Professional Documents
Culture Documents
Als Lyme
Uploaded by
Christine Anne HellmundOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Als Lyme
Uploaded by
Christine Anne HellmundCopyright:
Available Formats
Letters Palpebral narrowing associated with peripheral facial nerve palsy showed a varying degree of drooping of the eyebrow.
There were patients with a severe degree of narrowing. This indicates that the frontal muscle which has been considered as an accessory muscle, occasionally plays a very important part in eyelid elevation. We suspect that this is closely related to the skin condition of the upper face. Some Japanese people, especially elderly ones, have excessively loose skin of the upper eyelid or forehead, resulting in masked palpebral narrowing. In such a situation, occurrence of frontal muscle weakness can produce severe palpebral narrowing. In conclusion, the eyebrow lifting test is simple but very helpful in diVerentiating between two types of palpebral narrowing due to weakness of the frontal muscle and levator palpebrae superioris.
SHINGO OHKAWA HIROSHI YAMASAKI YUKIO OHSUMI MASAYASU TABUCHI TAKASHI YOSHIDA Neurology Service, Hyogo Brain and Heart Center at Himeji ATSUSHI YAMADORI Department of Disability Science, Tohoku University Graduate School of Medicine, Sendai KOHKICHI HOSODA SHIGEKIYO FUJITA Neurosurgery Service, Hyogo Brain and Heart Center at Himeji Correspondence to: Dr Shingo Ohkawa, Neurology Service, Hyogo Brain and Heart Center at Himeji, 520, Saisho-Ko, Himeji 670, Japan.
257
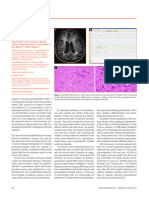
Severe atrophy of hand muscles. arthralgias, his physician tested him for Borrelia burgdorferi specic antibodies in the serum because he lived in an endemic region. The test disclosed high concentrations of specic IgG antibodies (1:1200, cut oV <1:200). The patient was treated with doxycyclin for two weeks. A control examination performed in a diVerent laboratory still disclosed high concentrations of specic IgG antibodies (1:160, cut oV 1:40). Treatment was started again with cefotaxim (2g intravenously for ve days). Six months later he was admitted to our hospital because of persisting paresis and muscle atrophy. On admission, clinical examination disclosed hyperactive deep tendon reexes with a clonus in both ankles. The muscles of both hands and forearms showed atrophy and severe paresis (gure). His gait was clumsy and stiV, and there was mild spastic paraparesis. There was no sensory loss, and cerebellar function was normal. Needle EMG disclosed severe active denervation in the small hand muscles bilaterally. Mild to moderate signs of axonal damage were seen in the right anterior tibial muscle and in the left masseter muscle. Motor and sensory nerve conduction velocities were normal. No conduction block could be detected. F waves were abolished. Visual and sensory evoked potentials were normal. Motor evoked potentials disclosed prolonged central conduction times to both anterior tibial muscles and to the left abductor digiti minimi muscle. Summary of antibody tests
Examination Serum: B burgdorferi IgG antibodies B burgdorferi IgM antibodies CSF: Total cell count (/l) CSF/serum albumin ratio (103) IgG-local (%) IgA-local (%) B burgdorferi IgG antibodies B burgdorferi IgM antibodies B burgdorferi IgG antibody index B burgdorferi IgA antibody index 14 kDa fragment IgG antibody index *Five months after treatment. Normal <1:16 <1:48 <5 <7.5 0 0 <1:16 <1:48 <1.5 <1.5 <1.5 Initial study 1:64 1:12 5 4.7 75 20 1:16 <1:2 24.6 2.2 35.6 Follow up* <1:16 <1:12 3 3.5 65 10 1:2 <1:2 18.0 7.2 Negative
1 Adams RD, Victor M. Diseases of the cranial nerves. In: Adams RD, Victor M, eds. Principle of neurology, 4th ed. New York: McGraw-Hill, 1989:1081. 2 Ohkawa S, Yoshida T, Yamadori A. Acute stage Bells palsy and narrowing of the palpebral ssure caused by drooping of the eyebrow and the upper eyelid. J Neurol Neurosurg Psychiatry 1992;55:976. 3 Caplan LR. Ptosis. J Neurol Neurosurg Psychiatry 1974;37:17. 4 Miller NR. Anatomy and physiology of normal and abnormal eyelid position and movement. In: Miller NR, ed. Walsh and Hoyt s clinical neuro-ophthalmology. 4th ed. Baltimore: Williams and Wilkins, 1985:933. 5 Averbuch-Heller L, Poonyathalang A, von Maydell RD, Remler BF. Herings law for eyelids: Still valid. Neurology 1995;45:17813.
