Professional Documents
Culture Documents
Homework 3
Uploaded by
soph0 ratings0% found this document useful (0 votes)
3 views5 pagesThe document contains 5 multiple choice questions about organic chemistry reactions including Friedel-Crafts reactions, electrophilic aromatic substitution, and nucleophilic aromatic substitution. The questions test understanding of reaction mechanisms, directing effects of functional groups, and identification of reaction intermediates.
Original Description:
有機
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document contains 5 multiple choice questions about organic chemistry reactions including Friedel-Crafts reactions, electrophilic aromatic substitution, and nucleophilic aromatic substitution. The questions test understanding of reaction mechanisms, directing effects of functional groups, and identification of reaction intermediates.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
3 views5 pagesHomework 3
Uploaded by
sophThe document contains 5 multiple choice questions about organic chemistry reactions including Friedel-Crafts reactions, electrophilic aromatic substitution, and nucleophilic aromatic substitution. The questions test understanding of reaction mechanisms, directing effects of functional groups, and identification of reaction intermediates.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You are on page 1of 5
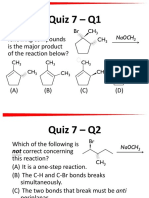
Quiz 3 – Q1
Which of the following compounds would
most likely be used in Friedel-Craft
reaction without skeletal rearrangement?
(A) (CH3)2CHCOCl
(B) (CH3)2CHCH2Cl
(C) (CH3)2CHCH2Br
(D) CH3CH2CH2CH2Cl
Quiz 3 – Q2
Which of the following compounds will
undergo Friedel-Crafts alkylation with
(CH3)3CCl and AlCl3 most rapidly?
(A) Toluene (C6H5-CH3)
(B) Iodobenzene (C6H5-I)
(C) Benzenesulfonic acid (C6H5-SO3H)
(D) Cyanobenzene (C6H5-CN)
Quiz 3 – Q3
In electrophilic aromatic substitution reactions
the hydroxyl group is an o,p-director because
it donates electron density to the ring by
_______ and stabilizes ______ transition
state(s).
(A) induction; ortho and para
(B) resonance; meta
(C) resonance; ortho and para
(D) induction; meta
Quiz 3 – Q4
In the addition of an electrophile to
acetophenone (C6H5-CO-CH3), we expect that
the _______ positions are more _______ for
the attack by the electrophile.
(A) ortho, para; activated
(B) meta; activated
(C) ortho, para; deactivated
(D) meta; deactivated
Quiz 3 – Q5
Which of the following is an intermediate when
1,2-dibromo-4-nitrobenzene is heated with NaOH
in a nucleophilic aromatic substitution reaction?
(A) (B) (C) (D)
You might also like
- All India Test Series For Iit-JeeDocument16 pagesAll India Test Series For Iit-JeeApex Institute100% (1)
- 09-Final With SolutionsDocument27 pages09-Final With SolutionsDanielle Wood100% (2)
- Alcohols, Phenols Ethers: Multiple Choice QuestionsDocument30 pagesAlcohols, Phenols Ethers: Multiple Choice QuestionsUrja MoonNo ratings yet
- 235practice Exam 2 AnswerDocument9 pages235practice Exam 2 Answernbobs7No ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Class 12 Chem QBDocument160 pagesClass 12 Chem QBRohit KumarNo ratings yet
- Entry Test Chemistry Answer KeyDocument4 pagesEntry Test Chemistry Answer KeyMudassir HussainNo ratings yet
- Haloalkanes and Haloarenes Question BankDocument16 pagesHaloalkanes and Haloarenes Question BankBrown HustlerNo ratings yet
