Professional Documents
Culture Documents
Laboratory For Medical Tests: Test Report
Uploaded by
Thornblad33Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Laboratory For Medical Tests: Test Report
Uploaded by
Thornblad33Copyright:
Available Formats
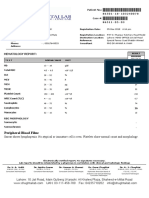
LABORATORY FOR MEDICAL TESTS
PSIHOMED BACAU SRL
Str. Buciumului 25 Bacau
Telefon: 0723121782
Email: psihomedbacau@yahoo.com
TEST REPORT
No. 76703 date 2021-12-22
Copy 0
Name: MARES SORIN-VIOREL PNC: 1880505132887 Chart No. -
Address: Age: 33 years 7 months
Sent by: Psihomed Constanta Physic
Sampling date and time: 2021-12-22 10:09 Validation date and 2021-12-23 00:19
External Internal sampling LUTCHI ALEXANDRA IZABELA
Nature and condition of Compliant Remarks: 05.05.1988
Phone 0728175152 Email Passport 055760129
MOLECULAR BIOLOGY
Parameter Sample Value Unit Reference
range
Depistare SARS-Cov-2(RT PCR) - DLAB Accurate 96 PCR
*SARS-Cov-2 detection(RT PCR) Nasophary Negative - Negative
ngeal
swab
*Gene N Nasophary Not detected - Not detected
ngeal
swab
*Gena E Nasophary Not detected - Not detected
ngeal
swab
*Gena ORF 1ab Nasophary Not detected - Not detected
ngeal
swab
Validated by biologist TUDORACHE ADRIAN CONSTANTIN
END OF TEST REPORT NO. 76703
Validated,
Laboratory manager:
COSTACHE RĂZVAN
Notes:
1. Results are only for analyzed samples.
2. SC Psihomed SRL is authorized for molecular biology tests by authorization 1458/18.11.2020.
3. Tests marked with ** are analyzed at SANTE laboratory
4. Opinions and interpretations are not RENAR certified
5. Partial reproduction of this document is permited only with the approval of the laboratory
6. Interpretation of the results is made in concordance with the methodology of Public Health for the surveillance of Sars Cov 2
Antigen Sars Cov 2 tests have the following tehnical specifications: Sensitivity: 92.9% ( 84%-97%), Specificity: 99,9%(94%-100%),
Accuracy: 96,2%(91,3-98,7), Producer: HANGZHOU ALLTEST BIOTECH CO., LTD.
PCR tests for Sars Cov 2 have the following tehnical specifications:
- Product performance:
1. Specificity: The primers and probes provided in this kit are designed based on the conserved sequence of the novel coronavirus
Name: MARES SORIN-VIOREL, PNC: 1880505132887, No. 76703/ 2021-12-22 Page 1 of 2
(SARS-CoV-2), and has a high detection rate of the target gene fragment. This kit has no cross-reactions among positive samples of
Coronavirus(NL63, HKU1, 229E, OC43), Influenza A virus, Influenza B virus, Respiratory syncytial virus, Adenovirus, Parainfluenza virus,
Klebsiella pneumoniae, Chlamydia pneumoniae. The negative and positive rates of detecting commercial reference materials were 100%.
2. Minimum detection limit: 200 copies/mL
- Positive treshhold: According to the study of the reference value, the Ct reference value for the target gene detected by this kit is 40,
and the Ct reference value of internal control is 40.
