Professional Documents
Culture Documents
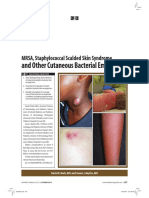
Staphylococcal Scalded Skin Syndrome
Staphylococcal Scalded Skin Syndrome
Uploaded by
M Daffa Abhista ReviansyahOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Staphylococcal Scalded Skin Syndrome
Staphylococcal Scalded Skin Syndrome
Uploaded by
M Daffa Abhista ReviansyahCopyright:
Available Formats
See discussions, stats, and author profiles for this publication at: https://www.researchgate.
net/publication/51327036
Staphylococcal scalded skin syndrome
Article in Archives of Disease in Childhood · January 1998
DOI: 10.1136/adc.78.1.85 · Source: PubMed
CITATIONS READS
70 2,253
2 authors:
Shamez Ladhani Robert W Evans
Public Health England 140 PUBLICATIONS 6,097 CITATIONS
536 PUBLICATIONS 21,168 CITATIONS
SEE PROFILE
SEE PROFILE
Some of the authors of this publication are also working on these related projects:
Therapeutic recommendations in HFE hemochromatosis: specialist-patient knowledge transfer View project
Point of Care Diagnostics View project
All content following this page was uploaded by Robert W Evans on 15 August 2014.
The user has requested enhancement of the downloaded file.
Arch Dis Child 1998;78:85–88 85
CURRENT TOPIC
Staphylococcal scalded skin syndrome
Shamez Ladhani, Robert W Evans
The disease Potential problems with antibiotic resistance
Staphylococcal scalded skin syndrome (SSSS) have also recently arisen with the isolation of a
is the clinical term used for a spectrum of blis- methicillin resistant toxin producing strain of S
tering skin diseases induced by the exfoliative aureus in a case of SSSS in Japan.12
(epidermolytic) toxins (ET) of Staphylococcus
aureus.1 Current synonyms include Ritter’s dis-
ease, bullous impetigo, pemphigus neonato-
rum, and staphylococcal scarlatiniform rash. It The toxins
is a disease primarily aVecting infants and The role of the exfoliative toxins in SSSS was
young children,2 but cases have been reported demonstrated as far back as 1970,1 but their
in adults.3 It seems that the location of lesions mechanism of action remains elusive 25 years
depends on age. In neonates, the lesions are later, even though their physicochemical prop-
mostly found on the perineum or periumbili- erties and, more recently, aspects of their mode
cally, or both, while the extremities are more of action have been extensively studied. The
commonly aVected in older children.2 The dis- two known toxins (ETA and ETB) act specifi-
ease begins with erythema and fever, followed cally in the zona granulosa of the epidermis,13
by formation of large fluid filled bullae which and even intraperitoneal inoculation will result
quickly rupture on slightest pressure (Nikolsky in exfoliation. Immunocytochemical studies
sign) to leave extensive areas of denuded skin. have shown that the toxin binds to the filaggrin
The form and severity of SSSS will vary with group of proteins in keratohyalin granules,14
the route of delivery of the toxin to the skin, and because filaggrins act as intracellular
ranging from the localised bullous impetigo to anchors of desmosomes, many investigators
generalised SSSS involving the entire skin have speculated that epidermal splitting is a
surface.2–4 In the latter, patients are susceptible result of rupture of these desmosomes, prob-
to poor temperature control, extensive fluid ably from proteolytic activity of the toxins.15–17
losses, and secondary infections. They may also The toxins in their native form, however, do
develop sepsis and present with hypotension, not have any significant proteolytic activity,18 19
neutropenia, and respiratory distress.5 Anti- and the hypothesis that they may be serine
biotic treatment with â-lactamase resistant proteases comes from indirect evidence: (1)
semisynthetic penicillins such as flucloxacillin
both toxins show significant sequence homol-
is usually eVective.3 6 Outbreaks of SSSS
ogy with the staphylococcal V8 protease,
involving a large number of babies in neonatal
particularly in the region of the serine–aspartic
wards are not uncommon and may persist for a
acid–histidine catalytic triad that forms the
long time if carriers of toxin producing S aureus
active site of trypsin-like serine proteases15 ; (2)
are not rapidly identified and treated.6–9
Considering the severity of the disease and replacing any of the three amino acids that
the amount of published work, it is surprising form the catalytic triad of the toxins results in
how little progress has been made in under- complete loss of biological activity when
standing the mode of action of the toxin and injected into newborn mice16 17 19; (3) incuba-
the epidemiology of the disease. Much work tion of ETA with neonatal mouse epidermis18
over the years on SSSS has been uneventful or neonatal mouse epidermal extract19 results
and repetitive—a large outbreak or a fatal out- in the induction of caseinolytic activity in the
come usually results in a surge of interest in the supernatant; and (4) recent computer model-
disease, which soon declines until the next ling20 and crystallographic studies21 22 on the
Division of
Biochemistry and event. There have been no major break- three dimensional structure of the toxins have
Molecular Biology, throughs in management or prevention over revealed a high degree of structural similarity
UMDS, Guy’s the past 25 years. It is true that because of the with known glutamate specific trypsin-like ser-
Campus, London ease of treatment of SSSS with eVective antibi- ine proteases. Deletion studies have shown that
Bridge, London the highly charged amino terminal of the exfo-
S Ladhani