Generalised motor neuron disease as an unusual manifestation of Borrelia burgdorferi infection Lyme borreliosis is a well known multisystem disease caused by the spirochete Borrelia burgdorferi and can produce a wide array of neurological abnormalities in humans. The most frequent are meningitis, cranial neuritis, and painful radiculoneuritis.1 Other clinical manifestations include chronic encephalomyelitis, spastic paraplegia, and axonal polyneuropathy. Our report concerns what we think to be the rst case of a patient with upper and lower motor neuron disease and Borrelia burgdorferi infection of the CNS. A causal relation is strongly supported by an evaluation of the Borrelia burgdorferi specic antibody index and the patients favourable response to medical treatment. Fifteen months before admission a 33 year old patient noticed weakness in his right hand followed by weakness of the left hand and a progressive gait disturbance. Although he had no pain or sensory disturbance and no history of a tick bite, an erythema migrans, or
Routine blood chemistry was normal. Serum Treponema pallidum and GM1-specific antibodies were not detected. Borrelia serology tests showed slightly raised concentrations of Borrelia burgdorferi specic IgG antibodies in serum and clearly raised concentrations in the CSF. At the time of the rst lumbar puncture (after antibiotic treatment), CSF contained ve white blood cells/l and a total protein concentration of 410 mg/l. Reiberformula analysis2 indicated an intrathecal synthesis of IgG and IgA. Employing a sensitive aYnity blotting technique most of the oligoclonal IgG bands in the CSF were shown to be specic for Borrelia burgdorferi This nding was conrmed by western blotting using identical concentrations of IgG in the CSF and serum. A higher number of Borrelia burgdorferi specic antibody bands were found in the CSF than in serum. Calculation of the Borrelia burgdorferi specic antibody index from enzyme linked immunosorbent assay (ELISA) studies disclosed raised values for IgG and IgA. Specicity of intrathecally produced IgG antibodies for Borrelia burgdorferi was conrmed by employing a highly specic 14 kDa fragment of the agellin as antigen in enzyme linked immunosorbent assay (ELISA).3 The table shows the detailed data of the antibody tests on admission and on follow up examination ve months later. MRI of the cervical spinal cord and the brain disclosed no abnormalities. The patient was treated with ceftriaxone intravenously for two weeks, followed by oral
258
Letters
R KAISER C H LCKING Department of Neurology and Clinical Neurophysiology, University of Freiburg , Germany G DEUSCHL Department of Neurology and Clinical Neurophysiology, University of Kiel, Germany Correspondence to: Dr Franz X Glocker, Neurologische Universittsklinik Breisacher Strasse 64, D-79106 Freiburg, Germany. 1 Pachner AR, Steere AC. The triad of neurologic manifestations of Lyme disease: meningitis, cranial neuritis, and radiculoneuritis. Neurology 1985;35:4753. 2 Reiber H, Felgenhauer K. Protein transfer at the blood cerebrospinal uid barrier and the quantitation of the humoral immune response within the central nervous system. Clin Chim Acta 1987;163:31928. 3 Kaiser R, Rasiah C, Gassmann G, Vogt A, Lcking C. Intrathecal antibody synthesis in Lyme neuroborreliosis: use of recombinant p41 and a 14-kDa agellin fragment in ELISA. J Med Microbiol 1993;39:2907. 4 ElAlaouli F, Medejel A, AlZemmouri K, Yahyaoui M, Chkili T. Syphilitic lateral amyotrophic sclerosis. A study of 5 cases. Rev Neurol 1990;146:414. 5 Fredrikson S, Link H. CNS-borreliosis selectively aVecting central motor neurons. Acta Neurol Scand 1988;78:1814. 6 Halperin JJ, Kaplan GP, Brazinsky S, et al. Immunologic reactivity against Borrelia burgdorferi in patients with motor neuron disease. Arch Neurol 1990;47:58694.