- device name is Accurate 96, produced by DLAB
Name: MARES SORIN-VIOREL, PNC: 1880505132887, No. 76703/ 2021-12-22 Page 2 of 2
You might also like
- Haematology: Investigation Observed Value Unit Biological Reference IntervalDocument10 pagesHaematology: Investigation Observed Value Unit Biological Reference IntervalVanshitaNo ratings yet
- MethamphetamineDocument17 pagesMethamphetamineSulis Tio100% (1)
- Prerna Doparkar Female52 26629Document13 pagesPrerna Doparkar Female52 26629drsrikanthreddyNo ratings yet
- Hba1c (Glycosylated Hemoglobin)Document1 pageHba1c (Glycosylated Hemoglobin)NaeemNo ratings yet
- PoojaDocument9 pagesPoojaRupinder KaurNo ratings yet
- Ms SEEMABALAYAN 9 24 2022 7 04 10 PMDocument22 pagesMs SEEMABALAYAN 9 24 2022 7 04 10 PMMohammad Ali NPNo ratings yet
- 57f031c3-14a8-4cd3-88b0-f96c1afbe475Document3 pages57f031c3-14a8-4cd3-88b0-f96c1afbe475Spam GmailNo ratings yet
- Dengue CertficateDocument1 pageDengue Certficateyashwanth saiNo ratings yet
- MAX Health CheckupDocument10 pagesMAX Health Checkuppriyanka.singh1996.pssNo ratings yet
- 43ttkxj2joqnljbdueumkilwDocument4 pages43ttkxj2joqnljbdueumkilwDivyansh SinghNo ratings yet
- Department of Hematology Preliminary Health Checkup Test Name Result Unit Bio. Ref. IntervalDocument7 pagesDepartment of Hematology Preliminary Health Checkup Test Name Result Unit Bio. Ref. IntervalVarun YadavNo ratings yet
- A03 - FPSC Muir Road FIRST FLOOR, SHOP NO.-G/3,89/276, MUIR ROAD, ALLAHABAD - 211002, U.P AllahabadDocument11 pagesA03 - FPSC Muir Road FIRST FLOOR, SHOP NO.-G/3,89/276, MUIR ROAD, ALLAHABAD - 211002, U.P AllahabadAnkit RameeraNo ratings yet
- Apollo247 249052700 LabreportDocument7 pagesApollo247 249052700 LabreportASHWAQ JAANNo ratings yet
- Diagnostic Report: FinalDocument3 pagesDiagnostic Report: FinalDr Amit SinghNo ratings yet
- TestReport 2100101650Document1 pageTestReport 2100101650Kashi RajpootNo ratings yet
- Investigation Observed Value Unit Biological Reference Interval CRP - C Reactive Protein 14.25Document2 pagesInvestigation Observed Value Unit Biological Reference Interval CRP - C Reactive Protein 14.25Sandip VaghelaNo ratings yet
- Cellsummativereview AnswersDocument5 pagesCellsummativereview Answersapi-422802678No ratings yet
- Max Lab ReportDocument8 pagesMax Lab ReportKallu PrasadNo ratings yet
- MadhavDocument1 pageMadhavmadhav maheshwariNo ratings yet
- Age Related Cerebral Cortical Atrophy Is SeenDocument5 pagesAge Related Cerebral Cortical Atrophy Is SeenTamanash ChowdharyNo ratings yet
- Nohailic Dorin 500213dd8146202Document2 pagesNohailic Dorin 500213dd8146202Dorin NohailîcNo ratings yet
- Department of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodDocument2 pagesDepartment of Molecular Biology. The Automotive Reasearch India - Covid 19 RT PCR - Pune - Fy2122 Test Name Result Unit Bio. Ref. Range MethodanilsgaikwadNo ratings yet
- ReportDocument10 pagesReportAnkita GoyalNo ratings yet
- Lectures On EnzymesDocument123 pagesLectures On EnzymesProf Rakesh Sharma100% (2)
- ALT UrineDocument1 pageALT UrineUmar BhattiNo ratings yet
- Test ReportsDocument90 pagesTest ReportsRohitNo ratings yet
- Sample Type: Visit Id: R8539558Document1 pageSample Type: Visit Id: R8539558Ravi KumarNo ratings yet
- Report ViewerDocument1 pageReport ViewerZeeshan JunejoNo ratings yet