otics and the lack of long term sequelae such as
R W Evans scarring, there is very little pressure for liative toxins is essential for their activity,22 and
research into the disease and the toxins that recent structural studies have led to specula-
Correspondence to: cause it. However, the mortality is still 3% in tion that this region may be responsible for
Dr Shamez Ladhani, 65 children,10 11 and over 50% in adults, reaching binding an epidermal receptor, which in turn
Littlemede, Cold Harbour
Estate, Mottingham, London almost 100% in those with underlying disease may result in a conformational change that
SE9 3ED, UK (see below), despite antibiotic treatment.3 exposes the toxin’s active catalytic site.21
86 Ladhani, Evans
Epidemiology Healthy adults rarely develop SSSS, and the
Staphylococcus aureus is a Gram positive coccus only two reported cases have been due to
which colonises the nose, perineum, axillae, ETB,3 although it is not known whether this is
eyes, wound sites, and toe webs.23 Two toxin a coincidence or whether ETB is more
serotypes are known—ETA and ETB—but commonly able to aVect healthy adults. Re-
others may exist.24 In the West, 80–85% of ported risk factors for adult SSSS include
toxin producing S aureus belong to phage immunosuppression (including immunosup-
group II,3 and around 90% of toxin producing pressive drugs and AIDS), renal failure, malig-
strains produce ETA.25 In contrast, in Japan nant disease, chronic alcohol abuse, and
there is a predominance of ETB producing intravenous drug addiction.3 However, it is still
strains, which are less likely to be of phage not clear whether the disease is the result of a
group II.26 27 A recent Nigerian study showed higher carriage rate of toxin producing S
that healthy animals can carry exfoliatin aureus, increased susceptibility to the toxins,
producing S aureus,28 and a previous study lack of protective antitoxin antibodies, or just a
identified toxin producing strains in inanimate manifestation of a generalised increase in the
objects such as ventilation shafts in a hospital.29 risk of infections.
Both may act as reservoirs of infection, but
their impact on human SSSS has not been Diagnosis
determined. In the United Kingdom, SSSS diagnosis is
Large epidemiological studies have found currently based mainly on clinical grounds,
toxin producing strains of S aureus in 5.1% of supported by the presence of S aureus in nasal,
944 isolates from 577 dermatological patients conjunctival, pharyngeal, umbilical, or other
screened,10 6.2% of 2362 isolates from hospital swabs, although these criteria are not always
inpatients,30 3% of isolates from 500 antenatal reliable.34 If confirmation of the diagnosis is
required, or when outbreaks occur, isolates can
women,31 and 4.0% of isolates from various
be sent to the Public Health Laboratory
clinical sources in Nigeria.28 Hargiss and
(PHL), London, where the S aureus will be
Larson found that up to 60% of newborn
phage typed (this service is not routinely avail-
babies discharged from the hospitals may be
able in hospital laboratories). The presence of
nasal carriers of S aureus.32 If approximately 5%
isolates in phage group II will strongly support
of the strains produce the exfoliative toxins, the diagnosis of SSSS, even though other phage
then as many as 3% of all neonates may carry types have been shown to produce an identical
toxin producing strains of S aureus. clinical picture.35 If further confirmation of the
However, the incidence of SSSS does not diagnosis is required, the PHL may test for
seem to be as common as might be expected. toxins using the immunological Ouchterlony
This might be the result of any combination of method, which lacks sensitivity and specificity.
poor data collection and reporting of the Other detection systems have been devel-
disease, a lower than reported prevalence of oped for the exfoliative toxins, including
toxin producing S aureus carriage, or a low serological methods of gel immunoprecipita-
incidence of disease in carriers, possibly tion, radioimmunological assays, enzyme
because of high levels of protective antitoxin linked immunosorbent assays, and detection of
antibodies in the population. Studies in 1981 gene sequences by DNA hybridisation and
showed ETA antibody in 88% of cord blood polymerase chain reaction.36 False positive
samples, reflecting maternal antibody status.11 results may occur in serological studies due to
This level dropped to 30% at 3 months to 2 protein A, a 42 kDa protein produced by over
years, then rose steadily to 91% at 40 years. In 90% of S aureus, which binds non-specifically
cases of generalised SSSS, ETA antibody was to the constant (Fc) domain of
absent in acute sera (five days) and present in immunoglobulins.37 These detection systems
convalescent sera (14 days). This contrasts were developed for use in research laboratories
with localised infection, when antibody was rather than clinical settings and tend to be
present in over 60% of acute samples (four to either very expensive or too time consuming for
10 days) but was no predictor of spread of dis- routine use.
ease. Perhaps maternal antibodies play an
important role in preventing SSSS, as has been Research
shown in mice,33 and if so breast feeding may be While a great deal of information is still
protective. lacking, two particular areas of research merit
Factors other than antibodies may also gov- more attention: the development of a standard
ern the development and severity of SSSS. assay for the exfoliative toxins, and elucidation
Organism factors may include subtle diVer- of their mechanism of action.
