Professional Documents
Culture Documents
AGECHM LAB1 QFR For Expt #6
Uploaded by
Jasper Alicnas0 ratings0% found this document useful (0 votes)
7 views2 pagesThe document summarizes key aspects of solvent extraction including:
1) Five good extracting solvents (ethanol, acetone, toluene, chloroform, hexane) and the specific substances they can extract.
2) Five characteristics of a good extracting solvent including immiscibility, solubility, volatility, and toxicity.
3) Four factors that can affect extraction results: temperature/inert solutes, pH, ion-pair formation, and synergistic extraction. Temperature and pH can alter solubility while ion-pair formation and synergistic extraction lead to unexpected extractions.
Original Description:
Original Title
AGECHM LAB1 QFR for Expt #6
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document summarizes key aspects of solvent extraction including:
1) Five good extracting solvents (ethanol, acetone, toluene, chloroform, hexane) and the specific substances they can extract.
2) Five characteristics of a good extracting solvent including immiscibility, solubility, volatility, and toxicity.
3) Four factors that can affect extraction results: temperature/inert solutes, pH, ion-pair formation, and synergistic extraction. Temperature and pH can alter solubility while ion-pair formation and synergistic extraction lead to unexpected extractions.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
7 views2 pagesAGECHM LAB1 QFR For Expt #6
Uploaded by
Jasper AlicnasThe document summarizes key aspects of solvent extraction including:
1) Five good extracting solvents (ethanol, acetone, toluene, chloroform, hexane) and the specific substances they can extract.
2) Five characteristics of a good extracting solvent including immiscibility, solubility, volatility, and toxicity.
3) Four factors that can affect extraction results: temperature/inert solutes, pH, ion-pair formation, and synergistic extraction. Temperature and pH can alter solubility while ion-pair formation and synergistic extraction lead to unexpected extractions.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
ALICNAS, Jasper F.
September 24, 2022
QFR for Expt. #6
1. Enumerate five compounds that are good extracting solvents and give the specific
substance they can extract. Write your answers in a tabulated form.
COMPOUNDS THAT ARE GOOD SPECIFIC SUBSTANCE THAT THEY CAN
EXTRACTING SOLVENTS EXTRACT
1. Ethanol - Wine and whipped cream
2. Acetone - Resins, sulfur, mineral oils,
waxes, pine tar, some dyestuffs,
organic accelerators,
antioxidants, and peptizing
agents
3. Toluene - Crude petroleum
4. Chloroform - Nucleic acids from proteins and
lipids
5. Hexane - Extract more oil from oilseeds
compared to ethyl acetate and
ether.
2. Enumerate five characteristics of a good extracting solvent.
1) Immiscible pair of solvents: water and low polarity organic solvents.
2) Good solubility of the target compound.
3) Poor solubility of impurities.
4) Volatility of the extraction solvent.
5) Toxicity and safety properties of the extraction solvent.
3. Enumerate four factors that can affect the process of extraction and explain how
they can affect the results.
1) Effect of temperature and inert solutes.
Physical and chemical interactions can alter a solute's apparent partition
coefficient between two solvents. This must be considered at all costs
when selecting a proper extraction technique.
2) Effect of pH on extraction.
It has been established that organic acids and bases can exist in aqueous
solutions as equilibrium mixtures of their corresponding neutral and ionic
forms. Because these neutral and ionic forms might not have the same
equal partition coefficients in a second solvent, the amount of a chemical
that is extracted alone depends on the location of the acid-base
equilibrium and, ultimately, upon the pH of the resulting solution.
3) Effect of ion-pair formation.
Since ion-pair formation commonly leads to unexpected extractions, it
needs to be acknowledged properly. An ion-pair frequently arises in either
a polar or non-polar solvent since it is essentially a close interaction
between an anion and a cation.
4) Effect of synergistic extraction.
