Professional Documents
Culture Documents
HPCT311 Midterms Lec
Uploaded by
Juzhley PerezOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
HPCT311 Midterms Lec
Uploaded by
Juzhley PerezCopyright:
Available Formats
WEEK 7 | TISSUE PROCESSING
Tissue Processing
• This are the procedures employed for you to produce a
good quality of slide prior to analytical phase which is
the reading of the histopathology slides.
• For the examination of fresh tissue it involves the
grossing of the specime.
Methods of Fresh Tissue Examination
• Teasing
o A process whereby a selected tissue specimen is
Introduction to Tissue Processing immersed in a watch glass containing isotonic salt
solution, carefully dissected or separated, and
• Overview examined under a microscope.
o Processing of Tissue – a better and more effective o Unstained by Phase Contrast or Bright Field
means of studying tissues whether normal or Microscopy, or stained with differential dyes.
abnormal is by examination of their sections and
smears which have been permanently preserved,
stained and mounted on glass slides with cover
slips for permanent keeping.
o Processing of tissues involves our tissues and also
the smears of the client/patient preserved
permanently for diagnosis for reading of the
pathologist and for the future used.
o Involves the sample being placed on the glass
slides permanently.
• Why examine histopathologic specimens? • Squash Preparation (crushing)
o Pathology attempts to explain the why’s and o A process whereby small pieces of tissue not more
wherefores of the signs and symptoms manifested than 1 mm in diameter are placed in a microscopic
by patients while providing a sound foundation for slide and forcibly compressed with another slide or
rational clinical care and therapy. with a cover glass.
o Examined the samples to arrive on a certain o A vital stain may be placed at the junction of the
diagnosis and therapy of the patients and also to slide and the cover glass, and allowed to be
suggest the therapy for the client. absorbed by the tissue through capillary attraction.
o With the used of histopathology specimens it can be • Smear Preparation
tested using immunohistochemical staining – this o The process of examining sections or sediments
are stains that used for the treatment of the client. whereby cellular materials are spread lightly over a
o Determine if the sample is benign or malignant. slide by means of a wire loop or applicator, or by
Methods of Tissue Examination making an apposition smear with another slide.
o Especially useful in cytologic examinations,
Fresh Tissue particularly for cancer diagnosis.
▪ Streaking
• Usually examined when there is an immediate need for With an applicator stick or platinum loop,
evaluation. the material is rapidly and gently applied
• We use fresh tissue to diagnose the patient immediately. in a direct or zigzag line throughout the
• We use fresh tissue with a preservatives for the slide.
specimen. Attempts to obtain a relatively uniform
• Advantages: distribution of secretion.
o Examined in the living state thereby allowing Too thin or too thick smears have to be
protoplasmic activities such as: motion, mitosis, avoided, since they make the tissue
phagocytosis and pinocytosis to be observed. unsuitable for examination.
• Disadvantages: Commonly encountered when you have
o Its use has been limited Gynecological specimens for
o Liable to develop the changes that have usually Papanicolaou stain.
been observed after death. ▪ Spreading
A selected portion of the material is
transferred to a clean slide and gently
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 7 | TISSUE PROCESSING
spread into a moderately thick film by surgeon and the client can decide to perform
teasing the mucous strands apart with an modified radical mastectomy to remove the
applicator stick. entire breast.
A little more tedious than streaking but ▪ Routine biopsy can take up to 3-5 days or
maintains cellular interrelationships of the sometimes 1 month before the pathologist can
material to be examined. release a result.
For fresh sputum and bronchial aspirates, ▪ Frozen section is for rapid diagnosis we cut
and also for thick mucoid secretions. slices of the tissue around 10-15 micrometers
Spread evenly the sputum and the instrument that we used is the cryostat
AFB smear - blue at -10°C to -20°C depending on the sample
Mycobacterial tuberculosis - pink/red sometimes it can go up to -25°C.
