Professional Documents
Culture Documents
M2 A2
M2 A2
Uploaded by
Kyon KunOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
M2 A2
M2 A2
Uploaded by
Kyon KunCopyright:
Available Formats
Blood volume must be restored in a person who has lost large amounts of blood
due to serious injury. This is often accomplished by infusing isotonic NaCl
solution into the blood. Why is this better than infusing an isoosmotic solution of
a penetrating solute, such as urea?
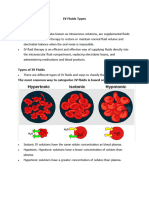
Isotonic solutions are IV fluids that have a similar concentration of dissolved particles as
blood. Such as a solution of 0.9% Normal Saline (0.9% NaCl). Due to the concentration of the
IV fluid being similar to blood, the fluid stays in the intravascular space and osmosis does not
cause fluid movement between compartments.
It is better to infuse isotonic NaCl solution into the blood because it is a nonpenetrating
solute, it restores blood volume without causing a redistribution of water between body fluid
compartments due to osmosis. An isoosmotic solution of a penetrating solute would only
partially restore blood volume because some water would enter the intracellular fluid by osmosis
as the solute enters cells. And could also result to damaging the cells as their volume expands
beyond normal.
References
Open Resources for Nursing (Open RN). (n.d.). 15.3 intravenous solutions. Nursing
Fundamentals. Retrieved February 14, 2022, from
https://wtcs.pressbooks.pub/nursingfundamentals/chapter/15-3-intravenous-solutions/
You might also like
- Fluids and Electrolytes: An Easy and Intuitive Way to Understand and Memorize Fluids, Electrolytes, and Acidic-Base BalanceFrom EverandFluids and Electrolytes: An Easy and Intuitive Way to Understand and Memorize Fluids, Electrolytes, and Acidic-Base BalanceRating: 5 out of 5 stars5/5 (2)
- I.V Fluid: PathologyDocument7 pagesI.V Fluid: PathologyruchikaNo ratings yet
- Fundamentals of Nursing Transes 2Document13 pagesFundamentals of Nursing Transes 2Louise Torres100% (1)
- Intravenous Fluid TherapyDocument9 pagesIntravenous Fluid TherapyLeviNo ratings yet
- What Are IV FluidsDocument5 pagesWhat Are IV FluidsRegine Mae Encinada100% (1)
- The Body Fluid CompartmentsDocument6 pagesThe Body Fluid CompartmentsAlya Putri KhairaniNo ratings yet
- Iv Fluids and Solutions With Nursing ResponsibilitiesDocument33 pagesIv Fluids and Solutions With Nursing ResponsibilitiesJennie JalemNo ratings yet
- Iv FluidsDocument11 pagesIv FluidsDianna Rose BelenNo ratings yet
- PROJECT REPORT ON I.V. FLUID UNIT (Ringer's Lactate, Normal Saline, Dextrose 4.3%, 5%, 10%, 50% & Darrows Solution)Document13 pagesPROJECT REPORT ON I.V. FLUID UNIT (Ringer's Lactate, Normal Saline, Dextrose 4.3%, 5%, 10%, 50% & Darrows Solution)EIRI Board of Consultants and PublishersNo ratings yet
- OsmosisDocument14 pagesOsmosisSuhada IdayuNo ratings yet
- Vomiting of Blood, (Hematemesis) A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandVomiting of Blood, (Hematemesis) A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Different Types of IV FluidsDocument10 pagesDifferent Types of IV FluidsMarinill SolimanNo ratings yet
- Intravenous Fluid Selection PDFDocument12 pagesIntravenous Fluid Selection PDFVASGJGNo ratings yet
