Professional Documents
Culture Documents
Cobalt: D. Nicholls, Complexes and First-Row Transition Elements © D. Nicholls 1974
Uploaded by
Pedro0 ratings0% found this document useful (0 votes)
19 views2 pagesOriginal Title
978-1-349-02335-6_16
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
19 views2 pagesCobalt: D. Nicholls, Complexes and First-Row Transition Elements © D. Nicholls 1974
Uploaded by
PedroCopyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 2
16 Cobalt
16.1 The Element
Cobalt compounds have been used in coloured glass for at least 4000 years but the
metal has been produced industrially only during this century. It is a widely
distributed but relatively uncommon element in the earth's crust. It occurs
biologically in vitamin B12 , which contains Co 3+ bonded octahedrally to five nitrogen
atoms (four from pyrroline rings and one from a benzimidazole ring) and the carbon
atom of a eN- group. The industrial extraction of the metal is usually an ancillary
process to the extraction of other metals such as copper and lead.
Cobalt (4s2 3d7 ) stands between iron and nickel in group VIII and above rhodium
and iridium. It shows high oxidation states even less willingly than iron, +3 being the
highest oxidatio:-- state of any significance. Cobalt resembles iron and nickel in
appearance, and like these metals it is ferromagnetic; it finds uses in a variety of steels
designed to have specific magnetic properties. The massive metal is oxidised in air
above 300° with the formation of Co 3 0 4 and CoO. Steam forms CoO at red heat.
Many nonmetals react when heated with cobalt; fluorine gives CoF 3 , the other
halogens giving CoX 2 . Cobalt is more resistant than iron to attack by mineral acids,
and it is not attacked by dilute alkalis.
16.2 Compounds of Cobalt(III) (d 6 )
The important oxidation states of cobalt in aqueous solution are +3 and +2. The
nature of the ligand bonded to cobalt has a dramatic effect on the stabilities of
these oxidation states. Simple compounds of cobalt(III) are strong oxidising agents,
and in water the [Co(H 2 0) 6 ] 3 + ion is a very powerful oxidising agent; it oxidises
water to oxygen
[Co(H20) 6 ]3+ + e -+ [Co(H20)6 j2+ (E' = +1.84 V)
However, other ligands, for example ammonia, stabilise the cobalt(III) state to varying
extents in aqueous solution
[Co(NH 3 ) 6 ]3+ + e -+ [Co(NH 3 ) 6 j2+ (E' = +0.11 V)
With some ligands, for example eN-, the stabilisation of the +3 state is so great that
the cobalt(II) complex reduces water to hydrogen
[Co(CN)6 j3- + e -+ [Co(CN) 5 j3- + CW (F = -0.8 V)
Further, the cobalt(II)-cyanide complex will even react with hydrogen to produce
what is regarded as a cobalt(III) complex
2[Co(CN) 5 ]3- + H2 -+ 2[Co(CN) 5 Hj3-
There is thus no simple aqueous chemistry of cobalt(III) but there is an
extensive range of complexes. For cobalt(II) the reverse is true; simple salts and
aqua-complexes are stable in aqueous solution but the addition of other ligands
makes for more facile conversion into the +3 state.
185
D. Nicholls, Complexes and First-Row Transition Elements
© D. Nicholls 1974
186 COMPLEXES AND FIRST-ROW TRANSITION ELEMENTS
16.2.1 Simple Compounds
Cobalt(III) fluoride. CoF 3 is a light-brown powder which reacts violently with
water with evolution of oxygen. It is obtained by the action of fluorine on the
heated cobalt(II) halides CoF 2 or CoC12 • It is a useful fluorinating agent; for
example, it converts hydrocarbons into fluorocarbons
4CoF 3 + -CH 2 - -+ -CF2 - + 4CoF 2 + 2HF
With N2 0 5 the green crystalline nitrate Co(N0 3 h is formed.
A blue hydrate CoF 3 .3.5H2 0 has been prepared by electrolytic oxidation of
CoF 2 in 40 per cent HF. It oxidises water like the simple fluoride.
The other cobalt(III) halides are unknown, cobalt(m) being too strong an
oxidising agent to exist solely with chloride, bromide, or iodide ions.
Cobalt(III) oxide. The oxidation of Co(OH) 2 in alkaline media gives a brown
precipitate of uncertain structure; it is best written as Co 2 0 3 .aq. When this is
dried at 150° the composition Co 2 0 3 .H2 0 is formed, which is probably CoO(OH).
