Professional Documents
Culture Documents
Activity No. 11 Answer Sheet
Uploaded by
Nashiba SaripOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Activity No. 11 Answer Sheet
Uploaded by
Nashiba SaripCopyright:
Available Formats
Activity No.
11
POTENTIOMETRIC TITRATION OF A WEAK ACID
Error, % = ___________
Rating, % = ___________
Deductions, % = ___________
Net Rating, % = ___________
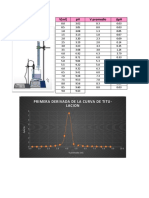
A. POTENTIOMETRIC TITRATION
Volume of NaOH, mL pH
0.00 3.78
0.60 4.11
1.20 4.38
1.60 4.55
2.20 4.76
2.70 4.91
3.50 5.20
4.10 5.45
4.30 5.52
4.40 5.58
4.50 5.67
4.70 5.80
4.90 6.01
5.10 6.32
5.20 6.59
5.30 7.13
5.40 8.10
5.60 9.76
6.00 10.4
6.50 10.75
7.00 10.91
7.90 11.11
B. STANDARDIZATION
I II III
Wt. of bottle + sample, g __________ __________ __________
Wt. of bottle + sample minus sample, g __________ __________ __________
Weight of sample taken, g __________ __________ __________
Final reading, mL NaOH __________ __________ __________
Initial reading, mL NaOH __________ __________ __________
Volume, mL NaOH __________ __________ __________
Normality of NaOH __________ __________ __________
STATISTICAL EVALUATION OF RESULTS (NNaOH):
Mean, x̄ ____________
Median, M ____________
Standard Deviation, s ____________
Rel. Std. Deviation, RSD (%) ____________
Std. Deviation of the Mean, S ____________
REPORTED VALUE (NNaOH) ____________
You might also like
- Math Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesFrom EverandMath Practice Simplified: Decimals & Percents (Book H): Practicing the Concepts of Decimals and PercentagesRating: 5 out of 5 stars5/5 (3)
- Annexure B - Sucrose Conversion TableDocument21 pagesAnnexure B - Sucrose Conversion TableLim Chen Gin100% (1)
- Frequencies TableDocument3 pagesFrequencies TableKelly lamNo ratings yet
- Curva de Titulacion PotenciometricaDocument3 pagesCurva de Titulacion PotenciometricaKatsumyNo ratings yet
- OutputDocument5 pagesOutputAnother YogaNo ratings yet
- UTS PraktikumEkonometrika Nadia Rachmani Putri - ES2 - 204105020056Document9 pagesUTS PraktikumEkonometrika Nadia Rachmani Putri - ES2 - 204105020056Nadia Rahmani PutriNo ratings yet
- Tugas Statistik BABYDocument4 pagesTugas Statistik BABYHanifah FahrunisaNo ratings yet
- HistogrameDocument9 pagesHistogrameLara PaleNo ratings yet
- SpssDocument5 pagesSpssIrwanNo ratings yet
- Datos Potenciometria 25-05-23Document6 pagesDatos Potenciometria 25-05-23Angela VillegasNo ratings yet
- Experiment 6Document5 pagesExperiment 6Rza AbdullayevNo ratings yet
- Water SupplyDocument12 pagesWater SupplyyusufNo ratings yet
- Gambar DB Di LPDocument3 pagesGambar DB Di LPSintong Leonardo SitungkirNo ratings yet
- Ion Selective ElectrodesDocument11 pagesIon Selective ElectrodesOm PhileNo ratings yet
- 4B - 920173142 - Afiyanti Riyana DewiDocument16 pages4B - 920173142 - Afiyanti Riyana DewiNawa EvalatulNo ratings yet
- Lampiran 1 Latihan SpssDocument6 pagesLampiran 1 Latihan SpssErwin NataNo ratings yet
- 1 Frequencies BDocument3 pages1 Frequencies BNazia SyedNo ratings yet
- Stats AssignmentDocument15 pagesStats AssignmentShumaila KhanNo ratings yet
- Analisa SaringanDocument2 pagesAnalisa SaringanNP Project Music26No ratings yet
- 4a - 920173038 - Puput Puji RahayuDocument16 pages4a - 920173038 - Puput Puji RahayuPuput Puji RahayuNo ratings yet
- SBL1023 Techniques in Biological and Biochemistry LaboratoryDocument9 pagesSBL1023 Techniques in Biological and Biochemistry Laboratoryapi-383715002No ratings yet
- NSGA and Optimization DocumentDocument6 pagesNSGA and Optimization DocumentAnutthara RatnayakeNo ratings yet
- 4a 920173027 s1 Keperawatan KelvinaDocument16 pages4a 920173027 s1 Keperawatan Kelvinakelvin vinaNo ratings yet
- AP CHEM Lab Acid Base Titration PDFDocument4 pagesAP CHEM Lab Acid Base Titration PDFMelnykNo ratings yet
- Lab Report BoiDocument7 pagesLab Report BoiNORHIDAYATI BINTI MD GHAZALI MoeNo ratings yet
- Statistics 1Document3 pagesStatistics 1CYNTHIA DEVI SIMANJUNTAK 19520283No ratings yet
- Frekuensi: Variety SeekingDocument3 pagesFrekuensi: Variety SeekingMekarayucNo ratings yet