- Organic 11th Level 3Document2 pagesOrganic 11th Level 3Chinmay YadavNo ratings yet
- 09 Oct 2021 Chemistry BJ9jLJEDocument4 pages09 Oct 2021 Chemistry BJ9jLJEMore SmithaNo ratings yet
- MCQ, Case-Case Study and Assertion Reason Question Bank For Periodic Assessment 1Document42 pagesMCQ, Case-Case Study and Assertion Reason Question Bank For Periodic Assessment 1Mohammed AmmaarNo ratings yet
- This Test Contains A Total of 15 Objective Type Questions. Each Question Carries 1 Mark. There Is NO NEGATIVE MarkingDocument8 pagesThis Test Contains A Total of 15 Objective Type Questions. Each Question Carries 1 Mark. There Is NO NEGATIVE MarkingvarunkohliinNo ratings yet
- Cls Jeead-16-17 Xii Che Target-7 Set-2 Chapter-11Document38 pagesCls Jeead-16-17 Xii Che Target-7 Set-2 Chapter-11Neeru SehgalNo ratings yet
- Class 12 Chemistry Sample PaperDocument8 pagesClass 12 Chemistry Sample Paperabhinavdahiya77No ratings yet
- Chemistry SQP 1Document8 pagesChemistry SQP 1Purnima PandaNo ratings yet
- Chemistry, Mathematics & Physics: Class IIT-JEE April, 2010 Solution To Paper I Marks PatternDocument26 pagesChemistry, Mathematics & Physics: Class IIT-JEE April, 2010 Solution To Paper I Marks PatternSURAJ SINGHNo ratings yet
- Chapter13 (11th Ed) Practice ProblemsDocument22 pagesChapter13 (11th Ed) Practice ProblemslianahajjNo ratings yet
- MCQ - Halo Alkanes& Haloarenes: Velammal Vidyalaya-Viraganoor Sub-Xii ChemistryDocument12 pagesMCQ - Halo Alkanes& Haloarenes: Velammal Vidyalaya-Viraganoor Sub-Xii ChemistryKrishna Moorthy RamaiahNo ratings yet
- Haloalkanes and Haloarenes Class 12 MCQs Questions With AnswersDocument8 pagesHaloalkanes and Haloarenes Class 12 MCQs Questions With AnswersThrik esh100% (1)
- Dec 2013Document90 pagesDec 2013sridharR hahahaNo ratings yet
- Chemistry XII Pre Board II Paper (2023-2024)Document10 pagesChemistry XII Pre Board II Paper (2023-2024)leothiveshNo ratings yet
- HaloDocument17 pagesHaloadityakatariya157No ratings yet
- Aieee 2009 Model Paper 1Document7 pagesAieee 2009 Model Paper 1Vicky_Munnetul_7889No ratings yet
- Cls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Document34 pagesCls Jeead-17-18 Xi Che Target-4 Set-2 Chapter-13Jotiraj Parihar50% (2)
- Aldehyde J Ketone Amd Carboxylic MCQDocument5 pagesAldehyde J Ketone Amd Carboxylic MCQSaransh KumarNo ratings yet
- Haloalkanes and Haloarenes Class 12 Chemistry MCQs PDFDocument33 pagesHaloalkanes and Haloarenes Class 12 Chemistry MCQs PDFSanjana Sanjay100% (1)
- 11-JEE-Adv Grand Test 11 Question Paper (P 1) - 18-05-2014Document18 pages11-JEE-Adv Grand Test 11 Question Paper (P 1) - 18-05-2014Ranjan PrasadNo ratings yet
- Xii Chem QPDocument6 pagesXii Chem QPnrusinghsamal2006No ratings yet
- University of Bahri Second Year Second Semester Main Exam 2015 Organic Reaction Type Date: / /2015 Time Allowed: 3 HrsDocument5 pagesUniversity of Bahri Second Year Second Semester Main Exam 2015 Organic Reaction Type Date: / /2015 Time Allowed: 3 HrsmanafadulNo ratings yet
- Maharashtra SET Exam Paper II Chemical Science Question Paper November 2011Document15 pagesMaharashtra SET Exam Paper II Chemical Science Question Paper November 2011pednekarprakashNo ratings yet
- Kvs Sample Paper Chemistry Page 2 - 6Document5 pagesKvs Sample Paper Chemistry Page 2 - 6Rohan BaghelNo ratings yet
- Lifescience GATEDocument35 pagesLifescience GATEhaleemaayubNo ratings yet