On further heating to 300°, oxygen begins to be evolved (as well as water) and
black Co3 0 4 is formed. The dark-brown CoO(OH) is formed when Co(OH) 2 is
heated in air at 100°; it has a layer lattice in which each cobalt is surrounded
octahedrally by oxygen atoms.
Cobalt(m) sulphate. This is the most readily available cobalt(III) salt. The
hydrate Co2 (S0 4 h .18H2 0 is prepared by oxidation of cobalt(II) sulphate in
8N H2 S0 4 either electrolytically or with ozone or fluorine. It is stable in the dry
state but liberates oxygen from water. Some alums, for example KCo(S0 4 ) 2 .12H2 0,
can be crystallised from sulphuric acid solutions; they, like the hydrated sulphate,
are believed to contain the [Co(H 2 0) 6 ] 3 + ion. As well as being acidic (hydrolysis
to [Co(OH) (H 2 0) 5 ] 2 + occurs in O.IM perchloric acid), this ion is a strong
oxidising agent. Therefore no salts of organic anions such as formate, tar-
trate, and citrate can be obtained since these anions are oxidised to carbon
dioxide and water by [Co(H 2 0)6 p+. However, cobalt(m) acetate is believed to be
present in dilute acetic acid solutions of cobalt(II) acetate that have been oxidised
with ozone.
16.2.~ Complex Compounds
We discussed in chapter 1 the part played by cobalt(III) complexes in the historical
development of co-ordination chemistry. The fact that cobalt (III) forms an enor-
mous number of octahedral compiexes having the inert t 2 g6 configuration has
resulted in these being used extensively for rate and mechanistic studies on octa-
hedral substitution reactions (chapter 8).
Cobalt(III) has a great affinity for nitrogen donors especially ammonia, amines,
nitro -N0 2 , and -NCS groups. Other ligands such as water molecules, halide,
hydroxide, or carbonate ions may also be present in these complexes with
nitrogen donors, and various stereoisomers can often be isolated from such mixed
ligand systems. The preparation of these complexes usually involves the addition
of the nitrogen donor to a cobalt(ll) solution followed by oxidation with air or
hydrogen peroxide. Perhaps the most famous of these compounds are the
cobaltammines. The orange hexammines, containing the [Co(NH3 ) 6 ] 3+ ion, are
obtained from solutions of cobalt(II) salts in aqueous ammonia in the presence of
You might also like
- Cobalt: This Article Is About The Chemical Element. For Other Uses, SeeDocument28 pagesCobalt: This Article Is About The Chemical Element. For Other Uses, SeeChuck FernandezNo ratings yet
- Group III Elements-1Document10 pagesGroup III Elements-1Brian NyagaNo ratings yet
- BAI 4 ĐỒNG VÀ HỢP CHẤTDocument41 pagesBAI 4 ĐỒNG VÀ HỢP CHẤTLinhh ChiiNo ratings yet
- One Mark Questions: Subject: Chemistry Chapter - 11: P-Block ElementDocument13 pagesOne Mark Questions: Subject: Chemistry Chapter - 11: P-Block ElementudaysrinivasNo ratings yet
- Group 16 ElementsDocument40 pagesGroup 16 Elementstapas kunduNo ratings yet
- Cobalt MetalDocument6 pagesCobalt MetalzidaaanNo ratings yet
- Chemistry Class 11 The P Block Elements PDFDocument8 pagesChemistry Class 11 The P Block Elements PDFAnonymous vRpzQ2BLNo ratings yet
- 19 CarbonDocument7 pages19 CarbonRonak Raj RauniyarNo ratings yet
- P Block NotesDocument4 pagesP Block NotesKunalKumarSinghNo ratings yet
- P Block2012 457Document143 pagesP Block2012 457Abhishek Bansal100% (1)
- P Block2012 457Document145 pagesP Block2012 457AaravNo ratings yet
- Metal Carbonyls & Its Derivatives: Inorganic Chemistry (CHEM - 363) Atif - Zia@uos - Edu.pkDocument19 pagesMetal Carbonyls & Its Derivatives: Inorganic Chemistry (CHEM - 363) Atif - Zia@uos - Edu.pkMutiva YyNo ratings yet