ences in S aureus strains or the toxins
themselves, requirement of a triggering factor DEVELOPING A STANDARDISED ASSAY
for toxin production, requirement of a cofactor The availability of a sensitive, specific, reliable
for the toxins, or the quantity of toxin in the clinical diagnostic kit which is simple to use
blood. Host factors may include an inappropri- would considerably alter the management of
ate immune response to bacteria or their SSSS. Such a kit would allow a confirmed
toxins, the presence of concurrent infections diagnosis of SSSS, with simple tests to detect
which would lower the host immune response, toxin levels in the blood or bullae, to be
or a genetically variable site of action of the available within a few hours. DiVerential diag-
exfoliative toxins, which would make some nosis of generalised exfoliation includes drug
individuals more susceptible than others. induced and virus mediated toxic epidermal
Staphylococcal scalded skin syndrome 87
necrolysis, burns, epidermolysis bullosa, bul- haematological disorders. Furthermore, if anti-
lous erythema multiforme, listeria and syphilis toxin antibodies are shown to protect against
infections, diVuse cutaneous mastocytosis, and SSSS, then inactive toxoids may be developed
graft versus host rejection.38 Rapid diagnosis of to provide active immunisation in susceptible
individual cases, particularly in adults where populations.
mortality rates are high, is important for The localised blistering seen in most healthy
initiating appropriate treatment and reducing individuals with SSSS could be exploited
mortality. Similarly, the rapid identification to produce a localised and controlled
and treatment of asymptomatic carriers—who exfoliation—for example, to remove a superfi-
may be numerous—should reduce the risk of cial oVending skin lesion.
large outbreaks of infection. Finally, discovering the mechanism of action
The diagnostic kit would improve our of these toxins may lead to the development of
understanding of the organism responsible, its eVective antitoxins. Given concurrently with
toxins, and the diseases they cause. For exam- antibiotics, antitoxins may abort exfoliation if
ple, simple epidemiological data on SSSS, such SSSS is diagnosed early, or may decrease the
as age distribution, sex ratio, ethnic suscepti- extent of exfoliation in severe cases, as may
bilities, and community as well as nursery occur in late presentation or delayed diagnosis
attendant carrier rates, are still lacking. The kit of the disease, in antibiotic resistant S aureus
would also speed up research into the possible strains that do not respond to conventional
role of staphylococcal toxins in other condi- antibiotics and in patients with underlying dis-
tions such as eczema39 and sudden infant death ease, where mortality rates are high.
syndrome.40
ELUCIDATING THE MECHANISM OF ACTION Conclusions
All the evidence for the exfoliative toxins being Although SSSS is relatively uncommon, usu-
glutamate specific trypsin-like serine proteases ally easily diagnosed on clinical grounds, and
is indirect and speculative and needs to be sub- readily treated with conventional antibiotics, it
stantiated with more research. The problem is important to emphasise that at present
lies in the lack of a suitable model to study the mortality rates are still unacceptably high, out-
mechanism of action of the toxins. The gold breaks are diYcult to control, and the second-
standard model still remains newborn1 or ary complications, which are particularly com-
hairless41 mice, developed over two decades mon in neonates, can often be lethal.
ago. Since then, various attempts have been Furthermore, clinicians should be aware of
made to develop a simpler and more humane three recently emerging trends. Firstly, al-
model to work on; human epidermis from sur- though phage group II staphylococci only
gery, keratinocyte cultures, and mouse epider- rarely develop antibiotic resistance,3 one
mis have been used to demonstrate epidermal such case has already been described recently,12
splitting at the zona granulosa.13 14 42 43 All these and it is well known that hospital outbreaks of
assays are diYcult to perform and replicate, multiresistant organisms are diYcult and
thus hampering research. expensive to control and carry a high mortality
However, research using the neonatal mouse rate.
epidermis assay18 19 could be extended to inves- Secondly, in the 1980s several studies
tigate whether the exfoliative toxins are indeed showed that the use of antiseptic neonatal
serine proteases and whether they bind to any umbilical cord care delayed the time of cord
receptors,44 and to elucidate events following separation,46–48 and this may produce parental
binding. The model could also be used to concern and increase midwives’ workload.
determine any changes occurring in ETA, Since then, there has been a gradual decline in
including a conformational change after recep- the use of antiseptic umbilical cord care in the
tor binding to expose the active catalytic site, as United Kingdom. However, several studies
has recently been speculated from structural have shown that this practice has led to a
models.21 22 The main diYculty is the use of significant increase in staphylococcal umbilical
mouse epidermis, but keratinocytes cell cul- colonisation, which in turn may lead to an
tures, for example, may provide an alternative increase in neonatal infections, including SSSS
substitute. and methicillin resistant S aureus outbreaks.49–51
Understanding the mechanism of action of Finally, the recent pressure to discharge
the toxins has several important implications. patients early from hospitals, particularly
For example, their ability to target a specific mothers and their newborn babies, may serve
layer within the epidermis could be used to to dissipate outbreaks (including SSSS)—
investigate the normal physiology of the skin by caused by hospital staV in close contact with
identifying, marking, and isolating specific neonates—into the community.6 While indi-
groups of cells and cell structures. Similarly, if vidual cases can easily be treated by the
the toxins’ targeting domain can be identified, primary health care team, delays in recognising
it may be possible to attach and deliver potent an outbreak mean that carriers still working in
drugs, such as chemotherapeutic agents, to the hospital will continue to infect more
specific sites within the skin, thereby reducing patients until identified, isolated, and treated.
the dose required and systemic side eVects. StaV should therefore ensure rigorous aseptic
Other toxins, such as diphtheria and pseudo- technique with hospital patients, particularly
monas exotoxin A,45 have already served a with neonates, and clinicians should beware of
similar purpose and provided novel treatments a possible outbreak, even if patients present
for various carcinogenic, immunological, and with infection after hospital discharge.
