endometrial cancers. Thus, the objectives of management of amenorrhea are
as follows:
• To treat the underlying disease
• To achieve resumption of menstruation and fertility, when desired
• To prevent complications of amenorrhea.
MANAGEMENT OF END ORGAN DISORDERS
• Imperforate hymen: This condition requires a simple surgical procedure of
cruciate incision and drainage.
• Transverse vaginal septum: A pelvic ultrasound or pelvic MRI should be done
to rule out hematometra and hematosalpinges. Excision of septum and
primary anastomosis of upper and lower vaginal canals over the defect is
done. If defect is large, application of graft may be required.
• Cervical atresia: This is a rare condition which is difficult to treat as it is difficult
to make a sustainable passage between the vagina and uterus and
hysterectomy is often required. Young girls with cervical atresia and severe
dysmenorrhea can be treated with hormones to stop mensturation till surgery
is undertaken.
• Mullerian agenesis: Development of normal secondary sexual characters
along with history and local examination showing an absent or small but blind
vagina clinches the diagnosis. However, final confirmation can be done by a
pelvic ultrasound and the presence of normal 46XX karyotype. An ultrasound
should be done to rule out associated renal anomalies. Primary goal of
treatment is to create a functional vagina which can be done by either of
following procedures:
– Frank's vaginal dilators are used to gradually deepen the vaginal dimple.
– McIndoe procedure: In this procedure neovagina is created by dissection in
the rectovesical space followed by either skin graft from the inner side of the
thigh or amniotic membrane used as graft.
– Vecchietti operation: This procedure is originated in Italy and it involves
surgical traction to dilate the rudimentary vaginal canal. It was earlier
described as an open procedure but then laparoscopic Vecchietti became
more popular due to surgeon's ease and patient's comfort. An ellipsoid bead
or olive is placed on vaginal dimple outside with inward traction applied
through two sutures one at each end. These sutures are taken inside through
the peritoneal cavity and then taken out extraperitoneally to be attached to a
traction device placed on the abdominal wall. Postoperatively the traction is
increased every day in such a way that vaginal cavity is created at the rate of
1 cm/day, hence by the end of 7 days a 7 cm vagina is created.15 In these
patients fertility is possible only with the help of surrogacy.
• Androgen insensitivity syndrome: Women with AIS are phenotypically tall and
usually pretty but their chromosomal analysis reveals a 46XY or male
karyotype. These women have presence of testis within their abdomen which
produces both testosterone and AMH which on one hand leads to either
increased or normal serum testosterone or dihydrotestosterone (DHT) levels
and on the other hand secretion of AMH prevents the development of the
uterus and upper vagina. Imaging studies, such as pelvic ultrasound, confirm
the absence of fallopian tubes and uterus due to the AMH secreted by these
testes. The testes usually remain in the abdomen, or occasionally move into
the inguinal canals. The testes make sufficient amounts of testosterone but no
androgenic sexual differentiation occurs and internal male genital ducts fail to
form because of lack of testosterone receptors. Puberty tends to begin slightly
later as some of the testosterone is converted to the estradiol, inducing normal
breast development. Little or no pubic hair or other androgenic hair appears
and acne is rare but what is the most conspicuous feature of AIS is absence
of vagina. The vagina varies from a dimple at perineum to almost normal
length but is always blind ending.
– Genetically in these cases of AIS the androgen receptor is rendered
insensitive to the action of androgens secreted from the testes because of the
presence of mutation of androgen receptor gene, which is situated on the X
arm of the male sex chromosome. There could be complete or partial gene
deletions, point mutations, or small insertions or deletions of the affected area
on the X chromosome. DNA tests can be done for mutation analysis for
androgen receptor gene on X chromosome.16
– Developmental arrest of fetal germ cells in AIS can lead to development of
carcinoma in situ in the gonocytes which can further progress into malignant
germ cell tumors, i.e. gonadoblastoma, seminoma, teratoma, and embryonal
tumors. Benign tumors such as hamartomas, Sertoli cell adenomas, and
rarely Leydig cell tumors have also been reported in association with AIS.