- Kendriya Vidyalaya Sanghthan, Ahmedabad Region SAMPLE PAPER (2022-23) Chemistry Theory (043) MM:70 Time: 3 HoursDocument8 pagesKendriya Vidyalaya Sanghthan, Ahmedabad Region SAMPLE PAPER (2022-23) Chemistry Theory (043) MM:70 Time: 3 Hoursharsh.mahori09No ratings yet
- 15 CHEMISTRY Some Basic Principles & Techniques HydrocarbonsDocument3 pages15 CHEMISTRY Some Basic Principles & Techniques HydrocarbonsHasan shaikhNo ratings yet
- Class 12chemistry - Haloalkanes and Haloarenes - McqsDocument22 pagesClass 12chemistry - Haloalkanes and Haloarenes - McqsDivyam Garg100% (1)
- Class Xii Chemistry Mcqs and Assertion Reason Questions Feb 24Document43 pagesClass Xii Chemistry Mcqs and Assertion Reason Questions Feb 24Soumya PNo ratings yet
- 12 04 14 SR - Iplco Chemistry Assignment 2Document6 pages12 04 14 SR - Iplco Chemistry Assignment 2Gadde Gopala Krishna0% (1)
- 11 Cbse Chemistry Organic ChemistryDocument22 pages11 Cbse Chemistry Organic ChemistryKrish KakkarNo ratings yet
- Organic ChemistryDocument45 pagesOrganic ChemistryAnubhav Sinha0% (1)
- Class XI Chem SAMPLEDocument4 pagesClass XI Chem SAMPLEFIITJEE DPSNo ratings yet
- WS Class 11 Org ChemDocument4 pagesWS Class 11 Org ChemJavedNo ratings yet
- Iitjee Chemistry Sample Paper - IDocument7 pagesIitjee Chemistry Sample Paper - IdharamtanujNo ratings yet
- A (2e, 4e) B (2Z, 4Z) C (2Z, 4e) D (2e, 4Z)Document1 pageA (2e, 4e) B (2Z, 4Z) C (2Z, 4e) D (2e, 4Z)Agatha chilesheNo ratings yet
- Class 12 QDocument8 pagesClass 12 QR.KABILANNo ratings yet
- CBSE Class 12 Chemistry 14 Apr Sample Paper 2023 24Document8 pagesCBSE Class 12 Chemistry 14 Apr Sample Paper 2023 24aknishad71385No ratings yet
- CLS Aipmt-18-19 XII Che Study-Package-7 SET-2 Chapter-12 PDFDocument44 pagesCLS Aipmt-18-19 XII Che Study-Package-7 SET-2 Chapter-12 PDFJagnesh BhardwajNo ratings yet
- Revision 1 Chemistry Class 12Document6 pagesRevision 1 Chemistry Class 12saravanan.gNo ratings yet
- Orgsynth KeysDocument27 pagesOrgsynth KeysDebalina DassNo ratings yet
- Chemistry Practice Question Paper Class 12Document7 pagesChemistry Practice Question Paper Class 12tony starkNo ratings yet
- QP - Sol - NSEC 2012-13Document10 pagesQP - Sol - NSEC 2012-13Vardaan Bhatnagar100% (1)
- 11-Chemistry-A1-Annual Exam 2023-24Document8 pages11-Chemistry-A1-Annual Exam 2023-24harshitsharmasportsNo ratings yet
- CLASS 12 Chemistry-PQDocument24 pagesCLASS 12 Chemistry-PQJeremiah ShibuNo ratings yet
- Chemistry PQDocument13 pagesChemistry PQAman SilayachNo ratings yet
- Class 12 - HHDocument74 pagesClass 12 - HHgujjarvikram123456No ratings yet
- 2024 Set 2Document23 pages2024 Set 2Manab GhoshalNo ratings yet
- XIIth ChemistryDocument7 pagesXIIth ChemistryRiya MalikNo ratings yet
- Organic Chemistry: Section A: Straight Objective TypeDocument26 pagesOrganic Chemistry: Section A: Straight Objective TypeAmarNo ratings yet
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972From EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNo ratings yet
- Metallabenzenes: An Expert ViewFrom EverandMetallabenzenes: An Expert ViewL. James WrightNo ratings yet
- Homework 7Document5 pagesHomework 7sophNo ratings yet
- Homework 5Document5 pagesHomework 5sophNo ratings yet
- Homework 6Document5 pagesHomework 6sophNo ratings yet
- Homework 4Document5 pagesHomework 4sophNo ratings yet
- Bb. B BB BBBB BBB BB B Abbb A Doczzlg Utf OihuhfytdyrstsDocument1 pageBb. B BB BBBB BBB BB B Abbb A Doczzlg Utf OihuhfytdyrstssophNo ratings yet