Professional Documents
Culture Documents
Kami Export - Worksheet - Reaction Rates PDF
Kami Export - Worksheet - Reaction Rates PDF
Uploaded by
Osher ShalomOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Kami Export - Worksheet - Reaction Rates PDF
Kami Export - Worksheet - Reaction Rates PDF
Uploaded by
Osher ShalomCopyright:
Available Formats
Name
Period Date
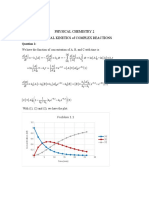
11.3 WORKSHEET – REACTION RATES
1. Which has a greater initial rate of disappearance? 3. Which chemical equation is consistent with this
graph? Explain briefly.
A or B Explain briefly.
(A) A B (B) B A
because it dropped down way (C) A 2B (D) B 2A
faster than A (E) 2A B (F) 2B A
concentration
concentration
B
time
B
2. This is a graph of the concentration of X over time.
time
concentration (M)
0.80 4. For the reaction C4H8 (g) 2 C2H4 (g), the
following data were collected:
0.60
0.40
Time (s) [C4H8] (mol/L)
0 1.000
0.20
10 0.913
0
0 1 2 3 4
20 0.835
time (s) 30 0.763
40 0.697
(a) At which time (t = 0 s, 1 s, 2 s, 3 s, or 4 s) is the 50 0.637
concentration of X the greatest (or are they the (a) What is the average rate of the reaction
same concentration)? Explain briefly. between 0 and 10 s? between 40 and 50 s?
if you look at the graph , you could
observe the fact that whenever the 0.087 less.
concentration reaches 0.8 it stays
the same .
(b) At which time (t = 0 s, 1 s, 2 s, 3 s, or 4 s) is the (b) What is the rate of formation of C2H4 between
rate of appearance of X the greatest (or is X 20 and 30 s?
changing at the same rate)? Explain briefly.
0.072
at 0.8 concentration the amount
stayed the same as time goes by so that is 5. For the reaction: Cl2 (g) + 3 F2 (g) 2 ClF3 (g)
the greatest point in this graph. ∆[Cl2]
∆t = ‒0.012 M/s
∆[F2]
(a) Find ∆t .
∆[ClF3]
(b) Find .
∆t
(c) Find Rate of Reaction.
You might also like
- Class 12 Chemistry Ch-3.Chemical KineticsDocument41 pagesClass 12 Chemistry Ch-3.Chemical Kineticskarnan karupiah0% (1)
- Unit and Dimensions Practice Problems (Physics)Document6 pagesUnit and Dimensions Practice Problems (Physics)Chandra Saini0% (2)
- Full Download Plates and Shells Theory and Analysis 4th Ugural Solution Manual PDF Full ChapterDocument36 pagesFull Download Plates and Shells Theory and Analysis 4th Ugural Solution Manual PDF Full Chaptergadere.prick.k2x7q100% (22)
- 13 Reaction Kinetics (S)Document32 pages13 Reaction Kinetics (S)Mr TanNo ratings yet
- S.S. Tutorials 2020-21: Student IDDocument3 pagesS.S. Tutorials 2020-21: Student IDSonuSharmaNo ratings yet
- Zero and First Order (Chemical Kinetics) : Assingment # 1 SyllabusDocument5 pagesZero and First Order (Chemical Kinetics) : Assingment # 1 Syllabusadityakumar krNo ratings yet
- SAMM HS15 Handout CharacteristicTimesDocument8 pagesSAMM HS15 Handout CharacteristicTimesSikanderMehmoodNo ratings yet
- 2020-21 S.S. Tutorials: Student ID Unit Test: 03 - ADocument3 pages2020-21 S.S. Tutorials: Student ID Unit Test: 03 - ASonuSharmaNo ratings yet