- Types of IV FluidsDocument6 pagesTypes of IV Fluidsharrisburrrg100% (1)
- Fluid Volume BalanceDocument73 pagesFluid Volume BalanceSalman HabeebNo ratings yet
- Osmosis: Dr. Afshan Gul Department of PhysiologyDocument31 pagesOsmosis: Dr. Afshan Gul Department of Physiologyfatima zahraNo ratings yet
- Dialysis NotesDocument9 pagesDialysis NotesJAYGONNo ratings yet
- Hypotonic Hypertonic Isotonic Solutions UsesDocument9 pagesHypotonic Hypertonic Isotonic Solutions UsesImtiaz AhmedNo ratings yet
- TonicityDocument6 pagesTonicitypavelbdsNo ratings yet
- Three Main Types of IVF: Isotonic Fluids Hypotonic Fluids Hypertonic FluidsDocument9 pagesThree Main Types of IVF: Isotonic Fluids Hypotonic Fluids Hypertonic FluidsIrina Luciana DumitriuNo ratings yet
- Lecture 1 NDHDocument3 pagesLecture 1 NDHTricia Jane OpinaldoNo ratings yet
- 1.4.A2 Tissues or Organs To Be Used in Medical Procedures Must Be Bathed in A Solution With The Same Osmolarity As The Cytoplasm To Prevent OsmosisDocument6 pages1.4.A2 Tissues or Organs To Be Used in Medical Procedures Must Be Bathed in A Solution With The Same Osmolarity As The Cytoplasm To Prevent OsmosisApollo AdairNo ratings yet
- T5 Fluid and Fluid Imbalances PTPDocument4 pagesT5 Fluid and Fluid Imbalances PTPangela adelantarNo ratings yet
- What Are IntravenousDocument11 pagesWhat Are IntravenousLynx Angeles RNNo ratings yet
- Osmosis and DialysisDocument5 pagesOsmosis and DialysisLinoel Izach GalfoNo ratings yet
- Colligative Properties of Electrolyte SolutionsDocument3 pagesColligative Properties of Electrolyte SolutionsLamiche EdalNo ratings yet
- Osmosis NotesDocument3 pagesOsmosis NotessmexiiloriNo ratings yet
- IV Infusion Preparations Fluid and Electrolytes: DR Anita JanadriDocument22 pagesIV Infusion Preparations Fluid and Electrolytes: DR Anita JanadriMohamed RasoolNo ratings yet
- Osmosis Presentation@Document20 pagesOsmosis Presentation@Dima ArafatNo ratings yet
- IV Fluids TheoryDocument13 pagesIV Fluids TheoryFRESY TRI SUGIANTONo ratings yet
- Fluid, Electrolyte and Acid - BaseDocument19 pagesFluid, Electrolyte and Acid - BaseNandita HalderNo ratings yet
- Biochemistry Molecules Solution Diffusion Dialysis Tubing Dialysis Semipermeable CelluloseDocument4 pagesBiochemistry Molecules Solution Diffusion Dialysis Tubing Dialysis Semipermeable CelluloseErick Rafael AncaNo ratings yet
- Intravenous Fluid Selection: Learning ObjectivesDocument11 pagesIntravenous Fluid Selection: Learning ObjectivesKristine AlejandroNo ratings yet
- OsmosisDocument9 pagesOsmosisgillianmarie.perez.pharmaNo ratings yet
- IB Biology D Continuity and Change pt1Document5 pagesIB Biology D Continuity and Change pt1rellomharazNo ratings yet
- Types of IvDocument1 pageTypes of IvFrancis GlinogaNo ratings yet
- Types of IV FluidsDocument15 pagesTypes of IV Fluidskirti thakurNo ratings yet
- Ú 6oss of Rbcs Diminishes O: Transfusion Medicine: Blood ProductsDocument4 pagesÚ 6oss of Rbcs Diminishes O: Transfusion Medicine: Blood ProductsshanabsinNo ratings yet
- Digital Scrapbook by Jacela BunayogDocument31 pagesDigital Scrapbook by Jacela BunayogJacela Annsyle J. BunayogNo ratings yet
- F & E Study Guide - SY 2022-2023Document16 pagesF & E Study Guide - SY 2022-20232A - Nicole Marrie HonradoNo ratings yet
- OsmosisDocument3 pagesOsmosisgrace.a.sun18No ratings yet
