Professional Documents
Culture Documents
(A) Calculate: (B) What Percentage of The Acid Is Ionized in This 0.10
Uploaded by
Cybrille Fleur Siobhan QúeensOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
(A) Calculate: (B) What Percentage of The Acid Is Ionized in This 0.10
Uploaded by
Cybrille Fleur Siobhan QúeensCopyright:
Available Formats
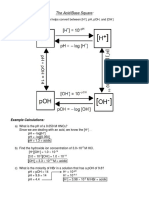
A 0.10 M solution of formic acid (HCHO2) has a pH of 2.
38 at 25°C
(a) Calculate Ka for formic acid at this temperature.
pH = - log [H+] = 2.38
log [H+] =-2.38
[H+] = 10 -2.38
= 4.1686 E-3
HCOOH H+ + HCOO-
Initial .1 M 0 0
C - 4.1686 E-3 M + 4.1686 E-3 M + 4.1686 E-3 M
E (0.1- 4.1686 E-3) M 4.1686 E-3 M 4.1686 E-3 M
Taking the dissociation as negligible-
( )( )
Ka= = 1.8 E-4
(b) What percentage of the acid is ionized in this 0.10 M solution?
% dissociation = (Concentration of H+/concentration of acid) x 100
= ( 4.1686 E-3/0.10 ) x 100 = 4. 16%
A 0.10 M solution of formic acid (HCHO2) has a pH of 2.50 at 25°C
(a) Calculate Ka for formic acid at this temperature.
pH = - log [H+] = 2.50
log [H+] =-2.50
[H+] = 10 -2.50
= 3.16227 E-3
HCOOH H+ + HCOO-
I .1 M 0 0
C - 3.16227 E-3 M + 3.16227 E-3 M + 3.16227 E-3 M
E (0.2- 3.16227 E-3) M 3.16227 E-3 M 3.16227 E-3 M
Taking the dissociation as negligible-
( )( )
Ka= = 1.0 x 10 -4
(b) What percentage of the acid is ionized in this 0.10 M
solution?
% dissociation = (Concentration of H+/concentration of acid) x 100
= ( 3.16227 E-3/0.10) x 100 = 3.16 %
A 0.10 M solution of formic acid (HCHO2) has a pH of 3.30 at 25°C
a) Calculate Ka for formic acid at this temperature.
pH = - log [H+] = 3.30
log [H+] =-3.30
[H+] = 10 -3.30
= 5.01187 E-4
HCOOH H+ + HCOO-
I .1 M 0 0
C - 5.01187 E-4 M + 5.01187 E-4 M + 5.01187 E-4 M
E (0.3- 5.01187 E-4) M 5.01187 E-4 M 5.01187 E-4 M
Taking the dissociation as negligible-
( )( )
Ka= = 2.5 E-6
(b) What percentage of the acid is ionized in this 0.10 M
solution?
% dissociation = (Concentration of H+/concentration of acid) x 100
= ( 5.01187 E-4/0.10 ) x 100 = 0.50 %
A 0.10 M solution of formic acid (HCHO2) has a pH of 3.2999999999999998at 25°C
(a) Calculate Ka for formic acid at this temperature.
pH = - log [H+] = 3.2999999999999998
log [H+] =-3.2999999999999998
[H+] 10 -3.2999999999999998 = 5.0118723362727209E-4
HCOOH H+ + HCOO-
.1 M 0 0
- 5.0118723362727209E-4 + +
M 5.0118723362727209E- 5.0118723362727209E-
4M 4M
(0.4- 5.0118723362727209E-
4) M 5.0118723362727209E- 5.0118723362727209E-
4M 4M
Taking the dissociation as negligible-
( )( )
Ka= =
2.511886431509578E-6
(b) What percentage of the acid is ionized in this 0.10 M
solution?