It may be defined as ‘the process whereby two different reagents when
employed together are capable of extracting a metal ion with a distinct
and marked efficiency, in comparison to a condition when the same two
reagents are used individually’.
https://www.brainkart.com/article/Factors-Influencing-Solvent-Extraction_30920/
https://labproinc.com/blogs/chemicals-and-solvents/hexane-an-effective-oil-extracting-
agent
https://wwwn.cdc.gov/TSP/MMG/MMGDetails.aspx?mmgid=157&toxid=29
http://www.chem.ualberta.ca/~orglabtutorials/Interactive%20Tutorials/separation/Theory/
theory6_2.htm
You might also like
- Advanced Method of Secondary Metabolite Extraction and Quality AnalysisDocument14 pagesAdvanced Method of Secondary Metabolite Extraction and Quality AnalysisroopashreeNo ratings yet
- Guidance On Essential OilsDocument18 pagesGuidance On Essential OilsJuanGuillermoCarmonaOcampoNo ratings yet
- Post Lab 26-31Document8 pagesPost Lab 26-31Christian Paulo D. PichayNo ratings yet
- All in One - 10th Class em - Fa-2Document35 pagesAll in One - 10th Class em - Fa-2sai ramxeroxNo ratings yet
- Chemical Formulations For Acrylic Matt and Acrylic Gloss PaintsDocument12 pagesChemical Formulations For Acrylic Matt and Acrylic Gloss PaintsDean HidayatNo ratings yet
- Influence of Base On Nitro-Aldol (Henry) Reaction Products For Alternative Clandestine PathwaysDocument11 pagesInfluence of Base On Nitro-Aldol (Henry) Reaction Products For Alternative Clandestine PathwaysYana PotemkinNo ratings yet
- TerpenoidsDocument17 pagesTerpenoidsSayli Sawant100% (1)
- Jurnal Oil 1Document11 pagesJurnal Oil 1Tri atikaNo ratings yet
- Fragrances: Jeanne Duus Johansen and Jean-Pierre LepoittevinDocument21 pagesFragrances: Jeanne Duus Johansen and Jean-Pierre LepoittevinJuanManuelAmaroLuisNo ratings yet
- Essential Oils in Food Processing: Chemistry, Safety and ApplicationsFrom EverandEssential Oils in Food Processing: Chemistry, Safety and ApplicationsSeyed Mohammed Bagher HashemiNo ratings yet
- Grade 9 Organic CompoundsDocument2 pagesGrade 9 Organic Compoundsking devesfruto82% (11)
- Plastic Compounding Masterbatches Pet and Other Plastic Processing IndustriesDocument3 pagesPlastic Compounding Masterbatches Pet and Other Plastic Processing Industrieshesham aldubaiNo ratings yet
- SEPARATION METHODS - AnimDocument58 pagesSEPARATION METHODS - AnimSandi Mahesa0% (1)
- Monophasic Dosage FormsDocument82 pagesMonophasic Dosage FormsJeeva RaviNo ratings yet
- Examination For Chemical Technician Checklist: KMPR - ChemistryDocument2 pagesExamination For Chemical Technician Checklist: KMPR - ChemistryK RiveraNo ratings yet
- 9 X October 2021Document5 pages9 X October 2021IJRASETPublicationsNo ratings yet
- Sem 3 Gtu B.pharm SyllabusDocument10 pagesSem 3 Gtu B.pharm SyllabusIshika RajputNo ratings yet
- Hydrocarbons and Functional Groups-: What's More What's NewDocument3 pagesHydrocarbons and Functional Groups-: What's More What's NewKIM Tae HYUNGNo ratings yet
- (Preformulation) : SeminarDocument54 pages(Preformulation) : SeminarAhmad AinurofiqNo ratings yet