- Chemistry of Carbon - Chem - f3 - v1 1Document25 pagesChemistry of Carbon - Chem - f3 - v1 1Lubanga N JamesNo ratings yet
- Lecture04c - Main-Group - Elements-PART3 - PenDocument48 pagesLecture04c - Main-Group - Elements-PART3 - PenAaf AbdRashidNo ratings yet
- Chemistry of CarbonDocument33 pagesChemistry of CarbonDavyieNo ratings yet
- Metal Pi Complexes - Dr. Archana Pandey PDFDocument56 pagesMetal Pi Complexes - Dr. Archana Pandey PDFdarshanNo ratings yet
- Carbon OxidesDocument12 pagesCarbon Oxidesprateek gangwaniNo ratings yet
- Carbon OxidesDocument12 pagesCarbon Oxidesprateek gangwaniNo ratings yet
- Notes On P-Block Elements GP 13 14Document10 pagesNotes On P-Block Elements GP 13 14Aditya Narayan50% (2)
- CHEMISTRY (XII) CHAPTER 03 (Group IIIA and IVA Elements Short QuestionsDocument6 pagesCHEMISTRY (XII) CHAPTER 03 (Group IIIA and IVA Elements Short QuestionsMajid HafeezNo ratings yet
- S-Block Elements & Their Compound - Done.p65Document6 pagesS-Block Elements & Their Compound - Done.p65Priyanshu SilNo ratings yet
- General Chemistry Part II 5 6Document109 pagesGeneral Chemistry Part II 5 6LUH EKA YANTHINo ratings yet
- Chemical Reactions and Equations With Answers Set 1Document6 pagesChemical Reactions and Equations With Answers Set 1Anjali JhaNo ratings yet
- Hsslive-Xi-Chem-Ch-11. P-Block Elements Q & ADocument8 pagesHsslive-Xi-Chem-Ch-11. P-Block Elements Q & A3093 Ayoob NNo ratings yet
- Ferrioxalate SystemDocument6 pagesFerrioxalate SystemRohit ChauhanNo ratings yet
- CLS JEEAD-19-20 XI Che Target-4 Level-1 Chapter-10 PDFDocument15 pagesCLS JEEAD-19-20 XI Che Target-4 Level-1 Chapter-10 PDFVinayNo ratings yet
- Group 4 Elements, Carbon, Silicon, Germanium, Tin and Lead: Physical PropertiesDocument18 pagesGroup 4 Elements, Carbon, Silicon, Germanium, Tin and Lead: Physical PropertiesPAUL KOLERE100% (1)
- Chlorine and Its CompoundsDocument19 pagesChlorine and Its Compoundskakembo hakimNo ratings yet
- Cobalt Metal: 191031003@duc - Edu.iqDocument10 pagesCobalt Metal: 191031003@duc - Edu.iqاديان كاظم جعفرNo ratings yet
- C Chemistry Synthesis Using Yttrium-Stabilized Catalyst: A ReviewDocument14 pagesC Chemistry Synthesis Using Yttrium-Stabilized Catalyst: A Reviewapi-3728640No ratings yet
- Physical Properties and Reactions of Period 3 OxidesDocument2 pagesPhysical Properties and Reactions of Period 3 OxidesShaNthini ManohaRan100% (1)
- Revision Notes Class 11 Chemistry Chapter 11 - The P-Block ElementsDocument44 pagesRevision Notes Class 11 Chemistry Chapter 11 - The P-Block ElementsDevashree NaikNo ratings yet
- Potassium: Physical PropertiesDocument6 pagesPotassium: Physical PropertiesJihad Ibrahim AYNo ratings yet
- Keep 511Document10 pagesKeep 511rajatguptNo ratings yet
- General PrincipalDocument6 pagesGeneral PrincipalthinkiitNo ratings yet
- Post MD TermDocument9 pagesPost MD TermmukunthaNo ratings yet
- Preparation of Metal CorbonylsDocument6 pagesPreparation of Metal Corbonylsyaqoob008No ratings yet
- GROUP 14 ELEMENTS (IVA Group Elements)Document8 pagesGROUP 14 ELEMENTS (IVA Group Elements)Premangshu GhoshalNo ratings yet
- ATOICV1 11 3 Important Reactions of Metal CarbonylsDocument12 pagesATOICV1 11 3 Important Reactions of Metal CarbonylsAYESHA MALIKNo ratings yet
- X ChemistryDocument5 pagesX Chemistrytejaswanigupta2011No ratings yet
- One MinuteDocument6 pagesOne MinuteEdy PurnomoNo ratings yet