prednisone for 10 weeks. After this treatment the patients condition improved slowly but continuously. At the time of the last clinical control examination 18 months after hospital discharge the patient was able to work without physical impairment. Clinical and electrophysiological ndings met all the criteria for the diagnosis of motor neuron disease. Clinical signs of lower motor neuron involvement were present in both arms. Electromyographic studies disclosed axonal loss at three diVerent levelsnamely, lumbar (anterior tibial muscle), cervical (hand muscles), and supraspinal (masseter muscle). Clear signs of damage to the upper motor neuron were also present. Although the symptoms of the patient could be explained by cervical myelitis the EMG ndings with evidence of axonal damage in the anterior tibial and masseter muscle as well as the lack of any sensory abnormalities argue strongly against this possibility. In addition, signs of inammation in the CSF were not consistent with a diagnosis of amyotrophic lateral sclerosis. We identied a Borrelia burgdorferi infection of the CNS as the cause of the inammation. Evidence included a raised specic IgG and IgA antibody index, the demonstration of Borrelia burgdorferi specic oligoclonal IgG bands in the CSF and the predominance of individual Borrelia burgdorferi specic antibody bands in CSF (as indicated by western blotting). The absence of a high white cell count and protein in the CSF could be attributed to prior antibiotic treatment. Optimising dose and duration, antibiotic treatment was renewed and combined with a long term steroid therapy. Four months later a CSF examination showed a considerable decrease in specic antibody concentrations, and the patients condition continued to improve. In the light of the evidence, it seems safe to conclude that the patients symptoms were due to a CNS Borrelia burgdorferi infection which merely mimicked amyotrophic lateral sclerosis. Several reports have been published on spirochetal diseases leading to isolated damage to the motor system. Spinal meningovascular lues has been reported to cause a clinical syndrome mimicking motor neuron disease.4 Fredrikson and Link published a case report of a patient with isolated upper motor neuron symptoms due to CNS borreliosis who responded favourably to antibiotic treatment.5 Cases of painful motor neuropathy due to Borrelia burgdorferi specic infection have also been reported.1 Halperin et al6 found serological evidence of exposure to Borrelia burgdorferi in nine of 19 patients with motor neuron disease. However, none of them showed signs of Borrelia burgdorferi specic immunoreactivity in the CSF or favourable response to treatment. It can be speculated that the spirochete Borrelia burgdorferi has the ability to induce an immune reaction that specically aVects motor neurons. This reaction may mimic different, non-curable diseases, such as spastic spinal paralysis, spinal muscle atrophy, and amyotrophic lateral sclerosis. Therefore, we suggest that patients diagnosed as having progressive motor neuron disease, who live in endemic areas, should be tested for Borrelia burgdorferi specic antibodies in serum and in CSF. The test could reliably detect a rare, but treatable disease mimicking motor neuron disease.