- Booklet Blood Culture 2018Document19 pagesBooklet Blood Culture 2018Karmass JallalNo ratings yet
- AHA1.0000286067 11981600 10108 19-Sep-2022 254 AYNOPP938597 AYN-OCS-761431 91-7001734967 SR 20220919101231.pdfpdf PDFDocument11 pagesAHA1.0000286067 11981600 10108 19-Sep-2022 254 AYNOPP938597 AYN-OCS-761431 91-7001734967 SR 20220919101231.pdfpdf PDFKoushik ChakrabortyNo ratings yet
- 99f5fd85-4ee0-4756-afcf-49c54943d6afDocument12 pages99f5fd85-4ee0-4756-afcf-49c54943d6afprint emitraNo ratings yet
- PCR Test Rooh UllahDocument1 pagePCR Test Rooh UllahRooh ullah janNo ratings yet
- Department of Genetics: Covid-19 RT PCRDocument1 pageDepartment of Genetics: Covid-19 RT PCRDv RasminaNo ratings yet
- 99fbcd52 Da4b 4be2 Bfd8 789c8ffdc7eeDocument7 pages99fbcd52 Da4b 4be2 Bfd8 789c8ffdc7eeG D HEALTH CARENo ratings yet
- Peripheral Blood Film:: Hematology ReportDocument2 pagesPeripheral Blood Film:: Hematology ReportusamaNo ratings yet
- ReportDocument16 pagesReportprachiNo ratings yet
- S28 - Sunshine Diagnostics Shop# G-10, Serene View Apartments, Road No12, Madhavpuri Hills, PJR Enclave. HYDERABAD 500050Document11 pagesS28 - Sunshine Diagnostics Shop# G-10, Serene View Apartments, Road No12, Madhavpuri Hills, PJR Enclave. HYDERABAD 500050Krish BoxbyNo ratings yet
- L60 - Yusuf Sarai Lab Home Visit 4/1-3, Aurobindo Marg, Yusuf Sarai DelhiDocument2 pagesL60 - Yusuf Sarai Lab Home Visit 4/1-3, Aurobindo Marg, Yusuf Sarai DelhiNishant bhardwajNo ratings yet
- Diagnostic Report: FinalDocument4 pagesDiagnostic Report: FinalRafik UchihaNo ratings yet
- Z021 PDFDocument2 pagesZ021 PDFAditya RudraNo ratings yet
- Sushil 2Document2 pagesSushil 2Raju BhuyanNo ratings yet
- Research & Development: Test Name Status Result Unit Reference Interval SARS-COV-2 Real-Time PCR, QualitativeDocument2 pagesResearch & Development: Test Name Status Result Unit Reference Interval SARS-COV-2 Real-Time PCR, QualitativeakashNo ratings yet
- Kaushalya SutharDocument2 pagesKaushalya SutharTesting PurposeNo ratings yet
- r139604587 - Maritza - Moreno - CUR139604587 2Document1 pager139604587 - Maritza - Moreno - CUR139604587 2MaritzaNo ratings yet
- Hdkgzt2ylj5kruld3lxjt3uuDocument10 pagesHdkgzt2ylj5kruld3lxjt3uuPRIYANSHU NAMANNo ratings yet
- Inv 7209351580Document3 pagesInv 7209351580HamzaNo ratings yet
- LabTest 02jul2022Document3 pagesLabTest 02jul2022harishNo ratings yet
- Interpretation: S74 - Healthnet CC Healthnet, Old No.34, New No.12, Besant Avenue Road, Opp To Royalenfield ShowroomDocument2 pagesInterpretation: S74 - Healthnet CC Healthnet, Old No.34, New No.12, Besant Avenue Road, Opp To Royalenfield ShowroomPrabhakaran ArumugamNo ratings yet
- Laboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Document1 pageLaboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Marupudi SaikrishnaNo ratings yet
- EMS ResultDocument2 pagesEMS ResultSmeeta AralikattiNo ratings yet
- Laboratory Test Report: Mrs. J SoujanyaDocument10 pagesLaboratory Test Report: Mrs. J SoujanyaJ SoujanyaNo ratings yet
- Temp-Pdf-Reports 431132449Document4 pagesTemp-Pdf-Reports 431132449badboyNo ratings yet
- D-11 Colorado Springs Voter GuideDocument5 pagesD-11 Colorado Springs Voter GuideCPR DigitalNo ratings yet