88 Ladhani, Evans
In conclusion, the chapter on SSSS is by no 22 Cavarelli J, Prevost G, Bourguet W, et al. The structure of
Staphylococcus aureus epidermolytic toxin A, an atypic
means closed. Much work has to be done by serine protease, at 1.7A resolution. Structure 1997;5:813–
both researchers and clinicians, and there is 24.
now added pressure to do so more quickly. 23 Noble WC. Microbiology of human skin. The micrococceae.
London: Lloyd-Luke, 1981:152–81.
SSSS researchers should be encouraged to 24 Sato H, Matsumori Y, Tanabe T, et al. A new type of staphy-
publish more of their work, including negative lococcal exfoliative toxin from a Staphylococcus aureus
findings, while clinicians should be collecting strain isolated from a horse with phlegmon. Infect Immun
1994;62:3780–5.
and combining more data and performing sim- 25 Willard D, Monteil H, Piemont Y, et al. L’exfoliatine dans
ple epidemiological studies on the disease and les staphylococcies néonatales. Nouv Presse Med 1982;11:
the organism responsible. It is only through 3769–71.
26 Sarai Y, Nikahara H, Ishikawa T, et al. A bacteriological
such collaborative work that wasteful repetitive study on children with staphylococcal toxic epidermal
research will be reduced and a more integrated necrolysis in Japan. Dermatologica 1977;154:161–7.
approach taken to tackle the disease once and 27 Murono K, Fujita K, Yoshioka H. Microbiological charac-
teristics of exfoliative toxin-producing Staphylococcus
for all. aureus. Pediatr Infect Dis J 1988;7:313–5.
28 Adesiyun AA, Lenz W, Schaal KP. Exfoliative toxin produc-
This work is the result of ideas and suggestions derived from tion by Staphylococcus aureus strains isolated from
group discussions and debates held within the Division of Bio- animals and human beings in Nigeria. Microbiologica 1991;
chemistry and Molecular Biology and the Division of Microbi- 14:357–62.
ology at UMDS, London. We would specifically like to thank Dr 29 Kaplan MH, Chmel H, Hsieh H, et al. Importance of exfo-
S Poston, Dr C L Joannou, and Miss J Cameron. The work on liative toxin A production by Staphylococcus aureus strains
SSSS has been supported by the Trustees of Guy’s Hospital, isolated from clustered epidemics of neonatal pustulosis. J
The Clothworkers’ Foundation, and The Agakhan Foundation. Clin Microbiol 1986;23:83–91.
30 Piemont Y, Rasoamananjara D, Fouace JM, et al. Epidemio-
logical investigation of exfoliative toxin-producing Staphy-
1 Melish ME, Glasgow LA. The staphylococcal scalded skin
syndrome: development of an experimental model. N Engl lococcus aureus strains in hospitalized patients. J Clin
J Med 1970;282:1114–9. Microbiol 1984;19:417–20.
2 Melish ME. Staphylococci, streptococci and the skin: review 31 Dancer SJ, Noble WC. Nasal, axillary, and perineal carriage
of impetigo and staphylococcal scalded skin syndrome. of Staphylococcus aureus among women: identification of
Semin Dermatol 1982:1:101–9. strains producing epidermolytic toxin. J Clin Pathol
3 Cribier B, Piemont Y, Grosshans E. Staphylococcal scalded 1991;44:681–4.
skin syndrome in adults: a clinical review illustrated with a 32 Hargiss C, Larson E. The epidemiology of Staphylococcus
new case. J Am Acad Dermatol 1984;30:319–24. aureus in a newborn nursery from 1970 through 1976.
4 Itani O, Crump R, Mimouni F, et al. Picture of the month: Pediatrics 1978;61:348–53.
SSSS. Am J Dis Child 1992;146:425–6. 33 Machida K. Immunological investigations on the pathogen-
5 Loughead JL. Congenital staphylococcal scalded skin esis of staphylococcal scalded skin syndrome. Jpn J Clin
syndrome: report of a case. Pediatr Infect Dis J 1992;11: Pathol 1995;43:547–56.
413–4. 34 Falk DK, King LE. Criteria for the diagnosis of staphyloco-
6 Bell FG, Fenton PA. Early hospital discharge and cross- ccal scalded skin syndrome in adults. Cutis 1983;31:421–4.
infection. Lancet 1993;342:120. 35 Curran JP, Al-Salihi FL. Neonatal staphylococcal scalded
7 Dancer SJ, Simmons NA, Poston SM, et al. Outbreak of sta- skin syndrome: massive outbreak due to an unusual phage
phylococcal scalded skin syndrome among neonates. J type. Pediatrics 1980;66:285–90.
Infect 1988;16:87–103. 36 De Azevedo JCS, Arbuthnott JP. Assays for epidermolytic
8 Dave J, Reith S, Nash JQ, et al. A double outbreak of exfo- toxin of Staphylococcus aureus. Methods Enzymol 1988;
liative toxin-producing strains of Staphylococcus aureus in 165:333–8.
a maternity unit. Epidemiol Infect 1994;112:103–14.