- Poc 2 QuestionsDocument5 pagesPoc 2 Questionspradeep36No ratings yet
- Technical Presentation Report On FFDCDocument24 pagesTechnical Presentation Report On FFDCApurv RajNo ratings yet
- Unit 11Document54 pagesUnit 11amna.qadri60No ratings yet
- EJMCM - Volume 9 - Issue 7 - Pages 8448-8453 PDFDocument6 pagesEJMCM - Volume 9 - Issue 7 - Pages 8448-8453 PDFVijayakumarNo ratings yet
- PPT3 Minyak AtsiriDocument9 pagesPPT3 Minyak AtsiriDwii NurfadilahNo ratings yet
- LAB QO 5 - Separation of A MixtureDocument11 pagesLAB QO 5 - Separation of A MixturemarioNo ratings yet
- Sci 7 Q1 WK3 Compounds Lea Tomas 1Document6 pagesSci 7 Q1 WK3 Compounds Lea Tomas 1Joyce CarilloNo ratings yet
- Assignement 2Document13 pagesAssignement 2api-374903028No ratings yet
- Phytochemical Study of ResinDocument24 pagesPhytochemical Study of ResinRajesh KumarNo ratings yet
- Gang Chen, Jiao Lin, Weimin Hu, Chao Cheng, Xuefan Gu, Weichao Du, Jie Zhang, Chengtun QuDocument5 pagesGang Chen, Jiao Lin, Weimin Hu, Chao Cheng, Xuefan Gu, Weichao Du, Jie Zhang, Chengtun QuTaha Lemdjed BelahçeneNo ratings yet
- 1 - 2 Introduction of Phytochemisry IDocument20 pages1 - 2 Introduction of Phytochemisry IZNA EntertainmentNo ratings yet
- Fragrance With Antiaging Benefits: Naturals-Driven Functionality For Added ValueDocument6 pagesFragrance With Antiaging Benefits: Naturals-Driven Functionality For Added Valuejasminai_17No ratings yet
- Lab Manual Inorganic and Organic ChemistryDocument57 pagesLab Manual Inorganic and Organic ChemistryKashif NazirNo ratings yet
- Paper 1Document46 pagesPaper 1ade saputra TanjungNo ratings yet
- Ekstraksi 3Document13 pagesEkstraksi 3Mira Ria AndrianiNo ratings yet
- Yousefi2019 PDFDocument12 pagesYousefi2019 PDFkhudhoriNo ratings yet
- Supercritical Fluid Extraction of Essential OilsDocument12 pagesSupercritical Fluid Extraction of Essential OilsFrancisco Acosta100% (1)
- Effect of Phytochemical Constituents of Argemone Mexicana Leaf ExtractDocument6 pagesEffect of Phytochemical Constituents of Argemone Mexicana Leaf ExtractIJRASETPublicationsNo ratings yet
- 1235341970528041Document26 pages1235341970528041Widayat DayatNo ratings yet
- JETIR2204669Document6 pagesJETIR2204669Fekadu DagnawNo ratings yet
- PreviewpdfDocument44 pagesPreviewpdfVeneta GizdakovaNo ratings yet
- Solutions - Active Pharmaceutical IngredientsDocument4 pagesSolutions - Active Pharmaceutical IngredientsMXLTRNo ratings yet
- Nano Size Range by Chemical MethodDocument1 pageNano Size Range by Chemical MethodMilan VadodariaNo ratings yet
- Surfactants and Their Use in Latex Technology: January 2013Document5 pagesSurfactants and Their Use in Latex Technology: January 2013herryNo ratings yet
- J of Applied Polymer Sci - 2003 - Kuriakose - Use of Rice Bran Oil and Epoxidized Rice Bran Oil in Carbon Black FilledDocument9 pagesJ of Applied Polymer Sci - 2003 - Kuriakose - Use of Rice Bran Oil and Epoxidized Rice Bran Oil in Carbon Black Filledjaswanth kumarNo ratings yet
- Secondary Metabolyte - Organic Compound AnalysisDocument12 pagesSecondary Metabolyte - Organic Compound AnalysisSisilia Anabina TariganNo ratings yet