Gonadectomy is thus essential after these girls achieve puberty due to more
than 30% risk of tumor in these dysgenetic gonads in adulthood.17 This allows
spontaneous pubertal development under effect of estrogen produced from
aromatization of the high levels of testosterone in blood. However, there is a
tendency to do gonadectomy as soon as the diagnosis is confirmed in
childhood and these girls then need to take estrogen replacement for pubertal
development.
– In addition, many women with AIS require vaginal lengthening procedures.
However, intersex advocates feel one must delay this reconstructive surgery
until children are old enough to decide for themselves, and only if they choose
to have it one of the techniques described above can be used.
These patients require both donor oocyte and surrogacy when fertility is
desired.
• Asherman's syndrome: Intrauterine adhesions can occur due to endometrial
infections such as genital tuberculosis or due to iatrogenic causes like
repeated curettage especially of a pregnant uterus or following operative
hysteroscopic procedure such as intrauterine polypectomy and myomectomy.
This condition was first described in 1894 by Heinrich Fritsch18 and further
characterized by Israeli gynecologist Joseph Asherman in 1948.19 It is also
known as Fritsch syndrome, or Fritsch-Asherman syndrome. This syndrome is
diagnosed when the cause of amenorrhea or hypomenorrhea is adhesions or
synechiae within the endometrial cavity. These synechiae can be picked up
quite often at 3D ultrasound and further confirmed by hysterosalpingography
or saline sonohysterography. Hysteroscopic division of intrauterine synechiae
considered the standard procedure for this disorder. Generally synechiolysis is
followed by high dose of exogenous estrogen given to the woman for 2–3
weeks followed by progesterone withdrawal. A study done on menstrual
outcomes after surgical intervention in Asherman's syndrome over a period of
10 years in Netherlands showed resumption of normal menses in 95% of
patients though recurrence was reported in 27% of them.20 Follow-up
assessment of uterine cavity is recommended preferably with hysteroscopy
after treatment of intrauterine adhesions.21
MANAGEMENT OF PCOS WITH AMENORRHEA
The role of lifestyle modification should be emphasized in detail to PCOS
women specially those who are overweight and obese. PCOS women with
weight loss of even 5% of initial weight have reported spontaneous resumption
of menses. The lifestyle changes include aerobic exercise for 150 minutes per
week and hypocaloric diet which could be to start with a 500 calorie daily deficit
in diet.22 Periodic treatment with progestin is given to these women to induce
regular menses and protect them from endometrial hyperplasia. The most
common used regime is tablet MPA 10 mg twice daily for 5–7 days every month
or alternate months. In women desiring contraception or those suffering from
acne and hirsutism combined oral contraceptive pills are recommended. Pills
containing cyproterone acetate as progestogen are preferred as they not only
ensure regular cycles but reduce circulating androgen levels too. When fertility
is desired ovulation induction is the way to it, which can be done by ovulation
inducing agents such as clomiphene citrate, letrozole or tamoxifen and if they
fail to induce ovulation then second-line drugs in form of injectable
gonadotropins are recommended to induce ovulation and attain fertility.
MANAGEMENT OF GONADAL AGENESIS AND DYSGENESIS
Turner's syndrome (46, XO): It is one of the common causes of primary
amenorrhea and mosaics can present with POF. It is imperative to look for
associated medical conditions which are additional features of Turner's
syndrome, hence a echocardiography, audiometry, thyroid profile, blood sugar
profile, liver and renal function tests, and ultrasonography should be done.
These girls need estrogen replacement therapy which must be started as soon
as the diagnosis of primary amenorrhea is confirmed, preferably before 15
years of age so that they can attain adult height. Estrogen is started at low dose
(0.25–0.5 mg micronized estradiol) and increased every 6 months till sexual
maturation is achieved. After 1–2 years of continuous estrogen, progestin is
added to induce withdrawal bleeding. Fertility is possible with donor oocytes
only.