37 Forsgren A. Significance of protein A production by staphy-
9 Mackenzie A, Johnson W, Heyes B, et al. A prolonged lococci. Infect Immun 1970;2:672–3.
outbreak of exfoliative toxin A-producing Staphylococcus
aureus in a newborn nursery. Diagn Microbiol Infect Dis 38 Easterley NB, Solomon LM. Neonatal dermatology II: blis-
1995;21:69–75. tering and scaling dermatoses. J Pediatr 1970;77:1075–88.
10 Gemell CG. Staphylococcal scalded skin syndrome. J Med 39 McFadden JP, Noble WC, Kemp RD. Superantigenic
Microbiol 1995;43:318–27. exotoxin-secreting potential of staphylococci isolated from
11 Melish ME, Chen FS, Sprouse S, et al. Epidermolytic toxin atopic eczematous skin. Br J Dermatol 1993;128:631–2.
in staphylococcal infection: toxin levels and host response. 40 Malam JE, Carrick GF, Telford DR, et al. Staphylococcal
In: Jelijaszewicz J, ed. Staphylococci and staphylococcal infec- toxins and sudden infant death syndrome. J Clin Pathol
tion (Zbl Bakt Suppl 10). Stuttgart: Gustav Fischer, 1993;45:716–21.
1981:287–98. 41 Arbuthnott JP, Kent J, Noble WC. The response of hairless
12 Yokota S, Imagawa T, Katakura S, et al. Staphylococcal mice to staphylococcal epidermolytic toxin. Br J Dermatol
scalded skin syndrome caused by exfoliative toxin 1973;88:481–5.
B-producing methicillin-resistant Staphylococcus aureus 42 Lillibridge CB, Melish ME, Glasgow LA. Site of action of
[letter]. Eur J Pediatr 1996;155:722. exfoliative toxin in the staphylococcal scalded skin
13 Dimond RL, WolV HH, Braun-Fulco O. The staphylococcal syndrome. Pediatrics 1972;50:728–38.
scalded skin syndrome. An experimental histochemical and
electron microscopic study. Br J Dermatol 1977;96:483–92. 43 Elias PM, Fritsch P, Dahl MV. Staphylococcal toxic epider-
mal necrolysis: pathogenesis and studies on the subcellular
14 Gentilhomme E, Faure M, Piemont Y, et al. Action of
staphylococcal exfoliative toxins on epidermal cell cultures site of action of exfoliatin. J Invest Dermatol 1965;65:501–
and organotypic skin. J Dermatol 1990;17:526–32. 12.
15 Dancer SJ, Garratt R, Saldanha J, et al. The epidermolytic 44 Tanabe T, Sato H, Ueda K, et al. Possible receptor for exfo-
toxins are serine proteases. FEBS Lett 1990;268:129–32. liative toxins produced by Staphylococcus hyicus and
16 Prevost G, Rifai S, Chaix ML, et al. Functional evidence Staphylococcus aureus. Infect Immun 1995;63:1591–4.
that Ser-195 residue of Staphylococcal exfoliative toxin A is 45 Woodworth TG, Nichols JC. Recombinant fusion toxins—a
essential for biological activity. Infect Immun 1991;59: new class of targeted biologic therapeutics. Cancer Treat Res
3337–9. 1995;68:145–60.
17 Redpath MB, Foster TJ, Bailey CJ. The role of serine 46 Lawrence CR. EVect of two diVerent methods of umbilical
protease active site in the mode of action of epidermolytic cord care on its separation time. Midwives Chron 1982;95:
toxin of S. aureus. FEMS Microbiol Lett 1991;81:151–6. 204–5.
18 Takiuchi I. Staphylococcal exfoliative toxin induces caseino- 47 Mugford M, Somchiwong M, Waterhouse I. Treatment of
lytic activity. J Infect Dis 1987;156:508–10. umbilical cords: a randomized controlled trial to assess the
19 Jaulhac B, Piemont Y, Prevost G. Staphylococcal exfoliative eVect of treatment methods on the work of midwives. Mid-
toxins induce proteolytic activity when combined with epi- wifery 1986;2:177–86.
dermis. In: Mollby, Flock, Nord, Christensson, eds. 48 Ronchea-Oms C, Hernandez C, Jimemez NV. Antiseptic
Staphylococci and staphylococcal infections. Proceedings of the cord care reduces bacterial colonisation but delays cord
7th International Symposium, Stockholm, June 29-July 3, 1992 detachment. Arch Dis Child 1994;70:F70.
(Zbl Bakt Suppl 26). Stuttgart: Gustav Fischer, 1994:
280–2. 49 Watkinson M, Dyas A. Staphylococcus aureus still colonises
20 Barbosa JARG, Saldanha JW, Garratt RC. Novel features of the untreated neonatal umbilicus. J Hosp Infect 1992;21:
serine protease active sites and specificity pockets: se- 131–6.
quence analysis and modelling studies of glutamate- 50 Allen KD, Ridgway EJ, Parsons LA. Hexochlorophane pow-
specific endopeptidases and epidermolytic toxins. Protein der and neonatal staphylococcal infection. J Hosp Infect
Engineering 1996;9:591–601. 1994;27:29–33.