B HEMMER F X GLOCKER
Severe but transient parkinsonism after tetanus vaccination A 38 year old metal worker with a history of hypertension and hyperthyroidism presented with uctuating fever and sweating, palpitations, tremor of the upper parts of both legs, and diplopia. These symptoms had been present for ve days and had started within hours after he had received the last of three vaccinations for tetanus (TE anatoxal berna, contents: 20 LF tetanus toxoid, 2 mg aluminium phosphate, and 0.1 mg thiomersal, Primmed BV, Almere, The Netherlands). These vaccinations were given because of an injury to his right index nger one month before. There was no family history of movement disorders. Physical examination showed profuse sweating, normal consciousness, a temperature of 37.3C, symmetric rigidity of all four limbs, and a painful tremor in the upper parts of his legs. Muscle strength, tendon reexes, and sensation were normal. Within a week he progressed to severe hypokinetic dysarthria, a mask-like face, and a resting tremor of both hands, and he had bradykinesia and generalised rigidity, together with a cogwheel phenomenon in the arms. Laboratory examination showed a creatine phosphokinase activity of 2682 U/l (normal <190 U/l) and normal blood concentrations of manganese, copper, ceruloplasmin, and carbon monoxide. His CSF showed 50 lymphocytes (normal range 0-3), slightly raised total protein (0.54 g/l; normal range 0.15-0.45 g/l), normal IgG index (0.43; normal <0.66), and negative serological tests on Epstein-Barr virus, herpes zoster virus, herpes simplex virus, syphilis, Borrelia burgdorferi, and Mycoplasma pneumoniae. Brain MRI was normal. Single photon emission computed tomography (SPECT) with 123Iiodobenzamide (IBZM), specically binding to the cerebral dopamine receptor (D2), showed a decreased ganglia:cortex ratio, indicating a postsynaptic disorder. Nevertheless, biperidine, levodopa and carbidopa, and pergolide were prescribed, result-
ing in gradual but impressive clinical improvement within several weeks. The clinical syndrome was unclear during the rst few days after admission, but gradually developed into a hypokinetic rigid syndrome with resting tremor, generalised bradykinesia, and rigidity. This responded well to treatment with levodopa/carbidopa and a dopamine agonist. Possible causes of a rapidly progressive form of parkinsonism are encephalitis, intoxication, head trauma, tumour, ischaemia, or hydrocephalus.1 Imaging studies showed no abnormalities, thereby excluding the last three possible causes. Repeated history taking failed to disclose head trauma. The profession of the patient might suggest poisoning, but blood concentrations of manganese and copper were normal. The CSF showed mild pleiocytosis, but serological testing for various specic microorganisms did not show any recent infection. Radionuclide imaging showed a pattern similar to that seen in progressive supranuclear palsy or multiple system atrophy.2 The sequence of events strongly suggests a relation between the vaccination and the neurological syndrome, although the causal nature is diYcult to prove.3 To our knowledge, there are no reports of parkinsonism after tetanus immunisation. Alves et al reported a 5 year old boy who developed a postencephalitic rigid akinetic syndrome 15 days after vaccination for measles with live attenuated virus. Again, cause and eVect remained open for debate.4 5 The tetanus vaccine used in our patient does not contain any living microorganisms. However, repeated injections with the tetanus toxoid might have caused hypersensitivity, and also an immunological cross reaction of antibodies with neuronal tissue directly after the last injection. This might also explain the pleiocytosis and raised protein and IgG content in CSF. The alternative explanation is that one of the substances in the vaccine vehicle, thiomersal or aluminiumphosphate, had a neurotoxic eVect. Although we are aware that a causal relation between the vaccine and the hypokinetic rigid syndrome is far from established, we have no better explanation. We wish to record the patient history as a reference, in case analogous patients might be seen in the future.