▪ Pull-apart ▪ Freezing medium example, liquid nitrogen,
Done by placing a drop of secretion or isopentane cooled by the liquid nitrogen,
sediment upon one side and facing it to carbon dioxide gas and aerosol spray.
another clean slide. o Frozen sections are commonly used for:
Material disperse evenly over the surface ▪ Rapid pathologic diagnosis during surgery
of the two slides. ▪ Diagnostic and research enzyme
Slight movement of the 2 slides in histochemistry
opposite directions may be necessary to ▪ Diagnostic and research demonstration of
initiate the flow of materials. soluble such as lipids and carbohydrates
2 slides are then pulled apart in a single ▪ Immunofluorescent and immunohistochemical
uninterrupted motion. staining (IHC)
Most commonly used method in ▪ Some specialized silver stains, particularly in
preparation of fluid slide/cell slide for neuropathology.
cytology specimen. o Commonly used methods of freezing:
When you received sample (fluid cytology) ▪ Liquid nitrogen
always remember to prepare glass/cell ▪ Isopentane cooled by liquid nitrogen
slide for cytology and cell block. ▪ Carbon dioxide gas
Pull the 2 slides on different direction ▪ Aerosol sprays – sometimes it has liquid
Ex: Peritoneal fluid nitrogen at a matter of 2-5 secs the sample it
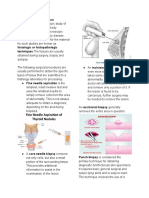
▪ Touch Preparation (Impression smear) will turn to rough as a stone.
One of the routine procedures done in the
laboratory. Preserved Tissue
A special method of smear prep whereby • Solid structures and tissues must be preserved and
the surface of a freshly cut piece of tissue carefully processed in the following order:
is brought into contact and pressed on to o Fixation
the surface of a clean glass slide, allowing o Decalcification (optional) – this is optional if the
the cells to be transferred directly to the sample has calcification or it has a rough portion
slide for examination by Phase Contrast o Dehydration – remove the excess water that you
microscopy or stained for light obtain during the fixation
microscopic study. o Clearing – to remove the excess dehydrating agent
Ex: Breast mass o Infiltration (impregnation)
Cells may be examines without destroying o Embedding
their actual intercellular relationship. o Trimming
• Frozen Section o Section-cutting
o Normally utilized when a rapid diagnosis of the o Staining
tissue in question is required, and is especially o Mounting
recommended when lipids and nervous tissue o Labeling
elements are to be demonstrated. o Fixation and infiltration can sometimes take up to
▪ Very thin slices, around 10-15 in thickness 12 hours
are cut from a fresh tissue frozen on a Cryostat o Frozen section at a matter of 30 minutes you have
(instrument or machine to be used) , a cold a result.
chamber kept at an atmospheric temperature of • Receiving, custody and identification of tissues
-10 °C to -20°C. o Receipt of Specimens
▪ Cryostat is a microtome inside the freezer. ▪ Specimen can be received fresh (without
▪ Within 15-30 minutes released a result if a fixative) or in formalin.
sample is benign or malignant so that if the ▪ Never put fixative/formalin for frozen
sample is benign the doctor/surgeon or even specimen or frozen section specimens.
the client can decide not to remove the entire I. Fresh
breast if he/she wants to preserve the breast.
But if the sample turn out to be malignant the
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 7 | TISSUE PROCESSING
For frozen sections, tissue is received o The specimen is then cut into representative
fresh for immediate microscopic sections and is put in small plastic cassette to hold
evaluation by the Pathologist. the tissue
May be received for an OR consultation. ▪ 3.0 x 2.0 x 0.4 cm – std of cassette
The pathologist will look at the specimen ▪ Specimen should not be more than 0.3 mm in
and make a gross diagnosis. thickness.
❖ You may weigh and measure the
specimen Responsibility of a Technician
❖ You may also ink the specimen, if 1. Specimen preservation.
necessary; however, always check 2. Specimen labeling, logging and identification.
with the Pathologist before making 3. Preparation of the specimen to facilitate their gross and
any of these decisions. microscopy.
II. In formalin 4. Record keeping.
Proceed as a routine surgical specimen
Ideally, the specimen should have at least Specimen – Types
20x its volume of formalin.
Volume of tissue to fixative of choice: 1:20 • Excision specimens (surgical biopsies), where whole
organs or affected areas are removed at operation. (we
Specimen Accessioning used blade)
• Incisional biopsy specimens, where tissue is removed
• Each specimen receives an accession number. for diagnosis from within an affected area.