Swyer syndrome and AIS (46XY): AIS has been discussed in end organ
disorders. In Swyer syndrome, SRY region on Y chromosome is missing so
testes fail to develop and there are only streak gonads present. In absence of
testicular development there is neither testosterone nor AMH secreted which
leads to failure of development of male secondary sexual characteristics, hence
the female phenotype. In addition in absence of AMH, the mullerian system
develops in these girls resulting in functional uterus and vagina which responds
to hormones. Menstruation follows sequential hormone replacement therapy
(HRT) with estrogen and progesterone in these girls as well as the development
of endometrium, to support a pregnancy through a donated oocyte or embryo
by in vitro fertilization (IVF). In Swyer syndrome unlike AIS gonadectomy is
recommended as soon as the diagnosis is made to avoid the high risk of
malignant transformation in these nonfunctional gonads as hormonal
replacement for development of secondary sex characters is mandatory.
MANAGEMENT OF PREMATURE OVARIAN FAILURE
These women require HRT both estrogen and progestin either in continuous or
cyclic manner. They should also take calcium and vitamin D3 supplementation
and exercise regularly to protect bones. In case fertility is desired, IVF with
donor oocytes is the only option to have children.
MANAGEMENT OF HYPOGONADOTROPIC HYPOGONADISM
In case of anorexia nervosa or severe malnutrition, behavioral and nutritional
therapy alone can bring back the menstrual pattern once an optimal weight gain
is achieved. If the condition is due to stressful situations they have to be treated
by a psychiatrist to reverse the condition. Women with hyperprolactinemia are
treated with dopamine agonists (cabergoline or bromocriptine) and ovulation
and menses are resumed after 2–3 months of therapy. Women with
hyperprolactinemia who are experiencing hypoestrogenic symptoms can be
prescribed HRT also. Women with hypogonadotropic hypogonadism need to
undergo ovulation induction with gonadotropins which contain both FSH and LH
or HMG (human menopausal gonadotropin) when desirous of conception.
Other wise should be offered HRT to prevent adverse sequel of absence of
ovarian steroid hormones in body.
KEY NOTES
Amenorrhea can occur as a natural part of life, such as during pregnancy or
breastfeeding or it can be a sign of a health problem, hence it is pertinent to
know the underlying reasons to decide the appropriate treatment. However, in
some cases leading to amenorrhea it may not be possible to induce
menstruation, as for those who do not have a uterus, as in MRKH syndrome or
AIS or for girls with cervical atresia where hysterectomy needs to be undertaken
to relieve her from cyclical pain. We also need to identify the subset of women
who may not desire treatment for resumption of menses as far as they have no
health consequences such as those with end organ failure or Asherman's
syndrome. For all causes of amenorrhea which root from hypothalamus or
pituitary, lifelong HRT is advisable till conception is desired. Amenorrhea
stemming from ovary has most diverse management strategies; dysgenic
gonads as those with XY karyotype need to be removed and HRT instituted,
ovarian agenesis or failure requires HRT lifelong during the reproductive years
with oocyte donation and IVF for reproduction. Amenorrhea associated with
androgen excess conditions, such as PCOS, needs to be managed as per
presenting features and health issues. Ovulation induction in this group of
women is the best method to attain fertility. The key to management of women
with amenorrhea is to make a correct diagnosis especially in young girls where
primary amenorrhea is a very emotionally draining concern, not only for the girl
but also for the family and counseling is an integral part of management of all
such cases.