- Kinetic (Graphical Analysis) 21Document3 pagesKinetic (Graphical Analysis) 21滾滾滾滾滾滾No ratings yet
- 02 Chemical Kinetics II (Integrated Rate Law) Excel BatchDocument3 pages02 Chemical Kinetics II (Integrated Rate Law) Excel Batcharyanssharma235No ratings yet
- H2CO3 DissociationDocument3 pagesH2CO3 DissociationArisitica VinilescuNo ratings yet
- Adobe Scan 22 Nov 2023Document7 pagesAdobe Scan 22 Nov 2023avnishpratapsingh744No ratings yet
- Chemical KineticsDocument11 pagesChemical KineticsrajaNo ratings yet
- Chemical Kinetics - Chapter 14Document16 pagesChemical Kinetics - Chapter 14aniedorfNo ratings yet
- Average and Instantaneous Rates: Use The Following Table To Answer The Questions Regarding The Equation Below: A+BÆ2C+DDocument7 pagesAverage and Instantaneous Rates: Use The Following Table To Answer The Questions Regarding The Equation Below: A+BÆ2C+DMohit sadhNo ratings yet
- Modul Excellence Fizik 110222Document30 pagesModul Excellence Fizik 110222MOHAMMAD RAFIQ BIN MUIN MoeNo ratings yet
- Skema t4 TuitionDocument20 pagesSkema t4 TuitionshshshchinNo ratings yet
- Chemistry Answers PDFDocument126 pagesChemistry Answers PDFNurafiqah FarhaniNo ratings yet
- 0209 Physics Paper-With-Solution EveningDocument10 pages0209 Physics Paper-With-Solution EveningMom MomNo ratings yet
- Topic: Chemical Kinetic: DT) NH (D 2 DT) H (DDocument5 pagesTopic: Chemical Kinetic: DT) NH (D 2 DT) H (Dvictoria schoolNo ratings yet
- NEET States of Matter - Gases and Liquid Important QuestionsDocument28 pagesNEET States of Matter - Gases and Liquid Important QuestionsUjjwal KumarNo ratings yet
- Physics B.SCDocument2 pagesPhysics B.SCAkash Deep PandeyNo ratings yet
- FyuoDocument4 pagesFyuoshaunbphillips13No ratings yet
- Acjc H1 Phy P2 MSDocument5 pagesAcjc H1 Phy P2 MSTeh Kim Wee (Asrjc)No ratings yet
- Homework 9: Problem1Document12 pagesHomework 9: Problem1andres lopezNo ratings yet
- 4211 Solns 08Document7 pages4211 Solns 08Roy VeseyNo ratings yet
- Rates of Reaction Suroviec Spring 2014Document43 pagesRates of Reaction Suroviec Spring 2014enesffsNo ratings yet
- Chemical KineticsDocument4 pagesChemical KineticsSaurabh yadavNo ratings yet
- Chem Kinetic (Complex)Document5 pagesChem Kinetic (Complex)Trung VõNo ratings yet
- XII H 06 Chemical Kinetics - 64bbcfd208530Document18 pagesXII H 06 Chemical Kinetics - 64bbcfd208530aniketwadje8No ratings yet
- 1 Introduction The Spherical Collapse: Lectures On Effective Field Theory of Large Scale StructureDocument24 pages1 Introduction The Spherical Collapse: Lectures On Effective Field Theory of Large Scale StructureMatejaBoskovicNo ratings yet
- CPP12 Mixed Reactions-20220809124004923777Document6 pagesCPP12 Mixed Reactions-20220809124004923777Ashutosh SinghNo ratings yet
- Physical Chemistry DPP 112-20200420174656396309 PDFDocument47 pagesPhysical Chemistry DPP 112-20200420174656396309 PDFAnkit SharmaNo ratings yet
- Written Report in ChemistryDocument8 pagesWritten Report in Chemistrybunso padillaNo ratings yet
- Trig Test ANSDocument15 pagesTrig Test ANSJia VaishNo ratings yet