We thank Dr J W van Isselt, University Department of Nuclear Medicine, Utrecht, for kindly providing the SPECT. J C REIJNEVELD M J B TAPHOORN T U HOOGENRAAD J VAN GIJN University Department of Neurology, Utrecht, The Netherlands Correspondence to: Dr J C Reijneveld, Department of Neurology, University Hospital Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands. 1 Fahn S. Parkinsons disease and other basal ganglion disorders. In: Asbury AK, McKhann GM, McDonald WI, eds. Diseases of the nervous system. London: Heinemann, 1986;98:121728. 2 Buck A, Westera G, Sutter M, Albani C, Kung HF, von Schulthess GK. Iodine-123-IBF SPECT evaluation of extrapyramidal diseases. J Nucl Med 1995;36:1196-200. 3 Stratton KR, Howe CJ, Johnston RB, eds. Adverse events associated with childhood vaccines. Evidence bearing on causality. Washington, DC: National Academy Press, 1994. 4 Alves RSC, Barbosa ER, ScaV M. Postvaccinal parkinsonism. Mov Disord 1992;7:178-80. 5 Fenichel GM. Postvaccinal parkinsonism [letter]. Mov Disord 1993;8:253
You might also like
- Neurology Multiple Choice Questions With Explanations: Volume IFrom EverandNeurology Multiple Choice Questions With Explanations: Volume IRating: 4 out of 5 stars4/5 (7)
- BH 010060 ENGDocument2 pagesBH 010060 ENGCarlosNo ratings yet
- Ptosis Without Ophthalmoplegia: A Rare Manifestation of Guillain-Barré SyndromeDocument5 pagesPtosis Without Ophthalmoplegia: A Rare Manifestation of Guillain-Barré SyndromeMosNo ratings yet
- Cranial Nerve Enhancement in The Guillain-Barre Syndrome: DiscussionDocument3 pagesCranial Nerve Enhancement in The Guillain-Barre Syndrome: DiscussionAdesh KumarNo ratings yet
- Clinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945Document13 pagesClinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945aileenzhongNo ratings yet
- Mab200501 10Document8 pagesMab200501 10Muhammad Ardyansyah PratamaNo ratings yet
- Guillain-Barre Syndrome Presenting With Sensory Disturbance Following A Herpes Virus Infection: A Case ReportDocument5 pagesGuillain-Barre Syndrome Presenting With Sensory Disturbance Following A Herpes Virus Infection: A Case ReportAsep MetrikaNo ratings yet
- Pons LesionDocument4 pagesPons Lesiontjimen2No ratings yet
- Epilepsy & Behavior Case Reports: 10.1016/j.ebcr.2017.07.003Document21 pagesEpilepsy & Behavior Case Reports: 10.1016/j.ebcr.2017.07.003Faras ArinalNo ratings yet
- Encefalitis LimbicaDocument11 pagesEncefalitis LimbicaRandy UlloaNo ratings yet
- Syringomyelia in Neuromyelitis Optica Seropositive For Aquaporin-4 Antibody: A Case ReportDocument4 pagesSyringomyelia in Neuromyelitis Optica Seropositive For Aquaporin-4 Antibody: A Case ReportIJAR JOURNALNo ratings yet
- Neurohistiocytosis: Two Cases of A Rare DiseaseDocument8 pagesNeurohistiocytosis: Two Cases of A Rare DiseaseIJAR JOURNALNo ratings yet
- 2017 32 3 264 267 EngDocument4 pages2017 32 3 264 267 Engemmanuellagrace06No ratings yet
- PDF JNS 27Document7 pagesPDF JNS 27emreyavuz8745No ratings yet
- Seronegative Myasthenia Gravis Presenting With PneumoniaDocument4 pagesSeronegative Myasthenia Gravis Presenting With PneumoniaJ. Ruben HermannNo ratings yet
- Article or ReviewDocument3 pagesArticle or Reviewv-santNo ratings yet
- EMG in PolioDocument8 pagesEMG in PolioShauki AliNo ratings yet
- Diplejia BraquialDocument7 pagesDiplejia BraquialDiego Agustin ZarzaNo ratings yet