- Ssumangarg@gmail - Com 20220825193722Document13 pagesSsumangarg@gmail - Com 20220825193722Suman GargNo ratings yet
- Haematology: DR - Abhilash Kumar JainDocument1 pageHaematology: DR - Abhilash Kumar Jainseds5anuragNo ratings yet
- Tahenyat Karimkhan. 2-35-37 PMDocument1 pageTahenyat Karimkhan. 2-35-37 PMchildicuNo ratings yet
- Ljl0wdga4zgftfjlachhji4oDocument3 pagesLjl0wdga4zgftfjlachhji4oAmitNo ratings yet
- 03-12-2021 7:59 Am Covid-Sudharma Lab Wandoor: Molecular Biology ReportDocument2 pages03-12-2021 7:59 Am Covid-Sudharma Lab Wandoor: Molecular Biology ReportRinu jasNo ratings yet
- Confirmation Slip Kriiishna ShelkeeeDocument1 pageConfirmation Slip Kriiishna ShelkeeeR Khan KhanNo ratings yet
- Diagnostic Report: FinalDocument3 pagesDiagnostic Report: Finalrajesh kothariNo ratings yet
- Laboratory For Medical Tests: Test ReportDocument2 pagesLaboratory For Medical Tests: Test ReportAlexandru Ionuț PufuNo ratings yet
- Laboratory For Medical Tests: Test ReportDocument2 pagesLaboratory For Medical Tests: Test ReportConstantin CătălinNo ratings yet
- Laboratory For Medical Tests: Test ReportDocument2 pagesLaboratory For Medical Tests: Test ReportAdnana AndreeaNo ratings yet
- Dacia Spring Voted The Best Buy CAR OF 2022 Winner: Press Release 15/12/2021Document2 pagesDacia Spring Voted The Best Buy CAR OF 2022 Winner: Press Release 15/12/2021Thornblad33No ratings yet
- Wwe Alarm Clock RadioDocument14 pagesWwe Alarm Clock RadioThornblad33No ratings yet
- Grundig: SatellitDocument1 pageGrundig: SatellitThornblad33No ratings yet
- B40C1000G, B80C1000G, B125C1000G, B250C1000G, B380C1000G: Vishay SemiconductorsDocument4 pagesB40C1000G, B80C1000G, B125C1000G, B250C1000G, B380C1000G: Vishay Semiconductorsxbczvbc ertNo ratings yet
- Downloaded From - Datasheet Search EngineDocument3 pagesDownloaded From - Datasheet Search EngineThornblad33No ratings yet
- Semiconductor Technical Data: 0.5 Ampere Power Transistors NPN Silicon 250 - 300 - 350 VOLTS 20 WattsDocument5 pagesSemiconductor Technical Data: 0.5 Ampere Power Transistors NPN Silicon 250 - 300 - 350 VOLTS 20 WattsThornblad33No ratings yet
- Valid From 01.01.2022 Wages in US$ Contract Duration ApplicableDocument1 pageValid From 01.01.2022 Wages in US$ Contract Duration ApplicableThornblad33No ratings yet
- Valid From 01.01.2022 Wages in Euro Contract Duration ApplicableDocument1 pageValid From 01.01.2022 Wages in Euro Contract Duration ApplicableThornblad33No ratings yet
- World's Powerful Radio: TransmitterDocument3 pagesWorld's Powerful Radio: TransmitterThornblad33No ratings yet
- UntitledyyyDocument7 pagesUntitledyyyThornblad33No ratings yet
- UntitleduuuDocument2 pagesUntitleduuuThornblad33No ratings yet
- UntitledDocument5 pagesUntitledThornblad33No ratings yet
- Flight Schedule: Ticket For Miss Tea JurasDocument2 pagesFlight Schedule: Ticket For Miss Tea JurasThornblad33No ratings yet
- UntitledttDocument2 pagesUntitledttThornblad33No ratings yet
- UKMTO Vessel Position Reporting Form - Initial ReportDocument1 pageUKMTO Vessel Position Reporting Form - Initial ReportThornblad33No ratings yet
- Travel Announcement PDFDocument1 pageTravel Announcement PDFMladen RepacNo ratings yet
- UntitledgyyDocument2 pagesUntitledgyyThornblad33No ratings yet