- Some Atypical Properties of Beryllium Compounds - Chemistry LibretextsDocument6 pagesSome Atypical Properties of Beryllium Compounds - Chemistry Libretextsapi-368121935No ratings yet
- Boron GroupDocument37 pagesBoron GroupFidya Ahdiati UtamiNo ratings yet
- The P Block ElementsDocument4 pagesThe P Block ElementsAthulRKrishnanNo ratings yet
- Chemistry Investigatory ProjectDocument11 pagesChemistry Investigatory ProjectjujuNo ratings yet
- Class XI Chemistry Assignment On S and P - Block Elements PDFDocument4 pagesClass XI Chemistry Assignment On S and P - Block Elements PDFSadiq JavedNo ratings yet
- Chemistry of ChlorineDocument41 pagesChemistry of ChlorineKennedy ChitayiNo ratings yet
- 3d Transition MetalDocument2 pages3d Transition MetalDk Hazra HadzryaNo ratings yet
- 2023-24 Coordination CompoundsDocument36 pages2023-24 Coordination Compoundsthe Skulptor100% (1)
- Preparations and Properties of Mononuclear Metal Carbonyl CompoundsDocument18 pagesPreparations and Properties of Mononuclear Metal Carbonyl CompoundsVigyan Pravaha100% (3)
- Question Bank of Chapter 1Document4 pagesQuestion Bank of Chapter 1lovika malhotraNo ratings yet
- Metal-Π Complexes:: Metal Carbonyls: Structure and BondingDocument53 pagesMetal-Π Complexes:: Metal Carbonyls: Structure and BondingDonné van HeerdenNo ratings yet
- ATOICV1 11 1 Metal Carbonyls Structure and BondingDocument21 pagesATOICV1 11 1 Metal Carbonyls Structure and Bondingfa3814497No ratings yet
- Results and Discussion 11Document4 pagesResults and Discussion 11fengyuhengNo ratings yet
- The Boron Family & Its Physical and Chemical PropertiesDocument15 pagesThe Boron Family & Its Physical and Chemical PropertiesTr Mazhar PunjabiNo ratings yet
- Chem NotesDocument27 pagesChem NotesRaya DhanushNo ratings yet
- Class 10 Science Chapter 3 Previous Year Questions - Metals and Non-MetalsDocument24 pagesClass 10 Science Chapter 3 Previous Year Questions - Metals and Non-Metalsshaistudy1No ratings yet
- Iron Metabolism: From Molecular Mechanisms to Clinical ConsequencesFrom EverandIron Metabolism: From Molecular Mechanisms to Clinical ConsequencesRating: 5 out of 5 stars5/5 (1)
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
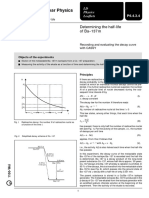
- Atomic and Nuclear Physics: Determining The Half-Life of Ba 137 MDocument4 pagesAtomic and Nuclear Physics: Determining The Half-Life of Ba 137 MPedroNo ratings yet
- Ra 010 D0ra03285aDocument6 pagesRa 010 D0ra03285aPedroNo ratings yet
- Franck-Hertz Experiment - NISERDocument7 pagesFranck-Hertz Experiment - NISERPedroNo ratings yet
- Electron Spin Resonance: Laboratory & Computational Physics 2Document15 pagesElectron Spin Resonance: Laboratory & Computational Physics 2PedroNo ratings yet
- LEP 5.1.12 Electron Spin Resonance: Related Topics ProblemsDocument5 pagesLEP 5.1.12 Electron Spin Resonance: Related Topics ProblemsPedroNo ratings yet
- Effect of Temperature and PH On The Tryptophan FluorescenceDocument19 pagesEffect of Temperature and PH On The Tryptophan FluorescencePedroNo ratings yet
- 30 580ln Fa08 PDFDocument14 pages30 580ln Fa08 PDFPedroNo ratings yet
- Journalof Pharmaceuticsans Drug Research 2020Document6 pagesJournalof Pharmaceuticsans Drug Research 2020PedroNo ratings yet
- Griffiths Cap3 Cinematica RelativistaDocument26 pagesGriffiths Cap3 Cinematica RelativistaPedroNo ratings yet
- Ferritic Stainless Steels: Soft MagneticDocument4 pagesFerritic Stainless Steels: Soft MagneticMohammadehsan SalarpourNo ratings yet
- Ferrite Content Measurement ProcedureDocument5 pagesFerrite Content Measurement ProcedureSenthil Periyasamy0% (1)