21 Vath GM, Earhart CA, Rago JV, et al. The structure of the 51 Stark V, Harrison SP. Staphylococcus aureus colonization of
superantigen exfoliative toxin A suggests a novel regulation the newborn in a Darlington hospital. J Hosp Infect
as a serine protease. Biochemistry 1997;36:1559–66. 1992;21:205–11.
View publication stats
You might also like
- The Black Death (PDFDrive)Document2,130 pagesThe Black Death (PDFDrive)Via et Veritas et VitaNo ratings yet
- Race Specific Biological Weapons: November 1970 Military Review - Ethnic Weapons ArticleDocument10 pagesRace Specific Biological Weapons: November 1970 Military Review - Ethnic Weapons ArticleJeff ScottNo ratings yet
- Stafilococo AureusDocument15 pagesStafilococo AureusJose MosqueraNo ratings yet
- Medical Microbiology ReviewerDocument3 pagesMedical Microbiology ReviewerJoan BularioNo ratings yet
- Arch Dis Child 1998 Ladhani 85 8Document5 pagesArch Dis Child 1998 Ladhani 85 8Dian Ayu Permata SandiNo ratings yet
- NitradorDocument13 pagesNitradorcristian fodasticoNo ratings yet
- Kakoullis 2019Document20 pagesKakoullis 2019Andi SusiloNo ratings yet
- Streptococcal Skin InfectionsssDocument6 pagesStreptococcal Skin InfectionsssteenaNo ratings yet
- Staphylococcus: Skin Infections Osteomyelitis Bloodstream Infection Food Poisoning Foreign Body Infections MRSADocument7 pagesStaphylococcus: Skin Infections Osteomyelitis Bloodstream Infection Food Poisoning Foreign Body Infections MRSAnour achkarNo ratings yet
- An Overview of The Immune SystemDocument14 pagesAn Overview of The Immune SystemMaría Camila ValenciaNo ratings yet
- Regis 2001Document5 pagesRegis 2001yifiyyfyi bhvxhddfiNo ratings yet
- Toxic Shock SyndromeDocument13 pagesToxic Shock SyndromeSrinivas PingaliNo ratings yet
- Salmonella Typhi: From A Human Pathogen To A Vaccine Vector: Cellular & Molecular Immunology May 2008Document8 pagesSalmonella Typhi: From A Human Pathogen To A Vaccine Vector: Cellular & Molecular Immunology May 2008noordin MukasaNo ratings yet
- Microbiologia de Murray 8va Edicion-194-227Document34 pagesMicrobiologia de Murray 8va Edicion-194-227Ailyn Montalvo FigueroaNo ratings yet
- Emg Piel PDFDocument7 pagesEmg Piel PDFRafael ValverdeNo ratings yet
- Staphylococci: R.Varidianto Yudo T., DRDocument36 pagesStaphylococci: R.Varidianto Yudo T., DRIndra MahaputraNo ratings yet
- Trop Med 140515Document10 pagesTrop Med 140515Arfa AlyaNo ratings yet
- Rotavirus: Structure & Composition PathogenesisDocument4 pagesRotavirus: Structure & Composition PathogenesisyusrinastitiNo ratings yet
- Schistosomiasis: ArticleDocument20 pagesSchistosomiasis: ArticleVincent ReyesNo ratings yet
- Basophils and Allergic Inflammation: Mechanisms of Allergic DiseasesDocument13 pagesBasophils and Allergic Inflammation: Mechanisms of Allergic DiseasesBasideu ByinajuNo ratings yet
- Austin AndrologyDocument4 pagesAustin AndrologyAustin Publishing GroupNo ratings yet
- Annals NY Academy of Science - 2021 - Muscella - Antitumor and Antimigration Effects of Salvia Clandestina L Extract OnDocument14 pagesAnnals NY Academy of Science - 2021 - Muscella - Antitumor and Antimigration Effects of Salvia Clandestina L Extract OnOstazNo ratings yet
- Sistiserkosis Taenia Solium PDFDocument10 pagesSistiserkosis Taenia Solium PDFOgix HilandoNo ratings yet
- 10.2165@00042310 200420040 00005Document4 pages10.2165@00042310 200420040 00005RSU Trijaya medical centerNo ratings yet
- Platelets y Escherichia ColiDocument12 pagesPlatelets y Escherichia Coliedson floresNo ratings yet
- Toxins: Exfoliative Toxins of Staphylococcus AureusDocument24 pagesToxins: Exfoliative Toxins of Staphylococcus AureusJhon Lipa MazaNo ratings yet
- J Cellular Molecular Medi - 2023 - LuDocument11 pagesJ Cellular Molecular Medi - 2023 - Lulars0511000No ratings yet
- Avances en El Tratamiento, Diagnóstico, Control y Comprensión Científica de Las Infecciones Por Parásitos Cestodos en Los Últimos 50 AñosDocument26 pagesAvances en El Tratamiento, Diagnóstico, Control y Comprensión Científica de Las Infecciones Por Parásitos Cestodos en Los Últimos 50 Añoswen_ucNo ratings yet
- T Lymphocyte Exhaustion During Human and Experimental Visceral LeishmaniasisDocument15 pagesT Lymphocyte Exhaustion During Human and Experimental Visceral LeishmaniasisLuis Carlos Calvanapon TerronesNo ratings yet