- College of Health Sciences: Urdaneta City UniversityDocument5 pagesCollege of Health Sciences: Urdaneta City UniversityDan Dan ManaoisNo ratings yet
- A Brief Guide & Summation Jackie Weisbein, D.O. Westchester General Hospital Miami, FloridaDocument35 pagesA Brief Guide & Summation Jackie Weisbein, D.O. Westchester General Hospital Miami, FloridaSalil MahajanNo ratings yet
- Aquino, Christian Jufers G. BSN3-NCA2: IsotonicDocument2 pagesAquino, Christian Jufers G. BSN3-NCA2: IsotonicJuf's Aquino IINo ratings yet
- 5% Dextrose in Water-A Carbohydrate Solution That: Key TermsDocument3 pages5% Dextrose in Water-A Carbohydrate Solution That: Key Termsamoosh_amani725No ratings yet
- 248 205Document27 pages248 205AtharvaNo ratings yet
- Fluid, Electrolyte and Acid - Base BalancesDocument65 pagesFluid, Electrolyte and Acid - Base BalancesNandita HalderNo ratings yet
- Intravenous Therapy PDFDocument4 pagesIntravenous Therapy PDFcatislandbigredNo ratings yet
- Iv Solutions: Hypotonic (280 Mosm/L)Document2 pagesIv Solutions: Hypotonic (280 Mosm/L)bebeNo ratings yet
- 03 - Isotonic SolutionDocument29 pages03 - Isotonic SolutionamirNo ratings yet
- 03a Cell TonicityDocument4 pages03a Cell TonicityMary Jewel0% (1)
- Lab 5Document15 pagesLab 5Suhada IdayuNo ratings yet
- Pharmacology IV FluidDocument44 pagesPharmacology IV FluidmelaniNo ratings yet
- Pharmacology 1Document25 pagesPharmacology 1Gul NazNo ratings yet
- Biochem Dela CruzDocument3 pagesBiochem Dela CruzValerie Gayle De la CruzNo ratings yet
- 26IV Fluids0706Document2 pages26IV Fluids0706Sofia AraujoNo ratings yet
- Fluids in Surgery 1 1Document43 pagesFluids in Surgery 1 1محمد عماد عليNo ratings yet
- Osmotic FragilityDocument22 pagesOsmotic Fragilityfatma522No ratings yet
- 12 - Hydration StatusDocument30 pages12 - Hydration StatusSelin SakarNo ratings yet
- IV FluidDocument49 pagesIV Fluidibrahimadnan040No ratings yet
- Challenge 4.1Document1 pageChallenge 4.1Kyon KunNo ratings yet
- M4. A4Document1 pageM4. A4Kyon KunNo ratings yet
- A3Document1 pageA3Kyon KunNo ratings yet
- What Would Happen To Heart Rate If Some Stimulus Caused Blood Pressure To Decrease? Would This Occur by Way of Positive or Negative Feedback?Document1 pageWhat Would Happen To Heart Rate If Some Stimulus Caused Blood Pressure To Decrease? Would This Occur by Way of Positive or Negative Feedback?Kyon KunNo ratings yet
- Explain How An Imbalance in Any Given Physiological Variable Might Produce A Change in One or More Other Variables. Provide A Specific ExampleDocument1 pageExplain How An Imbalance in Any Given Physiological Variable Might Produce A Change in One or More Other Variables. Provide A Specific ExampleKyon KunNo ratings yet
- M19 The Rizal Retraction Learning Activity 2Document1 pageM19 The Rizal Retraction Learning Activity 2Kyon KunNo ratings yet
- Challenge 2.1Document1 pageChallenge 2.1Kyon KunNo ratings yet
- The Two Faces of The 1872 Cavite Mutiny: Spanish PerspectiveDocument2 pagesThe Two Faces of The 1872 Cavite Mutiny: Spanish PerspectiveKyon KunNo ratings yet
- Bad Politics of Filipinos During The American Period Bad Politics of Filipinos Until TodayDocument2 pagesBad Politics of Filipinos During The American Period Bad Politics of Filipinos Until TodayKyon KunNo ratings yet