- A Middle Aged Woman With Nausea Weight Loss and Orthostatic HypotensionDocument7 pagesA Middle Aged Woman With Nausea Weight Loss and Orthostatic Hypotensioniri_balNo ratings yet
- Nokura 1997Document7 pagesNokura 1997Luis CsrNo ratings yet
- 急性呼吸障害を来した単純ヘルペス脳炎 において脳幹病変が病理学的に確認でき た 89 歳男性の 1 例 A case of an 89-year-old male with pathologically confirmed brainstem lesion in herpes simplex encephalitis with acute respiratory disorderDocument6 pages急性呼吸障害を来した単純ヘルペス脳炎 において脳幹病変が病理学的に確認でき た 89 歳男性の 1 例 A case of an 89-year-old male with pathologically confirmed brainstem lesion in herpes simplex encephalitis with acute respiratory disorderEdgar Azael GarciaNo ratings yet
- A Rare Presentation of Guillain-Barre Syndrome: Pharyngeal Cervical-Brachial VariantDocument2 pagesA Rare Presentation of Guillain-Barre Syndrome: Pharyngeal Cervical-Brachial VariantdzakiyahNo ratings yet
- Neuroscience AssignmentDocument7 pagesNeuroscience AssignmentAubrey Unique EvangelistaNo ratings yet
- ALS1Document4 pagesALS1Jhoana Rose Joaquin SantosNo ratings yet
- Exercise-Associated Numbness and Tingling in The Legs: For Editorial Comment See Page 1509Document4 pagesExercise-Associated Numbness and Tingling in The Legs: For Editorial Comment See Page 1509herpthederpNo ratings yet
- J Neurol Neurosurg Psychiatry 2005 Vanopdenbosch 1017 8Document3 pagesJ Neurol Neurosurg Psychiatry 2005 Vanopdenbosch 1017 8afiwahyuNo ratings yet
- Case ReportDocument5 pagesCase ReportsyahputriNo ratings yet
- Guillain Barr Syndrome Occurring Synchronously With Systemic Lupus Erythematosus As Initial Manifestation Treated Successfully With Low DoseDocument5 pagesGuillain Barr Syndrome Occurring Synchronously With Systemic Lupus Erythematosus As Initial Manifestation Treated Successfully With Low DoseTessa CruzNo ratings yet
- Journal of Clinical NeuroscienceDocument3 pagesJournal of Clinical NeurosciencejicarrerNo ratings yet
- 31-Year-Old Man With Balint'S Syndrome and Visual Problems: Clinical History and NeuroimagingDocument4 pages31-Year-Old Man With Balint'S Syndrome and Visual Problems: Clinical History and NeuroimagingwillygopeNo ratings yet
- Posterior Reversible Encephalopathy Syndrome (PRES) : Electroencephalographic Findings and Seizure PatternsDocument7 pagesPosterior Reversible Encephalopathy Syndrome (PRES) : Electroencephalographic Findings and Seizure PatternsJasper CubiasNo ratings yet
- Advance Publication by J-STAGE: Japanese Journal of Infectious DiseasesDocument8 pagesAdvance Publication by J-STAGE: Japanese Journal of Infectious DiseasesEdgar Azael GarciaNo ratings yet
- 52 Tripathy EtalDocument5 pages52 Tripathy EtaleditorijmrhsNo ratings yet
- Clinical Neurophysiology: Letter To The EditorDocument3 pagesClinical Neurophysiology: Letter To The EditorRubén Darío Arenas DíazNo ratings yet
- Art Acta - Neurol.belgicaDocument6 pagesArt Acta - Neurol.belgicaAna GabrielaNo ratings yet
- Hsan4 Neurophys CIPADocument5 pagesHsan4 Neurophys CIPADesyaNo ratings yet
- Jurnal GBSDocument5 pagesJurnal GBSDian Indrayani PoraNo ratings yet
- Epilepsia - 2019 - Hanin - Cerebrospinal Fluid and Blood Biomarkers of Status EpilepticusDocument13 pagesEpilepsia - 2019 - Hanin - Cerebrospinal Fluid and Blood Biomarkers of Status EpilepticusAmira GharbiNo ratings yet
- Subacute Sclerosing Panencephalitis A Clinical and Pathological Study - 101 108Document7 pagesSubacute Sclerosing Panencephalitis A Clinical and Pathological Study - 101 108Bhanu PratapNo ratings yet