- Epoxidation of Some Vegetable Oils and Their Hydrolysed Products With Peroxyformic Acid - Optimised To Industrial ScaleDocument13 pagesEpoxidation of Some Vegetable Oils and Their Hydrolysed Products With Peroxyformic Acid - Optimised To Industrial ScaleNovrilia Dwi PermatasariNo ratings yet
- Smith and Parsons, 1973Document3 pagesSmith and Parsons, 1973aaNo ratings yet
- Trịnh Huy Cường Đặng Nguyễn Minh Khôi: Natural ProductsDocument47 pagesTrịnh Huy Cường Đặng Nguyễn Minh Khôi: Natural ProductsAndré Luiz Meleiro PortoNo ratings yet
- Topic 10 - Organic ChemistryDocument102 pagesTopic 10 - Organic ChemistryLucia PesentiNo ratings yet
- EJCHEM - Volume 65 - Issue 5 - Pages 419-433Document15 pagesEJCHEM - Volume 65 - Issue 5 - Pages 419-433Maira IsmailNo ratings yet
- Applsci 12 10355Document17 pagesApplsci 12 10355Manoel LourençoNo ratings yet
- Preformulation Testing of Dosage FormsDocument101 pagesPreformulation Testing of Dosage FormsEndmotions07No ratings yet
- Studies On Interfacial Properties of Natural Surfactants and Its ApplicationsDocument56 pagesStudies On Interfacial Properties of Natural Surfactants and Its Applicationsmkray0No ratings yet
- Selection SurfactantDocument7 pagesSelection Surfactantabhis_gupta08No ratings yet
- Synthesis and Interfacial Properties of Bio-Based Zwitterionic Surfactants Derived From Different Fatty Acids in Non-Edible Vegetable OilsDocument13 pagesSynthesis and Interfacial Properties of Bio-Based Zwitterionic Surfactants Derived From Different Fatty Acids in Non-Edible Vegetable OilsSanjay singhNo ratings yet
- Lecture No 70, 71 Formulation of Semi Solids and Gels and JelliesDocument34 pagesLecture No 70, 71 Formulation of Semi Solids and Gels and JelliesAdinath ShirsatNo ratings yet
- CosmeticsDocument27 pagesCosmeticsShivangi VermaNo ratings yet
- Study of Oxidative Stability of Lubricants Blended With P-Substituted Phenolic AntioxidantsDocument8 pagesStudy of Oxidative Stability of Lubricants Blended With P-Substituted Phenolic AntioxidantsJohn BenderNo ratings yet
- Crompton1998 PDFDocument523 pagesCrompton1998 PDFMaria Inês Vasconcellos FurtadoNo ratings yet
- PCG 311.1 PDFDocument17 pagesPCG 311.1 PDFNigel ZaraNo ratings yet
- Syllabus - B - Pharmacy - 2017-18 (3-8TH SEM)Document77 pagesSyllabus - B - Pharmacy - 2017-18 (3-8TH SEM)Turn it OnNo ratings yet
- Cacorr2 Act1Document2 pagesCacorr2 Act1Jasper AlicnasNo ratings yet
- AGECHM LAB1 QFR For Expt. #7Document2 pagesAGECHM LAB1 QFR For Expt. #7Jasper AlicnasNo ratings yet
- Agechm 3-11-22Document1 pageAgechm 3-11-22Jasper AlicnasNo ratings yet
- 33Document1 page33Jasper AlicnasNo ratings yet
- 22Document1 page22Jasper AlicnasNo ratings yet
- 11Document1 page11Jasper AlicnasNo ratings yet
- AddBond LTWDocument1 pageAddBond LTWb3ry 17No ratings yet
- CWC TrainingDocument58 pagesCWC Trainingbalaji3110100% (1)
- Sterilisation and DisinfectionDocument11 pagesSterilisation and DisinfectionVaishali PrasharNo ratings yet
- United States: Patent OfficeDocument3 pagesUnited States: Patent OfficeIRIENE DELFITA TKIMNo ratings yet