% dissociation = (Concentration of H+/concentration of acid) x 100
= ( 5.0118723362727209E-4/0.10000000000000001) x 100 =
You might also like
- Critical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsFrom EverandCritical Evaluation of Some Equilibrium Constants Involving Organophosphorus ExtractantsNo ratings yet
- Chapter 3 Answers 2019-2020Document11 pagesChapter 3 Answers 2019-2020Nuraina NabihahNo ratings yet
- Physical Organic Chemistry — 3: Plenary Lectures Presented at the Third IUPAC Conference on Physical Organic Chemistry, Montpellier, France, 6 - 10 September, 1976From EverandPhysical Organic Chemistry — 3: Plenary Lectures Presented at the Third IUPAC Conference on Physical Organic Chemistry, Montpellier, France, 6 - 10 September, 1976A. FruchierNo ratings yet
- Acid-Base Unit Review QuestionsDocument4 pagesAcid-Base Unit Review QuestionsSamia KabirNo ratings yet
- Compound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesFrom EverandCompound Forming Extractants, Solvating Solvents and Inert Solvents: Iupac Chemical Data SeriesNo ratings yet
- Buffer solutions and acid-base titrationsDocument6 pagesBuffer solutions and acid-base titrationsLê Hoàng MinhNo ratings yet
- Answer To Problem SolvingDocument15 pagesAnswer To Problem SolvingKitkatNo ratings yet
- Acid Base Problems SolutionsDocument20 pagesAcid Base Problems SolutionsldfwykbhnuklerNo ratings yet
- Acid Base Problems SolutionsDocument20 pagesAcid Base Problems SolutionsAnusha PatelNo ratings yet
- Chem 2206 Unit 2Document69 pagesChem 2206 Unit 2Danica Rose ZapanzaNo ratings yet
- IdkDocument6 pagesIdkDanice LunaNo ratings yet
- Chapter 7 Tutorial AnswerDocument11 pagesChapter 7 Tutorial Answernaderaqistina23No ratings yet
- CH 302 Worksheet 10 Simple Equilibria CalculationsDocument2 pagesCH 302 Worksheet 10 Simple Equilibria CalculationsAbiegail Asas PelenioNo ratings yet
- Acid-Base Unit Review Questions Answer KeyDocument3 pagesAcid-Base Unit Review Questions Answer KeySamia KabirNo ratings yet
- Chemistry Teach Yourself Series - Topic 1 - PHDocument11 pagesChemistry Teach Yourself Series - Topic 1 - PHMia FENTONNo ratings yet
- Additional Aspects of Acid-Base Equilibria: Practice ExamplesDocument57 pagesAdditional Aspects of Acid-Base Equilibria: Practice ExamplesJudith Del Valle MorejonNo ratings yet
- Buffers ExplainedDocument4 pagesBuffers ExplainedAlejandra Gonzalez RuizNo ratings yet
- EDTA Titration AnalysisDocument12 pagesEDTA Titration AnalysisTamar A. VasquezNo ratings yet
- Practice Quiz 2 ANSWER KEY 2017Document3 pagesPractice Quiz 2 ANSWER KEY 2017KennethTrucillaCortezNo ratings yet
- 17 Petrucci10e CSMDocument104 pages17 Petrucci10e CSMElah PalaganasNo ratings yet
- Tugas Kimia FarmasiDocument4 pagesTugas Kimia FarmasiLtifaNo ratings yet
- TugasDocument7 pagesTugastemizzhNo ratings yet
- Ques192 212abe2frDocument3 pagesQues192 212abe2frKerimberdiNo ratings yet
- Group3 Equilibrium KeyDocument8 pagesGroup3 Equilibrium KeyMelwyn FranciscoNo ratings yet
- CHEM 213 Chemical Analysis Exam 2 Monday October 25, 2004Document11 pagesCHEM 213 Chemical Analysis Exam 2 Monday October 25, 2004Alan BaggioNo ratings yet
- LogC PH Diagrams Diprotic AcidsDocument20 pagesLogC PH Diagrams Diprotic AcidsPhạm Yên KhangNo ratings yet
- Titration Complex Systems Acid BaseDocument11 pagesTitration Complex Systems Acid BaseGeorge AggelisNo ratings yet
- Chem 213 Chemical Analysis Final June 9, 2003Document10 pagesChem 213 Chemical Analysis Final June 9, 2003ramesh pokhrelNo ratings yet
- Resolução Atkins Capitulo 11 (Ímpares)Document40 pagesResolução Atkins Capitulo 11 (Ímpares)JaoJaoNo ratings yet
- Almuete 202-0125 Activity1Document9 pagesAlmuete 202-0125 Activity1Jonh Jester MallariNo ratings yet
- Acid-Base EquilibriaDocument31 pagesAcid-Base EquilibriaKim Fan100% (1)