- 1 s2.0 S0146000598800037 MainDocument13 pages1 s2.0 S0146000598800037 MainEmilio Emmanué Escobar CruzNo ratings yet
- STREPTOCOCCUSDocument24 pagesSTREPTOCOCCUSTUSHAR MORESHWARNo ratings yet
- Bacterial ToxinsDocument29 pagesBacterial ToxinsShashi SharmaNo ratings yet
- Serratia Marcescensbacteraemia in Preterm Neonate - A Case ReportDocument4 pagesSerratia Marcescensbacteraemia in Preterm Neonate - A Case ReportIJAR JOURNALNo ratings yet
- Virulence Factors of Streptococcus PyogenesDocument6 pagesVirulence Factors of Streptococcus PyogenesAmador GielasNo ratings yet
- Fcimb 11 662766Document10 pagesFcimb 11 662766Gustavo AndresNo ratings yet
- B. PathologyDocument13 pagesB. PathologyWendi YuNo ratings yet
- FrontiersDocument15 pagesFrontiersValiio mazNo ratings yet
- 3 PDFDocument6 pages3 PDFsandra magdalenaNo ratings yet
- Fimmu 11 571816Document20 pagesFimmu 11 571816Ricardo GomezNo ratings yet
- Leptospirosis: Aspects of Innate Immunity, Immunopathogenesis and Immune Evasion From The Complement SystemDocument12 pagesLeptospirosis: Aspects of Innate Immunity, Immunopathogenesis and Immune Evasion From The Complement SystemUfarah Indah SariNo ratings yet
- Peter Hawkey: The Enemy Within - Hospital-Acquired, Antibiotic-Resistant BacteriaDocument3 pagesPeter Hawkey: The Enemy Within - Hospital-Acquired, Antibiotic-Resistant BacteriaCoffee CojjeeNo ratings yet
- Research Article: SOCS1 Mimetic Peptide Suppresses Chronic Intraocular Inflammatory Disease (Uveitis)Document16 pagesResearch Article: SOCS1 Mimetic Peptide Suppresses Chronic Intraocular Inflammatory Disease (Uveitis)Paulus SidhartaNo ratings yet
- J. Biol. Chem.-2019-Coelho-1202-17 PDFDocument17 pagesJ. Biol. Chem.-2019-Coelho-1202-17 PDFJoel Torres VillenaNo ratings yet
- Feline Infectious Peritonitis ABCD Guidelines On PDocument12 pagesFeline Infectious Peritonitis ABCD Guidelines On Pxn5q8nckvkNo ratings yet
- The Staphylococci: Lecture SixDocument8 pagesThe Staphylococci: Lecture SixjohnsmithprayNo ratings yet
- The Microbiome in Patients With Atopic Dermatitis: Current PerspectivesDocument10 pagesThe Microbiome in Patients With Atopic Dermatitis: Current PerspectivesPande Agung MahariskiNo ratings yet
- Culture and IdentificationDocument4 pagesCulture and IdentificationDewa Denis100% (1)
- Journal SchistosomiasisDocument11 pagesJournal SchistosomiasisCelissa Mauriz HilarioNo ratings yet
- 2004 Macrophages, Pathology and Parasite Persistence in Experimental Visceral LeishmaniasisDocument7 pages2004 Macrophages, Pathology and Parasite Persistence in Experimental Visceral LeishmaniasismclimacoNo ratings yet
- E00489-12 FullDocument10 pagesE00489-12 FulldoruolaruNo ratings yet
- K - 10 Important Pathogenic Bacteria During Child (Mikrobiologi)Document42 pagesK - 10 Important Pathogenic Bacteria During Child (Mikrobiologi)Josephine IrenaNo ratings yet
- Innate Immunity, Microbiology, Inflammation - ABSTRACTSDocument1 pageInnate Immunity, Microbiology, Inflammation - ABSTRACTSFebrian AlfaroNo ratings yet
- K20 Infeksi Bakteri Pada Kulit DR DR Enny Nugraheni, M BiomedDocument38 pagesK20 Infeksi Bakteri Pada Kulit DR DR Enny Nugraheni, M BiomedFajar Cristianta GintingNo ratings yet
- Nova Visão Da Patologia Da SepseDocument3 pagesNova Visão Da Patologia Da SepsePedro TadeuNo ratings yet
- Dynamics of Bovine Spleen Cell Populations DuringDocument12 pagesDynamics of Bovine Spleen Cell Populations DuringMony ElbazNo ratings yet
- Hyposplenic Review 2011Document12 pagesHyposplenic Review 2011IndhumathiNo ratings yet
- Parakeratosis Variegata: A Possible Role of Environmental Hazards?Document4 pagesParakeratosis Variegata: A Possible Role of Environmental Hazards?Ega AihenaNo ratings yet
- Cell Death 2011Document15 pagesCell Death 2011Paula Contreras MezaNo ratings yet
- Case Report SsDocument3 pagesCase Report SsmarselamgeNo ratings yet
- Ocular Manifestations in Systemic Lupus Erythematosus: Sukhum Silpa-Archa, Joan J Lee, C Stephen FosterDocument8 pagesOcular Manifestations in Systemic Lupus Erythematosus: Sukhum Silpa-Archa, Joan J Lee, C Stephen FosterNadya Regina PermataNo ratings yet