- Profound Olfactory Dysfunction in Myasthenia GravisDocument5 pagesProfound Olfactory Dysfunction in Myasthenia GravisAngie Sidney Naranjo GarciaNo ratings yet
- J Clinph 2013 03 031Document7 pagesJ Clinph 2013 03 031Andres Rojas JerezNo ratings yet
- Case Report: de Morsier Syndrome Associated With Periventricular Nodular HeterotopiaDocument4 pagesCase Report: de Morsier Syndrome Associated With Periventricular Nodular HeterotopiahypnonautNo ratings yet
- Clinical/Scientific Notes: Herpes Simplex Virus - 1 Encephalitis Can Trigger Anti-Nmda Receptor Encephalitis: Case ReportDocument3 pagesClinical/Scientific Notes: Herpes Simplex Virus - 1 Encephalitis Can Trigger Anti-Nmda Receptor Encephalitis: Case ReportMuhammad Imran MirzaNo ratings yet
- Algoritmo Diferencial de Debilidad Proximal en Paciente Con Miopatia Adquirida Por Nemalina.Document7 pagesAlgoritmo Diferencial de Debilidad Proximal en Paciente Con Miopatia Adquirida Por Nemalina.Farid Santiago Abedrabbo LombeydaNo ratings yet
- Clinical Reasoning: Rare Cause of Hemiparesis and Ataxia in A 36-Year-Old ManDocument8 pagesClinical Reasoning: Rare Cause of Hemiparesis and Ataxia in A 36-Year-Old MansamuelNo ratings yet
- 1 s2.0 S1388245722007714 MainDocument2 pages1 s2.0 S1388245722007714 MainAndres ChiribogaNo ratings yet
- Arm 37 717Document4 pagesArm 37 717ChrisNo ratings yet
- BioelectricidadDocument11 pagesBioelectricidadRubén TorresNo ratings yet
- 0717 9227 RCHNP 57 03 0283Document12 pages0717 9227 RCHNP 57 03 0283Araceli PerezNo ratings yet
- PBL NeuroDocument45 pagesPBL NeuroInsan_aqidNo ratings yet
- A Case of Male Sle: An Unusual PresentationDocument4 pagesA Case of Male Sle: An Unusual PresentationIJAR JOURNALNo ratings yet
- Syncope: How The EEG Helps in Understanding Clinical FindingsDocument3 pagesSyncope: How The EEG Helps in Understanding Clinical FindingsCarlos AcostaNo ratings yet
- Comment Letters: Similarity of Balloon Cells in Focal Cortical Dysplasia To Giant Cells in Tuberous SclerosisDocument4 pagesComment Letters: Similarity of Balloon Cells in Focal Cortical Dysplasia To Giant Cells in Tuberous SclerosisashfaqamarNo ratings yet
- Anti LGL1 Limbic EncephalitisDocument4 pagesAnti LGL1 Limbic EncephalitisasasakopNo ratings yet
- Status Epilepticus in Critically Ill PatientsDocument11 pagesStatus Epilepticus in Critically Ill PatientsAkbal Nur KarimNo ratings yet
- A New Familial Adult-Onset Leukodystrophy Manifesting As Cerebellar Ataxia and DementiaDocument9 pagesA New Familial Adult-Onset Leukodystrophy Manifesting As Cerebellar Ataxia and DementiaKristineNo ratings yet
- Cerebrospinal Fluid and Blood Biomarkers of Status Epilepticus - 2020Document40 pagesCerebrospinal Fluid and Blood Biomarkers of Status Epilepticus - 2020Reny Wane Vieira dos SantosNo ratings yet
- 1 Neuro A) IIHDocument3 pages1 Neuro A) IIHLoren boidNo ratings yet
- Cortical Laminar NecrosisDocument7 pagesCortical Laminar Necrosisbalajinagarajanas2001No ratings yet
- Pinocchio SyndromeDocument1 pagePinocchio SyndromeTakeshi ClairNo ratings yet
- Analytics Designer - Comment Deletion - SAP CommunityDocument6 pagesAnalytics Designer - Comment Deletion - SAP CommunityARPITA BISWASNo ratings yet
- Test Bank For The Psychology of Health and Health Care A Canadian Perspective 5th EditionDocument36 pagesTest Bank For The Psychology of Health and Health Care A Canadian Perspective 5th Editionload.notablewp0oz100% (37)
- E-Mobility and SafetyDocument77 pagesE-Mobility and SafetySantosh KumarNo ratings yet