- Worksheet 19 - Weak Acids and Bases: 2 4 2 0, C BX Form The of Equations For + +Document4 pagesWorksheet 19 - Weak Acids and Bases: 2 4 2 0, C BX Form The of Equations For + +Link 08769No ratings yet
- TSSM Topic 1Document11 pagesTSSM Topic 1sudotesterNo ratings yet
- Acid-Base Chemistry GuideDocument47 pagesAcid-Base Chemistry GuideSoniNo ratings yet
- 5 - EDTA Titrations 20130815Document12 pages5 - EDTA Titrations 20130815Husna Hafiza Bt. R.AzamiNo ratings yet
- Andre Anusta Barus (Chemistry Education 2010)Document42 pagesAndre Anusta Barus (Chemistry Education 2010)yasirhutapeaNo ratings yet
- HW8 Soln PDFDocument9 pagesHW8 Soln PDFPatricia de Leon100% (1)
- Chap 19Document4 pagesChap 19Jimini KimNo ratings yet
- Practice Test 2 Ad GGJDocument4 pagesPractice Test 2 Ad GGJjunomarsNo ratings yet
- Acid/Base Calculations: Strong vs WeakDocument9 pagesAcid/Base Calculations: Strong vs WeakGlen Mark MacarioNo ratings yet
- Acid-Base Group WorkDocument2 pagesAcid-Base Group WorkTebarek SitotawNo ratings yet
- PH CalculationsDocument4 pagesPH CalculationsVanandiNo ratings yet
- Exercises For Ionic Equilibria - Weak Acids and Bases-No AnswersDocument1 pageExercises For Ionic Equilibria - Weak Acids and Bases-No AnswersTerry Clarice Decatoria0% (1)
- WorkDocument31 pagesWorkBeauponte Pouky MezonlinNo ratings yet
- Assignment3 Solutions PDFDocument10 pagesAssignment3 Solutions PDFahmedNo ratings yet
- 2010 Stoichiometry Tut AnsDocument8 pages2010 Stoichiometry Tut AnsDomNo ratings yet
- 6 - Buffers, Common Ion and HHDocument34 pages6 - Buffers, Common Ion and HHKathryn Warner - Central Peel SS (2522)No ratings yet
- pH Measurement of Acid-Base BuffersDocument7 pagespH Measurement of Acid-Base BuffersAngel ManalotoNo ratings yet
- 41 To 60 Day 1Document6 pages41 To 60 Day 1Dominic Careo100% (1)
- Acid-Base FRQ 1970-2009 WTH AnsDocument37 pagesAcid-Base FRQ 1970-2009 WTH AnsbigNo ratings yet
- NCM 103 Final ExamDocument5 pagesNCM 103 Final ExamRichmond LacadenNo ratings yet
- ch19 - Lecture - 7e Ionic EquilibriaDocument88 pagesch19 - Lecture - 7e Ionic EquilibriabluemackerelNo ratings yet
- Homework 6 KeyDocument3 pagesHomework 6 Keychip_dale100% (1)
- Chemistry 101 Solutions for Acids and Bases AssignmentDocument3 pagesChemistry 101 Solutions for Acids and Bases AssignmentmikecostantiniNo ratings yet
- Cro Ag Cro S) 2 Ag (Aq) +CR O Aq) K K K KDocument4 pagesCro Ag Cro S) 2 Ag (Aq) +CR O Aq) K K K KCamiloNo ratings yet
- Ionic Equilibria in Aqueous SystemsDocument86 pagesIonic Equilibria in Aqueous SystemsDagnu DejeneNo ratings yet
- Lecture 9 - BuffersDocument12 pagesLecture 9 - BuffersKaizer NdoloNo ratings yet
- Chemistry Chapter 15 – Acid Base Equilibria in Solution – AP Questions ExplainedDocument10 pagesChemistry Chapter 15 – Acid Base Equilibria in Solution – AP Questions ExplainedSummaCumNo ratings yet
- Contoh Soal Buffer Kimia Kelas XI SMADocument3 pagesContoh Soal Buffer Kimia Kelas XI SMAMerry Br TariganNo ratings yet
- CHEM1070B - Assignment 4 KeyDocument7 pagesCHEM1070B - Assignment 4 Keymakabigail7No ratings yet
- ACID-BASE EQUILIBRIA (No Calculator)Document3 pagesACID-BASE EQUILIBRIA (No Calculator)Cybrille Fleur Siobhan QúeensNo ratings yet
- Stuff: Please Read Ahead and Don't Fall Behind, One Big Push at The End Will Help ManyDocument12 pagesStuff: Please Read Ahead and Don't Fall Behind, One Big Push at The End Will Help ManyCybrille Fleur Siobhan QúeensNo ratings yet
- Module 8 Integration Techniques Part 4 CHEM - 2Document20 pagesModule 8 Integration Techniques Part 4 CHEM - 2Cybrille Fleur Siobhan QúeensNo ratings yet
- Module 7 Trigonometric Integrals CHEM - 2Document33 pagesModule 7 Trigonometric Integrals CHEM - 2Cybrille Fleur Siobhan QúeensNo ratings yet
- MATH ANALYSIS 2 DEFINITE INTEGRALSDocument11 pagesMATH ANALYSIS 2 DEFINITE INTEGRALSCybrille Fleur Siobhan QúeensNo ratings yet
- Fe(s)|Fe2+(aq)||Cd2+(aq)|Cd(sDocument44 pagesFe(s)|Fe2+(aq)||Cd2+(aq)|Cd(sBagusprPrasetyoNo ratings yet