- Consumables For Inorganic Analysis Catalog WDocument60 pagesConsumables For Inorganic Analysis Catalog WTony C.No ratings yet
- As 3680-2008 Polyethylene Sleeving For Ductile Iron PipingDocument8 pagesAs 3680-2008 Polyethylene Sleeving For Ductile Iron PipingSAI Global - APACNo ratings yet
- Parkerizing 200: Technical BulletinDocument6 pagesParkerizing 200: Technical BulletinJony RahmanNo ratings yet
- 0620 CH 14 15 16 Past Papers 2020 2021Document20 pages0620 CH 14 15 16 Past Papers 2020 2021catdogdontusuckNo ratings yet
- Bridge Types - Historical Overviews - 2006 Pre1930metal PDFDocument18 pagesBridge Types - Historical Overviews - 2006 Pre1930metal PDFrobpallotNo ratings yet
- Cations: Ions and Charges Cations With Multiple ChargesDocument1 pageCations: Ions and Charges Cations With Multiple ChargesJohn Rey BayoguingNo ratings yet
- Lecture 3 Phyical Methods For Powder ProcessingDocument23 pagesLecture 3 Phyical Methods For Powder ProcessingguruNo ratings yet
- Atomizer Study For Processing PGMDocument6 pagesAtomizer Study For Processing PGMAFLAC ............No ratings yet
- Environment: KODAK Chemical Recovery CartridgesDocument12 pagesEnvironment: KODAK Chemical Recovery Cartridgesmarsbrown1No ratings yet
- Science: Quarter 2, WK 4 - Module 4Document36 pagesScience: Quarter 2, WK 4 - Module 4Ericha Solomon83% (6)
- 1 s2.0 S014181302201563X MainDocument7 pages1 s2.0 S014181302201563X Mainjulio cesar gurreonero fernandezNo ratings yet
- Theoretical Study of Electronic, Magnetic, and Structural Properties of α-Fe2O3 (Hematite)Document11 pagesTheoretical Study of Electronic, Magnetic, and Structural Properties of α-Fe2O3 (Hematite)Eder Ruiz HernandezNo ratings yet
- Astm A743 - A743m (98) (Ca15)Document2 pagesAstm A743 - A743m (98) (Ca15)ashsurya2008No ratings yet
- Fundamentals of Well Stimulation TechniqueDocument58 pagesFundamentals of Well Stimulation Techniquechemical todiNo ratings yet
- TTT Diagram Fe-C Phase DiagramDocument2 pagesTTT Diagram Fe-C Phase Diagramfarshid KarpasandNo ratings yet
- Model Answer Chapter 1 Final RevisionDocument67 pagesModel Answer Chapter 1 Final RevisionAhmed BasemNo ratings yet
- Cantidades de ObraDocument10 pagesCantidades de ObraAnderson Jota Romero MoralesNo ratings yet
- Comparison of Acid Mine Drainage Components in Botswana Coal MinesDocument57 pagesComparison of Acid Mine Drainage Components in Botswana Coal MinesGodfrey Moeng MonyakengNo ratings yet
- Fabrication of Silica Nanoparticles With Both Efficient Fluorescence and StrongDocument8 pagesFabrication of Silica Nanoparticles With Both Efficient Fluorescence and StrongAD DNo ratings yet
- Submersible Component PartsDocument1 pageSubmersible Component PartsFayez Al-ahmadiNo ratings yet
- Leach Precipitation and FlotationDocument4 pagesLeach Precipitation and FlotationMuhammad AndrianNo ratings yet
- Solidification of Iron Castings - 314983Document1 pageSolidification of Iron Castings - 314983Ankur PatelNo ratings yet
- HW 4Document3 pagesHW 4api-368121935No ratings yet
- Iron Steel Sector Report 2018 PDFDocument42 pagesIron Steel Sector Report 2018 PDFRam Deo AwasthiNo ratings yet
- Minerals and Energy Resources Padhai Ak Mazza Best Handwritten Notes 2023Document6 pagesMinerals and Energy Resources Padhai Ak Mazza Best Handwritten Notes 2023Prince SharmaNo ratings yet
- LUTH SCHOOL OF NURSING PAST QUESTIONS AND ANSWERS (Latest Copy)Document21 pagesLUTH SCHOOL OF NURSING PAST QUESTIONS AND ANSWERS (Latest Copy)Georges Abi JaoudeNo ratings yet
- Steelmaking PDFDocument228 pagesSteelmaking PDFVicbeau3No ratings yet