- 1 PBDocument7 pages1 PBIndah Purnama TaraNo ratings yet
- IQAc 04-05Document10 pagesIQAc 04-05ymcacollegewebsiteNo ratings yet
- Vygotsky EssayDocument3 pagesVygotsky Essayapi-526165635No ratings yet
- Benedict Anderson, Imagined CommunitiesDocument2 pagesBenedict Anderson, Imagined CommunitiesMonir Amine0% (1)
- (Isaac Asimov) How Did We Find Out About AntarcticDocument24 pages(Isaac Asimov) How Did We Find Out About AntarcticDrBabu PSNo ratings yet
- Project ProposalDocument4 pagesProject Proposaljiaclaire2998100% (1)
- X - WORMWOOD EVENT IMMEDIATE - Paranormal - 4chanDocument7 pagesX - WORMWOOD EVENT IMMEDIATE - Paranormal - 4chanAnonymous dIjB7XD8ZNo ratings yet
- Enzymes IntroDocument33 pagesEnzymes IntropragyasimsNo ratings yet
- The First Voyage Round The World by MageDocument405 pagesThe First Voyage Round The World by MageGift Marieneth LopezNo ratings yet
- Iec Codes PDFDocument257 pagesIec Codes PDFAkhil AnumandlaNo ratings yet
- Lightning Protection Measures NewDocument9 pagesLightning Protection Measures NewjithishNo ratings yet
- Sales Forecast Template DownloadDocument9 pagesSales Forecast Template DownloadAshokNo ratings yet
- Parallel Port Programming With DelphiDocument4 pagesParallel Port Programming With Delphiramadhan1933No ratings yet
- Operating Instructions: HTL-PHP Air Torque PumpDocument38 pagesOperating Instructions: HTL-PHP Air Torque PumpvankarpNo ratings yet
- Case Study - Montana Mountain BikingDocument6 pagesCase Study - Montana Mountain Bikingbonny MishNo ratings yet
- LPS 1131-Issue 1.2-Requirements and Testing Methods For Pumps For Automatic Sprinkler Installation Pump Sets PDFDocument19 pagesLPS 1131-Issue 1.2-Requirements and Testing Methods For Pumps For Automatic Sprinkler Installation Pump Sets PDFHazem HabibNo ratings yet
- Paul Wade - The Ultimate Isometrics Manual - Building Maximum Strength and Conditioning With Static Training-Dragon Door Publications (2020) - 120-146Document27 pagesPaul Wade - The Ultimate Isometrics Manual - Building Maximum Strength and Conditioning With Static Training-Dragon Door Publications (2020) - 120-146usman azharNo ratings yet
- Coal Bottom Ash As Sand Replacement in ConcreteDocument9 pagesCoal Bottom Ash As Sand Replacement in ConcretexxqNo ratings yet
- Final Test Level 7 New Format 2019Document3 pagesFinal Test Level 7 New Format 2019fabian serranoNo ratings yet
- Week - 2 Lab - 1 - Part I Lab Aim: Basic Programming Concepts, Python InstallationDocument13 pagesWeek - 2 Lab - 1 - Part I Lab Aim: Basic Programming Concepts, Python InstallationSahil Shah100% (1)
- Community Architecture Concept PDFDocument11 pagesCommunity Architecture Concept PDFdeanNo ratings yet
- Homeopatija I KancerDocument1 pageHomeopatija I KancermafkoNo ratings yet
- Ethical Conflicts in Psychology PDF DownloadDocument2 pagesEthical Conflicts in Psychology PDF DownloadAvory0% (2)
- Crafer. The Apocriticus of Macarius Magnes (S.P.C.K. Edition) - 1919.Document188 pagesCrafer. The Apocriticus of Macarius Magnes (S.P.C.K. Edition) - 1919.Patrologia Latina, Graeca et OrientalisNo ratings yet
- Days Papers 2001Document341 pagesDays Papers 2001jorgefeitoza_hotmailNo ratings yet
- Contemporary Philippine Arts From The Regions: Quarter 1Document11 pagesContemporary Philippine Arts From The Regions: Quarter 1JUN GERONANo ratings yet
- IOT Architecture IIDocument29 pagesIOT Architecture IIfaisul faryNo ratings yet