- Agrofood: Kajian Sifat Fisik Dan Organoleptik Penggunaan Tepung Jagung Pada Pembuatan Es Krim KelapaDocument5 pagesAgrofood: Kajian Sifat Fisik Dan Organoleptik Penggunaan Tepung Jagung Pada Pembuatan Es Krim KelapaTevania ShafiraNo ratings yet
- AnilineDocument5 pagesAnilinergNo ratings yet
- TDS OPT Accelerator 1-Aug15 PDFDocument1 pageTDS OPT Accelerator 1-Aug15 PDFTamer BidakNo ratings yet
- PVC LS100 by LGDocument1 pagePVC LS100 by LGabhaygupta1No ratings yet
- Operating the veltamat 3D RegulatorDocument11 pagesOperating the veltamat 3D Regulatorjamppajoo2No ratings yet
- Chemistry PHD Thesis FormatDocument5 pagesChemistry PHD Thesis Formatqrikaiiig100% (1)
- A2 Chemistry Coursework EvaluationDocument5 pagesA2 Chemistry Coursework Evaluationbcrqy80d100% (2)
- Presentation On Rayleighs Method of Dimensional AnalysisDocument13 pagesPresentation On Rayleighs Method of Dimensional Analysisabubakari meregulwaNo ratings yet
- Unit Two Acid-Base Equilibria: First Quarter Chemistry Test Two For Grade 12 Group ADocument7 pagesUnit Two Acid-Base Equilibria: First Quarter Chemistry Test Two For Grade 12 Group ANigatu MAmoNo ratings yet
- Rigorous Distillation Calculation For A Three-Component, Five-Stage Separation TowerDocument19 pagesRigorous Distillation Calculation For A Three-Component, Five-Stage Separation TowerAlex CoquisNo ratings yet
- Desmodur MAX-D XL1705Document2 pagesDesmodur MAX-D XL1705French CorvetteNo ratings yet
- Iso 8288 1986Document9 pagesIso 8288 1986Jim FrenkenNo ratings yet
- Chapter 6 Equilibrium ChemistryDocument28 pagesChapter 6 Equilibrium Chemistryinal arinalNo ratings yet
- Chemistry 1 11 Q2 M13Document14 pagesChemistry 1 11 Q2 M13Jessie CandawanNo ratings yet
- BASIS F31 Technical Data SheetDocument1 pageBASIS F31 Technical Data SheetVaittianathan MahavapillaiNo ratings yet
- BIOL 3090 Study Guide for Exam 1Document5 pagesBIOL 3090 Study Guide for Exam 1anhminhandnamNo ratings yet
- Major Process Equipment Selection and Sizing GuideDocument5 pagesMajor Process Equipment Selection and Sizing GuideMustafa AhsanNo ratings yet
- D Angelo Dongre 2009 Practical Use of Multiple Stress Creep and Recovery Test Characterization of Styrene ButadieneDocument10 pagesD Angelo Dongre 2009 Practical Use of Multiple Stress Creep and Recovery Test Characterization of Styrene Butadienebn23cem3r15No ratings yet
- Lydia 1-S2.0-S0022391320301669-MainDocument9 pagesLydia 1-S2.0-S0022391320301669-MainCherifNo ratings yet
- Stoichiometry Worksheet SolutionsDocument2 pagesStoichiometry Worksheet SolutionsQwert LimNo ratings yet
- Yr 12 Chemistry Holiday HomeworkDocument8 pagesYr 12 Chemistry Holiday HomeworkEsam ELNOAMANYNo ratings yet
- Lab Report Spectrophotometric and Potentiometric Determination The PH of An Unknown BufferDocument7 pagesLab Report Spectrophotometric and Potentiometric Determination The PH of An Unknown BufferMatthew GarnerNo ratings yet
- Spirit Academy Advanced Teaching Horizons Pairing Scheme Intermediate 2023 Biology Chemistry PhysicsDocument1 pageSpirit Academy Advanced Teaching Horizons Pairing Scheme Intermediate 2023 Biology Chemistry PhysicsAsif MureedNo ratings yet
- Manual Potenciometro Denver. OpMan - Basic - Meter PDFDocument24 pagesManual Potenciometro Denver. OpMan - Basic - Meter PDFVero VillarrealNo ratings yet
- 1996 Book EpisodesFromTheHistoryOfTheRarDocument255 pages1996 Book EpisodesFromTheHistoryOfTheRarBRUNA DA CUNHA PADOINNo ratings yet
- Euperlan PK 1200 UP - Eco-Friendly Pearl Wax Dispersion 2019-10Document1 pageEuperlan PK 1200 UP - Eco-Friendly Pearl Wax Dispersion 2019-10Marvin Dias SantosNo ratings yet
- Salt Analysis (Q.B.) 13thDocument6 pagesSalt Analysis (Q.B.) 13thRaju SinghNo ratings yet
- FRP Cable Tray - CatalogueDocument6 pagesFRP Cable Tray - CatalogueIshan PereraNo ratings yet
- RIA Final PDFDocument12 pagesRIA Final PDFAnjali SinghNo ratings yet