- Review: Nonideal Flow in A CSTRDocument49 pagesReview: Nonideal Flow in A CSTRFebrianti FitrianiNo ratings yet
- DPP23 Chemical KineticsDocument6 pagesDPP23 Chemical KineticsAbhishek SinglaNo ratings yet
- Chap 5 Collection Analysis of Rate DataDocument36 pagesChap 5 Collection Analysis of Rate DataSarah NeoSkyrerNo ratings yet
- Units and Dimensions - Practice Sheet - Arjuna JEE 3.0 2024Document3 pagesUnits and Dimensions - Practice Sheet - Arjuna JEE 3.0 2024technosumit18230No ratings yet
- Tejas: Practice Sheet JEE PhysicsDocument3 pagesTejas: Practice Sheet JEE PhysicsAshree KesarwaniNo ratings yet
- 1.0 Kinetics 2020 - 2021 (Lecturer)Document15 pages1.0 Kinetics 2020 - 2021 (Lecturer)siti aisyahNo ratings yet
- C Kinetics1Document5 pagesC Kinetics1Mahdiun MondolNo ratings yet
- 09 Gaseous State EngDocument5 pages09 Gaseous State EngMr XNo ratings yet
- DPPS-1 - Chemical KineticsDocument2 pagesDPPS-1 - Chemical KineticsHarsh Agarwal0% (1)
- Abstract: The Fungal Metabolite 3-Hydroxyanthranilic Acid (3-HAA) Was Used As A Redox MediatorDocument8 pagesAbstract: The Fungal Metabolite 3-Hydroxyanthranilic Acid (3-HAA) Was Used As A Redox MediatorhamzaabdalameerNo ratings yet
- Network Theory SolutionsDocument159 pagesNetwork Theory SolutionsSrijan DwivediNo ratings yet
- Physics: 25 July 2021 (SHIFT - 1) Question With AnswerDocument9 pagesPhysics: 25 July 2021 (SHIFT - 1) Question With Answereliezer hembromNo ratings yet
- Any Title: A. Partner, B. Partner, and C. Partner August 20, 2021Document4 pagesAny Title: A. Partner, B. Partner, and C. Partner August 20, 2021Pratiksha SinghNo ratings yet
- Exer 2Document1 pageExer 2prr.paragNo ratings yet
- 5 6070954144254919518Document32 pages5 6070954144254919518sujal thawareNo ratings yet
- MCD4390 Week 10 Tutorial QuestionsDocument5 pagesMCD4390 Week 10 Tutorial QuestionsGabbar100% (1)
- Chemistry QuestionDocument5 pagesChemistry QuestionPrithviraj GhoshNo ratings yet
- Indian School of PhysicsDocument2 pagesIndian School of PhysicsPrashant GargNo ratings yet
- Unit Dimensions and Measurement Practice Problem by Pristiadi UtomoDocument4 pagesUnit Dimensions and Measurement Practice Problem by Pristiadi UtomoPristiadi Utomo100% (3)
- Chemical KinematicsDocument3 pagesChemical KinematicsLyra GurimbaoNo ratings yet
- Plates and Shells Theory and Analysis 4th Ugural Solution ManualDocument36 pagesPlates and Shells Theory and Analysis 4th Ugural Solution Manualpirl.broom.sx9ir100% (43)
- Skill Builder Integrated Rate LawsDocument11 pagesSkill Builder Integrated Rate LawsBianca RolstonNo ratings yet
- MATH3331Document6 pagesMATH3331liamsk8dudeNo ratings yet
- On the Tangent Space to the Space of Algebraic Cycles on a Smooth Algebraic Variety. (AM-157)From EverandOn the Tangent Space to the Space of Algebraic Cycles on a Smooth Algebraic Variety. (AM-157)No ratings yet
- Gas Hydrates 1: Fundamentals, Characterization and ModelingFrom EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNo ratings yet