- Assignment: Importance of Biotransformation in The Human BodyDocument2 pagesAssignment: Importance of Biotransformation in The Human BodyMD REFATNo ratings yet
- Cy1104 - Engineering Chemistry Unit - 4 Fuels and Combustion Lecture PlanDocument22 pagesCy1104 - Engineering Chemistry Unit - 4 Fuels and Combustion Lecture PlanBeuna.No ratings yet
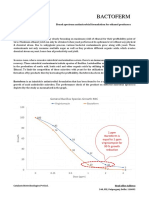
- BactofermDocument3 pagesBactofermPankaj RajbharNo ratings yet
- Chapter 7 Chemical Reactions Review Answer KeyDocument3 pagesChapter 7 Chemical Reactions Review Answer KeyRachelle QuiambaoNo ratings yet
- HydrocarbonDocument25 pagesHydrocarbonSoham NagNo ratings yet
- Citizens Protein Project A Self Funded,.15Document7 pagesCitizens Protein Project A Self Funded,.15na09b042No ratings yet
- Quotation BPEFT-FT-Q2009-83-1Document3 pagesQuotation BPEFT-FT-Q2009-83-1Has platiniumNo ratings yet
- Corning Cellgro-Catalog - Lista de Medios y FormulasDocument74 pagesCorning Cellgro-Catalog - Lista de Medios y FormulasDiego CompairedNo ratings yet
- Choking Agents and Their Countermeasures-2018 - 0Document1 pageChoking Agents and Their Countermeasures-2018 - 0Rajib AminNo ratings yet
- Test Moles and EquilibriaDocument2 pagesTest Moles and Equilibrianaeem mushtaqNo ratings yet
- Biochem-LabDocument76 pagesBiochem-LabannNo ratings yet
- Bioplastics IndiaDocument6 pagesBioplastics IndiaSanjana ShedgeNo ratings yet
- Research Proposal by Raniel FuerteDocument7 pagesResearch Proposal by Raniel FuerteGwenneth BrilloNo ratings yet
- Merged 429 1-7Document17 pagesMerged 429 1-7shubhamNo ratings yet
- Dunman High School Preliminary Examination 2018 Year 6 H2 ChemistryDocument18 pagesDunman High School Preliminary Examination 2018 Year 6 H2 ChemistryYao Le Titanium ChenNo ratings yet
- Nullifire SC902 - Safety Data Sheet Part A V9.0Document9 pagesNullifire SC902 - Safety Data Sheet Part A V9.0ANIBAL LOPEZNo ratings yet
- Acidity of Benzene Toluene Xylenes Solvent Naphthas Astm d847Document3 pagesAcidity of Benzene Toluene Xylenes Solvent Naphthas Astm d847Iraílson MatosNo ratings yet
- ORGANOMETALLIC TEST 2 50 New Questions OMCDocument16 pagesORGANOMETALLIC TEST 2 50 New Questions OMCLuCaNo ratings yet
- Role of Acidifiers in Livestock Nutrition and Health: A ReviewDocument12 pagesRole of Acidifiers in Livestock Nutrition and Health: A ReviewMaamar AmamraNo ratings yet
- Capstone MicroplasticsDocument52 pagesCapstone MicroplasticsChristian LimbaroNo ratings yet
- Company Name: C10 C10 C11 C12 C13 C14 C14Document1 pageCompany Name: C10 C10 C11 C12 C13 C14 C14Md Nazim UddinNo ratings yet
- Zinc Oxide Nanoparticle Incorporated On Graphene Oxide - An Efficient and Stable Photocatalyst For Water Treatment Through The Fenton ProcessDocument12 pagesZinc Oxide Nanoparticle Incorporated On Graphene Oxide - An Efficient and Stable Photocatalyst For Water Treatment Through The Fenton ProcessSenmarNo ratings yet
- AFCONA - 3700 TDS EngDocument1 pageAFCONA - 3700 TDS EngSantosh RajNo ratings yet
- Bleach and Incompatible FactSheet LSP 20 116Document4 pagesBleach and Incompatible FactSheet LSP 20 116Training & Recruitment GSINo ratings yet