- UKMTO BMP4 FINAL Reporting Data in Word FormatdocxDocument1 pageUKMTO BMP4 FINAL Reporting Data in Word Formatdocxsenthil kumarNo ratings yet
- Everything You Need To Know About:: Pay As You SailDocument28 pagesEverything You Need To Know About:: Pay As You SailThornblad33No ratings yet
- Pemilihan Biomarker Yang Efektif Untuk Penelitian Klinis 14042015Document66 pagesPemilihan Biomarker Yang Efektif Untuk Penelitian Klinis 14042015mahyarani dalimutheNo ratings yet
- Bio NotesDocument2 pagesBio NotesBobNo ratings yet
- 1 Aa Tesis Aravena Roman Maximiliano 2015Document339 pages1 Aa Tesis Aravena Roman Maximiliano 2015iqyuwidya100% (1)
- Taxonomy and Important Features of Probiotic Microorganisms in Food and NutritionDocument9 pagesTaxonomy and Important Features of Probiotic Microorganisms in Food and NutritionDhayu Mart Hindrasyah PandiaNo ratings yet
- Chloroplast Genes Reveal Hybridity in Mango (Mangifera Indica L.)Document5 pagesChloroplast Genes Reveal Hybridity in Mango (Mangifera Indica L.)Shailendra RajanNo ratings yet
- Atkins J F Gesteland R F Cech T R Eds Rna Worlds From Life S PDFDocument345 pagesAtkins J F Gesteland R F Cech T R Eds Rna Worlds From Life S PDFOleg TrifonovNo ratings yet
- Research Paper Topics in BiochemistryDocument7 pagesResearch Paper Topics in Biochemistryfzhw508n100% (1)
- Effect of Naringenin On Alcl3 Induced Alzheimir Disease in Drosophila FlyDocument27 pagesEffect of Naringenin On Alcl3 Induced Alzheimir Disease in Drosophila FlyPeter AiyedeNo ratings yet
- 2020 ImmuneEpitopeMapoftheReportedProteinDocument20 pages2020 ImmuneEpitopeMapoftheReportedProteinvivitri.dewiNo ratings yet
- Photosynthesis Basics, History and ModellingDocument27 pagesPhotosynthesis Basics, History and ModellingDario DiosNo ratings yet
- Lab Report 5 Protein Solubility and PHDocument3 pagesLab Report 5 Protein Solubility and PHDan Floyd FernandezNo ratings yet
- CytoscapeDocument86 pagesCytoscapethamizh555No ratings yet
- Marijuana Trace PDFDocument3 pagesMarijuana Trace PDFRicky Justin NgoNo ratings yet
- Anaerobic Respiration PDFDocument7 pagesAnaerobic Respiration PDFmanoj_rkl_07No ratings yet
- Study Questions For Test 1 BioDocument4 pagesStudy Questions For Test 1 BiokadooieNo ratings yet
- Protein Based NanostructuresDocument3 pagesProtein Based NanostructuresDannyMarlonJ100% (1)
- (1983) Stratum CorneumDocument275 pages(1983) Stratum CorneumfauzNo ratings yet
- Phycocyanin A Potential Drug For Cancer TreatmentDocument14 pagesPhycocyanin A Potential Drug For Cancer TreatmentSrivatsava RajagopalanNo ratings yet
- Biology Lab 1 Bioinformatic ReportDocument5 pagesBiology Lab 1 Bioinformatic ReportKasia DrewniakNo ratings yet
- Ap: Lab-Related Ap Exam Essays Lab 1. Osmosis and DiffusionDocument16 pagesAp: Lab-Related Ap Exam Essays Lab 1. Osmosis and DiffusionWutWutNo ratings yet
- L27 What Is Genetic Engineering 1Document37 pagesL27 What Is Genetic Engineering 1Kent Gabriel DumendenNo ratings yet
- RBC Maturation Stages (Tabulated)Document2 pagesRBC Maturation Stages (Tabulated)Claire GonzalesNo ratings yet
- JN2022819 26Document19 pagesJN2022819 26goutham an rajaNo ratings yet
- LipidsDocument86 pagesLipidssurajagtap01No ratings yet
- Fragi 01 610406Document6 pagesFragi 01 610406msNo ratings yet
- IeuyyDocument2 pagesIeuyyMaligat, John Joscel J.No ratings yet