- 4a - 920173024 - Ismaul WijayatriDocument16 pages4a - 920173024 - Ismaul WijayatriNawa EvalatulNo ratings yet
- Unknown Analysis LabDocument9 pagesUnknown Analysis LabTrixie LeNo ratings yet
- Lampiran SPSS (Fix)Document10 pagesLampiran SPSS (Fix)ebaddah trendcenterNo ratings yet
- Tugas Biostatistika Nama: Feronika Parastuti NIM: 920173065 1. Tabel Frekuensi Data A. Frekuensi KeseluruhanDocument16 pagesTugas Biostatistika Nama: Feronika Parastuti NIM: 920173065 1. Tabel Frekuensi Data A. Frekuensi KeseluruhanMita NisaNo ratings yet
- SPSS1Document3 pagesSPSS1Muhammad RizqiNo ratings yet
- Afna 4Document10 pagesAfna 4Babad BagusNo ratings yet
- Spreadsheet Program For Calculating The PH - Coagulant Dosage RelationshipDocument7 pagesSpreadsheet Program For Calculating The PH - Coagulant Dosage RelationshipMohamed TallyNo ratings yet
- Gebbrilla Anjeni Sadewa 002Document5 pagesGebbrilla Anjeni Sadewa 002GabbyNo ratings yet
- Laborat - 4a - 920173040 - Ririn Ayu Sofiyana NingsihDocument14 pagesLaborat - 4a - 920173040 - Ririn Ayu Sofiyana NingsihRirin PalasgaNo ratings yet
- Tugas Biostatistika Nama: Ririn Ayu Sofiyana Ningsih NIM: 920173040 1. Tabel Frekuensi Data A. Frekuensi KeseluruhanDocument14 pagesTugas Biostatistika Nama: Ririn Ayu Sofiyana Ningsih NIM: 920173040 1. Tabel Frekuensi Data A. Frekuensi KeseluruhanMita NisaNo ratings yet
- Post Lab Report: Determination of The Molar Mass of An Unknown Monoprotic Acid, HA.Document7 pagesPost Lab Report: Determination of The Molar Mass of An Unknown Monoprotic Acid, HA.Mohammad Fahim NurNo ratings yet
- Exp 1Document3 pagesExp 1shifaghannam2024No ratings yet
- 102L Spring 2021 16.2 Raw Data Unk TrialDocument32 pages102L Spring 2021 16.2 Raw Data Unk TrialDiya PatelNo ratings yet
- Experiment #02 To Verify The Theoretical Expression For The Force Exerted by Jet Striking Normally On The Flat Plate (Impact of Jet)Document2 pagesExperiment #02 To Verify The Theoretical Expression For The Force Exerted by Jet Striking Normally On The Flat Plate (Impact of Jet)Mir Masood ShahNo ratings yet
- 21.0101.0087 - Ujikom Temu 5Document5 pages21.0101.0087 - Ujikom Temu 5Dini HanifahNo ratings yet
- Activity For Pearson and SpearmanDocument3 pagesActivity For Pearson and SpearmanJoseph Peter BalangonNo ratings yet
- ProblemsDocument31 pagesProblemsAhmed Adham100% (1)
- PH de Reaccion MatematicaDocument3 pagesPH de Reaccion MatematicaJames J. Rojas SanchezNo ratings yet
- Act 5 - Titrimetric Determination of Acetylsalicyclic Acid - WorksheetDocument2 pagesAct 5 - Titrimetric Determination of Acetylsalicyclic Acid - WorksheetNitrogenNo ratings yet
- Tos FilipinoDocument6 pagesTos Filipinoayahzumi CuhNo ratings yet
- auto-CREATININE LiquicolorDocument8 pagesauto-CREATININE LiquicolorLemi MaluluNo ratings yet
- N H KB 1 ×10 HCL O: Base Debil Ácido FuerteDocument3 pagesN H KB 1 ×10 HCL O: Base Debil Ácido FuerteGonzalo Carlosama SandovalNo ratings yet
- Tarea E L-LDocument4 pagesTarea E L-LRoco neluNo ratings yet
- Output BaruDocument4 pagesOutput BarurismunandarNo ratings yet
- Tugas1 - Gebbriloa Anjeni Sadewa 002Document8 pagesTugas1 - Gebbriloa Anjeni Sadewa 002GabbyNo ratings yet
- Chapter 5 StatDocument6 pagesChapter 5 Statsyazwana atiyahNo ratings yet
- Standar Deviasi Pengukuran Variasi PDFDocument6 pagesStandar Deviasi Pengukuran Variasi PDFQonitah DhaimahwatiNo ratings yet
- Pavement Materials: Module 2, Lecture 10 Aggregate Properties (Part 4)Document11 pagesPavement Materials: Module 2, Lecture 10 Aggregate Properties (Part 4)Rohit VasudevaNo ratings yet
- Ride Price Calculation Sheet Inputs: Ride of Same Type in Park?Document7 pagesRide Price Calculation Sheet Inputs: Ride of Same Type in Park?Jhon IngNo ratings yet
- Notas Até 23-11-2020 - Publicada MoodleDocument3 pagesNotas Até 23-11-2020 - Publicada MoodleViviNo ratings yet
- Notas Até 23-11-2020 - Publicada MoodleDocument3 pagesNotas Até 23-11-2020 - Publicada MoodleViviNo ratings yet
- Lab Report 2 Group1Document7 pagesLab Report 2 Group1azamatNo ratings yet