You might also like
- Primary and Secondary AmenorrhoeaDocument72 pagesPrimary and Secondary Amenorrhoead clarkeNo ratings yet
- Chari Boru's - DR - Haasan Huseen - Microeconomics I PPT (PHD) ProgramDocument399 pagesChari Boru's - DR - Haasan Huseen - Microeconomics I PPT (PHD) ProgramCHARI BORUNo ratings yet
- Orisa OrunmilaDocument1 pageOrisa Orunmilaedutuca80No ratings yet
- Pega Intelligent BPM The Next Wave For Customer Centric Business Applicationskhoshafian11 140809041228 Phpapp02 PDFDocument215 pagesPega Intelligent BPM The Next Wave For Customer Centric Business Applicationskhoshafian11 140809041228 Phpapp02 PDFFred Marcus100% (1)
- AmenorrheaDocument11 pagesAmenorrheaDr.med SurduckiNo ratings yet
- Menstrual Disorders in Adolescents: The Internet Journal of Gynecology and ObstetricsDocument34 pagesMenstrual Disorders in Adolescents: The Internet Journal of Gynecology and ObstetricsNayla Adira04No ratings yet
- Assesment of Amen or RheaDocument49 pagesAssesment of Amen or Rheakhadzx100% (2)
- AmenorrheaDocument40 pagesAmenorrheaMeraol HusseinNo ratings yet
- Amenorrhea: Evaluation & Treatment: Jurnal Reading Mohamad Athaullah 11.2012.062Document28 pagesAmenorrhea: Evaluation & Treatment: Jurnal Reading Mohamad Athaullah 11.2012.062Athaullah IsmailNo ratings yet
- AmenorrheaDocument13 pagesAmenorrheaJanesel Plariza PanerioNo ratings yet
- Evaluation and Management of Primary AmenorrheaDocument11 pagesEvaluation and Management of Primary AmenorrheaLisa LestariNo ratings yet
- Amenorrhea & Heavy Menstrual BleedingDocument22 pagesAmenorrhea & Heavy Menstrual BleedingJanesel Plariza PanerioNo ratings yet
- Amenorrhea SGDDocument44 pagesAmenorrhea SGDMichelle Lariosa ChangNo ratings yet
- Infertility 001Document257 pagesInfertility 001Habtamu Nigussie100% (1)
- Evaluation and Management of Primary Amenorrhea - UpToDateDocument11 pagesEvaluation and Management of Primary Amenorrhea - UpToDateNatasya SugiantoNo ratings yet
- 14 - AmenorrhoeaDocument26 pages14 - AmenorrhoeaHimanshu MehtaNo ratings yet
- Amenorrhoea Primary & SecondaryDocument24 pagesAmenorrhoea Primary & SecondaryAnushaAgrawalNo ratings yet
- Evaluation and Management of Primary Amenorrhea - UpToDateDocument14 pagesEvaluation and Management of Primary Amenorrhea - UpToDateCristinaCaprosNo ratings yet
- Secondaryamenorrhoealectures 230316163724 360b79b3Document42 pagesSecondaryamenorrhoealectures 230316163724 360b79b3droc ahmedNo ratings yet
- Rimary Menorrhea: By: Zaineb Talib Nimaa Zahra Ali Hasoon Zina Safaa AldienDocument28 pagesRimary Menorrhea: By: Zaineb Talib Nimaa Zahra Ali Hasoon Zina Safaa AldienZaineb TalibNo ratings yet
- GROUP 3 LC AminoreDocument11 pagesGROUP 3 LC AminoreAAANo ratings yet
- AmenorrhoeaDocument38 pagesAmenorrhoeaheydydNo ratings yet
- OB 2nd SGDDocument4 pagesOB 2nd SGDJxyp MundoNo ratings yet
- Anemia in Pregnancy ACOGDocument126 pagesAnemia in Pregnancy ACOGrohitNo ratings yet
- Notes Infertility Delayed PubertyDocument9 pagesNotes Infertility Delayed PubertyTONY GO AWAYNo ratings yet
- Secamenhyper 120208102817 Phpapp02Document31 pagesSecamenhyper 120208102817 Phpapp02Mohan DassNo ratings yet
- AMENORRHOEADocument35 pagesAMENORRHOEAnyangaraNo ratings yet