- Research Article: SOCS1 Mimetic Peptide Suppresses Chronic Intraocular Inflammatory Disease (Uveitis)Document33 pagesResearch Article: SOCS1 Mimetic Peptide Suppresses Chronic Intraocular Inflammatory Disease (Uveitis)Paulus SidhartaNo ratings yet
- Non-inflammatory immunology: An introduction to the immune system and its pathologiesFrom EverandNon-inflammatory immunology: An introduction to the immune system and its pathologiesNo ratings yet
- A24 - Mathura Lab Home Collection 1st Floor, Tera Tower, Bhuteshwar Road, MathuraDocument2 pagesA24 - Mathura Lab Home Collection 1st Floor, Tera Tower, Bhuteshwar Road, MathuraAkki MauryaNo ratings yet
- Antifolate Drugs 17970Document19 pagesAntifolate Drugs 17970TES SENNo ratings yet
- Bacteriological Profile of Infections in Burns Unit - Plastic and Reconstructive Surgery Department - Mohammed Vi Chu MarrakechDocument7 pagesBacteriological Profile of Infections in Burns Unit - Plastic and Reconstructive Surgery Department - Mohammed Vi Chu MarrakechIJAR JOURNALNo ratings yet
- Rapid Laboratory Diagnosis of Cholera in The Field 0035-9203 (89) 90733-5Document2 pagesRapid Laboratory Diagnosis of Cholera in The Field 0035-9203 (89) 90733-5JUAN HERNANDEZNo ratings yet
- FNCP CHN Worksheet KatDocument6 pagesFNCP CHN Worksheet KatKatyana CesarNo ratings yet
- Epi Descriptive Study DesignsDocument4 pagesEpi Descriptive Study DesignsAndrea BardalesNo ratings yet
- Did Alexander The Great Die From Guillain-Barré Syndrome - HallDocument26 pagesDid Alexander The Great Die From Guillain-Barré Syndrome - HallJAVIER AGUILAR CASASOLANo ratings yet
- Nadja Durbach - Bodily Matters - The Anti-Vaccination Movement in England, 1853-1907 - SeminarDocument294 pagesNadja Durbach - Bodily Matters - The Anti-Vaccination Movement in England, 1853-1907 - SeminarMatthew ParnellNo ratings yet
- Acute Bacterial Meningitis in ChildrenDocument48 pagesAcute Bacterial Meningitis in ChildrenRadhika BatraNo ratings yet
- Vaccines in Swine ProductionDocument22 pagesVaccines in Swine ProductionRemelyn Rodrigo100% (1)
- Saifur Rahman Sabbir Tika Card - Masum BestDocument1 pageSaifur Rahman Sabbir Tika Card - Masum BestTowhidul Azam BabuNo ratings yet
- Community Health Nursing II NotesDocument11 pagesCommunity Health Nursing II NotesMarjorie UmipigNo ratings yet
- Table of Common Human PATHOGENS - Name, Type, Disease, Tissue, Method of Transmission, Virulence Factors, Normal Habitat, and TreatmentDocument3 pagesTable of Common Human PATHOGENS - Name, Type, Disease, Tissue, Method of Transmission, Virulence Factors, Normal Habitat, and TreatmentweslynnesmithNo ratings yet
- Pre - and Post Test Cardiac ArrhythmiasDocument6 pagesPre - and Post Test Cardiac ArrhythmiasEköw Santiago JavierNo ratings yet
- Overview of Public Health-Part 2Document44 pagesOverview of Public Health-Part 2omegasauronNo ratings yet
- ScabiesDocument6 pagesScabiesJayson ShieldsNo ratings yet
- Uganda Health Reporter October 2013Document6 pagesUganda Health Reporter October 2013Tobacco Control UgandaNo ratings yet
- Sepsis DoneDocument54 pagesSepsis DoneGiorgi PopiashviliNo ratings yet
- Kebersihan Tangan, APD, Peralatan Pasien, Dan Linen 16-2-2023 - CompressedDocument70 pagesKebersihan Tangan, APD, Peralatan Pasien, Dan Linen 16-2-2023 - CompressedSaripa TalaohuNo ratings yet
- Infection ControlDocument3 pagesInfection ControlHazelina VillamorNo ratings yet
- Chagas DiseaseDocument4 pagesChagas DiseaseAanuOluwapo EgberongbeNo ratings yet
- Module 3 Activity (Engage-Explore-Elaborate-Evaluate) (MIRABOLO)Document4 pagesModule 3 Activity (Engage-Explore-Elaborate-Evaluate) (MIRABOLO)MIRABOLO, MARK ANGELONo ratings yet
- 9 Bacteriological Analysis of WaterDocument9 pages9 Bacteriological Analysis of WaterGaurav MudaduNo ratings yet
- CSR of Dabur - NutanDocument16 pagesCSR of Dabur - NutanAkhilaChengotNo ratings yet
- Report On Global Sexually Transmitted Infection SurveillanceDocument74 pagesReport On Global Sexually Transmitted Infection SurveillanceSafaNo ratings yet
- LabyrinthitisDocument7 pagesLabyrinthitisChristie ZamoraNo ratings yet
- Micro ParasitologyDocument5 pagesMicro ParasitologyPlzstudylav SyedNo ratings yet
- Vaccine (Shot) For Pneumococcal DiseaseDocument7 pagesVaccine (Shot) For Pneumococcal DiseasecoooleNo ratings yet