- 無月經症 Amenorrhea: Reporter: FM R1 余明謙 Supervisor: VS 張德宇Document38 pages無月經症 Amenorrhea: Reporter: FM R1 余明謙 Supervisor: VS 張德宇余明謙No ratings yet
- Amenorrhea and SpaniomenorrheasDocument59 pagesAmenorrhea and SpaniomenorrheasIdiAmadouNo ratings yet
- MEETING 6 AmenorrheaDocument41 pagesMEETING 6 AmenorrheaNader KhouryNo ratings yet
- 3current Evaluation of Amenorrhea - A Committee OpinionDocument9 pages3current Evaluation of Amenorrhea - A Committee Opinionjorge nnNo ratings yet
- 1625064070jmpas May-June 2021Document5 pages1625064070jmpas May-June 2021Ikhlasul Achyar AjamiNo ratings yet
- Sexuality and IntersexualityDocument23 pagesSexuality and IntersexualityManu BharadwazNo ratings yet
- Investigating Primary Amenorrhea CaseDocument23 pagesInvestigating Primary Amenorrhea CaseMohammed AbdulNo ratings yet
- Amenorrhea, Hirsutism, VirilismDocument12 pagesAmenorrhea, Hirsutism, VirilismAly MorganNo ratings yet
- Physiology of Female ReproductionDocument3 pagesPhysiology of Female ReproductionAntoine JeriNo ratings yet
- Polycystic Ovary Syndrome: Dr. Rizwana ShaheenDocument34 pagesPolycystic Ovary Syndrome: Dr. Rizwana ShaheenAmaan Hakim100% (1)
- AmenorrheaDocument51 pagesAmenorrheabisharatNo ratings yet
- Amenorrea Evaluacion ActualDocument8 pagesAmenorrea Evaluacion ActualJosé María LauricellaNo ratings yet
- Secondary Amenorrhea: DR Hanaa AlaniDocument44 pagesSecondary Amenorrhea: DR Hanaa AlaniAakashNo ratings yet
- What Is AmenorrheaDocument5 pagesWhat Is AmenorrheaYhoyho Akhilun Dewa MimpiNo ratings yet
- Primary Amenorrhea: Rabika Almina RabiaDocument30 pagesPrimary Amenorrhea: Rabika Almina RabiaAlmina RehmanNo ratings yet
- Amenorrhea: UNC School of Medicine Obstetrics and Gynecology Clerkship Case Based Seminar SeriesDocument20 pagesAmenorrhea: UNC School of Medicine Obstetrics and Gynecology Clerkship Case Based Seminar Seriesbinadi vegaNo ratings yet
- AmenorrheaDocument41 pagesAmenorrheadoraNo ratings yet
- Primary Amenorrhea Is Failure of Menses To Occur by One of The FollowingDocument13 pagesPrimary Amenorrhea Is Failure of Menses To Occur by One of The FollowingMuhammad Ilyas AhmadNo ratings yet
- Rps138 Slide AmenorrheaDocument41 pagesRps138 Slide Amenorrheasyahidah nadiahNo ratings yet
- AUB StartedDocument13 pagesAUB StartedIbrahim AbdullahNo ratings yet
- Pcos - Clinical Case DiscussionDocument4 pagesPcos - Clinical Case Discussionreham macadatoNo ratings yet
- Female Infertility: Harsha Joseph M17LS25 Msc. Life ScienceDocument10 pagesFemale Infertility: Harsha Joseph M17LS25 Msc. Life ScienceHarsha JosephNo ratings yet
- Causes and Evaluation of AmenorrheaDocument44 pagesCauses and Evaluation of AmenorrheaasdfNo ratings yet
- Amenorrhea PDFDocument16 pagesAmenorrhea PDFOkky Winang SaktyawanNo ratings yet
- Gynecological Disorders in The Adolescent Gynecological Disorders in The AdolescentDocument31 pagesGynecological Disorders in The Adolescent Gynecological Disorders in The AdolescentarrypotterNo ratings yet
- 無月經症 Amenorrhea: Reporter: FM R1 余明謙 Supervisor: VS 張德宇Document37 pages無月經症 Amenorrhea: Reporter: FM R1 余明謙 Supervisor: VS 張德宇余明謙No ratings yet
- OB - GYN (5) - Alaa SalmanppDocument6 pagesOB - GYN (5) - Alaa Salmanppmotasem.med120No ratings yet
- InfertilityDocument10 pagesInfertilityHarish Labana100% (1)
- AMENORRHOEADocument16 pagesAMENORRHOEAĶHwola ƏľsHokryNo ratings yet
- Understand AmenorrheaDocument70 pagesUnderstand AmenorrheagimNo ratings yet
- AMENORRHEA CAUSES AND MANAGEMENTDocument41 pagesAMENORRHEA CAUSES AND MANAGEMENTsridhaniNo ratings yet
- Path o PhysiologyDocument36 pagesPath o Physiologywriters expertNo ratings yet
- Seminar: Guide: Co-Guide: Pramoted byDocument35 pagesSeminar: Guide: Co-Guide: Pramoted byDhara MeenaNo ratings yet
- Complementary and Alternative Medical Lab Testing Part 9: GynecologyFrom EverandComplementary and Alternative Medical Lab Testing Part 9: GynecologyNo ratings yet
- Vegetables 50 Vegetables Name in MarathiDocument3 pagesVegetables 50 Vegetables Name in Marathikutra3000No ratings yet
- Human HeartDocument4 pagesHuman Heartkutra3000No ratings yet
- 11Document1 page11kutra3000No ratings yet
- OSCE ScoreDocument1 pageOSCE Scorekutra3000No ratings yet
- NSE Margin Details ListDocument5 pagesNSE Margin Details Listkutra3000No ratings yet
- Refferal Audit Sep 2022Document2 pagesRefferal Audit Sep 2022kutra3000No ratings yet
- Labour Room InstrumentsDocument3 pagesLabour Room Instrumentskutra3000No ratings yet
- Month Wise Consolidated Report For CDocument1 pageMonth Wise Consolidated Report For Ckutra300050% (2)
- Meconium PpromDocument7 pagesMeconium Ppromkutra3000No ratings yet
- Human Milk Bank Update PDFDocument3 pagesHuman Milk Bank Update PDFkutra3000No ratings yet
- Referral Audit Sep 2022Document1 pageReferral Audit Sep 2022kutra3000No ratings yet
- August Refferal AuditDocument1 pageAugust Refferal Auditkutra3000No ratings yet
- 27Document55 pages27kutra3000No ratings yet
- New Labour MX Guidelines AcogDocument8 pagesNew Labour MX Guidelines Acogkutra3000No ratings yet
- KFOG Journal July 09Document16 pagesKFOG Journal July 09kutra3000No ratings yet
- KFOG Feb-2012-WebDocument16 pagesKFOG Feb-2012-Webkutra3000No ratings yet
- Pressure Pointer ManualDocument17 pagesPressure Pointer ManuallucymayNo ratings yet
- Hua Acog 2013Document6 pagesHua Acog 2013Francisco Chávez SolsolNo ratings yet
- The Relaxing Protein System of Striated Muscle: Mg2&-StimulatedDocument12 pagesThe Relaxing Protein System of Striated Muscle: Mg2&-Stimulatedkutra3000No ratings yet
- Web KFOG-jan-11Document16 pagesWeb KFOG-jan-11kutra3000No ratings yet
- Biophysical Profile - Wi..Document4 pagesBiophysical Profile - Wi..kutra3000No ratings yet
- WHO HIV Clinical Staging GuidelinesDocument49 pagesWHO HIV Clinical Staging Guidelinesdonovandube8235No ratings yet
- Final Rule - ABCDX CategoryDocument2 pagesFinal Rule - ABCDX Categorykutra3000No ratings yet
- Committee Opinion: Cesarean Delivery On Maternal RequestDocument4 pagesCommittee Opinion: Cesarean Delivery On Maternal Requestkutra3000No ratings yet
- Abnormal Uterine Bleeding: Palm CoeinDocument27 pagesAbnormal Uterine Bleeding: Palm CoeinPongpak SrisomsongNo ratings yet
- Adult Schedule Easy ReadDocument2 pagesAdult Schedule Easy Readtummalapalli venkateswara raoNo ratings yet
- Puerperial PyrexiaDocument4 pagesPuerperial Pyrexiakutra3000No ratings yet
- Prev Lscs RcogDocument17 pagesPrev Lscs Rcogkutra3000No ratings yet
- Robsons Criteria For Cesarean SectionDocument4 pagesRobsons Criteria For Cesarean Sectionkutra3000No ratings yet
- St. Mary'S University School of Graduate Studies: OCTOBER 2013, Addis Ababa, EthiopiaDocument88 pagesSt. Mary'S University School of Graduate Studies: OCTOBER 2013, Addis Ababa, EthiopiaRachel HaileNo ratings yet
- Sundqvist 2011 Review McKayDocument7 pagesSundqvist 2011 Review McKaySuriia SeyfullahNo ratings yet
- HRM Issues and Challenges in Cooperative Banks in IndiaDocument6 pagesHRM Issues and Challenges in Cooperative Banks in Indiaghanshamdas67% (3)
- Clipping (Advanced Grammar)Document3 pagesClipping (Advanced Grammar)Adriana MarcosNo ratings yet
- History of Anglo Saxon Literature English Assignment NUML National University of Modern LanguagesDocument15 pagesHistory of Anglo Saxon Literature English Assignment NUML National University of Modern LanguagesMaanNo ratings yet
- Backup and Restore Utility For RSView 4.0 or FactoryTalk View 5 PDFDocument11 pagesBackup and Restore Utility For RSView 4.0 or FactoryTalk View 5 PDFhipercortexNo ratings yet
- Political Science Paper Analyzes Society and Polity in IndiaDocument231 pagesPolitical Science Paper Analyzes Society and Polity in IndiaAhmed0% (1)
- Mise en place ingredients and equipment listDocument10 pagesMise en place ingredients and equipment listDavis Sagini ArtNo ratings yet
- Arihant Sample PaperDocument8 pagesArihant Sample PaperKhali GangueNo ratings yet
- Sorting Lesson PlanDocument4 pagesSorting Lesson PlanStasha DuttNo ratings yet
- Travels of RizalDocument13 pagesTravels of Rizaljjofgg100% (1)
- Maternal and Child PreboardDocument17 pagesMaternal and Child PreboardMichelle GambolNo ratings yet
- Pc128us 2 Sebm018419 PDFDocument1,029 pagesPc128us 2 Sebm018419 PDFLuis Carlos Ramos100% (1)
- Deglobalization of GlobalizationDocument20 pagesDeglobalization of GlobalizationAbdullah FarhadNo ratings yet
- Paul's View of Justification and Last JudgmentDocument13 pagesPaul's View of Justification and Last JudgmentMik3reyesNo ratings yet
- The Path To Dignity and Respect enDocument73 pagesThe Path To Dignity and Respect enStan BuddenNo ratings yet
- Edu 2012 Spring Fsa BooksDocument6 pagesEdu 2012 Spring Fsa BooksSivi Almanaf Ali ShahabNo ratings yet
- Knockspell Issue3Document68 pagesKnockspell Issue3Donald Marks100% (3)
- Aisha Isyaku Term PaperDocument27 pagesAisha Isyaku Term PaperUsman Ahmad TijjaniNo ratings yet
- SCA No. 8220-R - Petitioner's MemorandumDocument9 pagesSCA No. 8220-R - Petitioner's MemorandumElizerNo ratings yet
- L1 - Methods of Tissue Examination & Preparation and Tissue SamplingDocument47 pagesL1 - Methods of Tissue Examination & Preparation and Tissue SamplingsademNo ratings yet
- Department of History, III BA A' Paper-VI Ancient Karnataka History KNMNC, Shivamogga. Historical SourcesDocument7 pagesDepartment of History, III BA A' Paper-VI Ancient Karnataka History KNMNC, Shivamogga. Historical SourcesBharat KumarNo ratings yet
- Camera Obscura, Camera LucidaDocument297 pagesCamera Obscura, Camera LucidaFelix Garcia100% (3)
- ENGLSIH LISTENING SKILLS (Question Paper) PDFDocument2 pagesENGLSIH LISTENING SKILLS (Question Paper) PDFGagan H RNo ratings yet
- Exalt HimDocument2 pagesExalt HimSaxon Inga100% (1)
- Kurdonia Structural Design ReportDocument341 pagesKurdonia Structural Design ReportAnonymous nQ9RqmNo ratings yet