• Each number is unique to that particular case and is • Punch biopsies, to remove a small piece of suspicious
never reused. tissue for examination (often from the skin) – (we used
• The specimen container(s), the requisition slip and all circular blade; usually we used dermatological
cassettes are labeled with the case/accession number. specimen; it produce cylindrical shape sample)
Grossing and Section Cutting • Shave biopsies, where small fragments of tissue are
“shaved” from a surface (usually skin) (we used curve
• Grossing, often referred to as “cut-up”, it involves a razor to remove the sample on the skin)
careful examination and description of the specimen its • Curettings, where tissue is removed in small pieces
- appearance, - number of pieces and dimensions. from the lining of the uterus or cervix. (we used
• Gross Examination of Specimens - the most important curettage)
processes in which the pathologist arrives at a • Core biopsies, where a small tissue sample is removed
diagnosis. using a special needle sometimes through the skin
• Steps: (percutaneously)
o Identification of the specimen
▪ Patient’s surname, name birthday, hospital
number
▪ The specimen container must bear the same
name and accession number in the request
form
• Criteria for rejection of gross specimen
o Discrepancies between the requisition and
specimen label
o Specimen with no labels, or mislabeled
o Leaking specimen container
o Absent clinical data or history
o Inappropriately identified specimen
Grossing
Describing Specimens
• Orientation Markers
o Inks, nicking and suturing (Request form)
o Inks – to identify and orient the specimen
component
o Nicking – for indicating laterally
o Sutures attaches – represented as
▪ LL – long lateral
▪ SS – short superior
o We used ink for the margin or resection
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 8 | FIXATION OF TISSUE SAMPLE
Fixation o Specimen should be placed in fixative as soon as it
is removed from the body to prevent autolysis and
• Procedure adopted to kill, harden, and preserve putrefaction
materials for microscopic study by means of a o Removed from the specimen at least less than 1
substance known as a fixative. hour fixed the specimen to avoid autolysis and
• Shape, structure, intercellular relationship and chemical putrefaction.
constituents of tissues are preserved by preventing • Penetration
degeneration, decomposition and distortion of tissues o Formalin diffuses at 1 mm/hr
after death. o The rate of penetration of fixatives is 1 mm/hr
o The fixatives is preserved not only the structural • Volume
components but also the chemical integrity, o Amount of fixative – 10-25x the volume of tissue
chemical constituent of the sample. to be fixed
• The primary goal of fixation is to preserve the o The optimum is 1:20
morphologic and chemical integrity of the cell in as • Duration of Fixation
life-like a manner as possible. o Fibrous organs take longer to fix than biopsies or
• The second goal of fixation is to harden and protect the scrapings
tissue from the trauma of further handling, so that it is o Can be cut down using heat, vacuum, agitation or
easier to cut and process for microscopy. microwave.
Effects of Fixatives Effects of Fixatives
• Preserve the morphologic and chemical integrity of the • They harden soft and friable tissues
cell • Make the cells resistant to damage and distortion
• Harden soft and friable tissues • Inhibit bacterial decomposition
• Inhibit bacterial decomposition • They increase optical differentiation of cells and tissue
• Acts as mordants or accentuators components.
o With the used of fixatives it will enhance the • Act as mordant
staining affinity of the specimen to the dye that will
• Reduce the risk of infections during handling and
used.
processing
Main Factors Involved in Fixation
Characteristics of a Good Fixatives
• Hydrogen ion concentration
• Cheap, stable, safe to handle – 20x the volume of the
o Satisfactory fixation occurs between pH 6 and 8
specimen (it must be large amount)
o Commercial formalin is buffered with phosphate at
• Must be isotonic
a pH of 7
• Inhibit bacterial decomposition
• Temperature
• Must permit rapid and even penetration of tissues –
o Room temperature
specimen should penetrate the left and right side of the
• Thickness of section
uterus.
o Tissue blocks should be either small or thin
• Must make cellular components insoluble to hypotonic
o 1-2 mm2 for electron microscopy
solutions
o 2 cm2 wide for light microscopy
➢ So far, no single fixatives has yet been known to
o Large solid tissue
possess all the qualities of an ideal solution.
▪ Uterus – it cuts anteriorly to penetrate
properly the fixatives Types of Fixatives: According to Composition
o Brain (suspended whole in 10% buffered formalin
for 2-3 weeks) 1. Simple Fixatives: One Component
• Aldehydes
Main Factors Involved in Fixation o Formaldehyde
o Glutaraldehyde
• Osmolality
• Metallic Fixatives
o Slightly hypertonic solutions (400-450 mOsm)
o Mercuric chloride
• Concentration
o Chromate fixatives
o Formaldehyde – 10%
▪ Potassium dichromate
o Glutaraldehyde – 3%
▪ Chromic acid
• Duration of fixation
o Simple fixatives
o Usually for 2-6 hours
▪ Acetone
o Formalin an be washed after fixation for 24 hours.
▪ Alcohol
Practical Considerations of Fixation ▪ Picric acid
▪ Acetic acid
• Speed ▪ Osmium tetroxide (Osmic acid)
2. Compound Fixatives: 2 or More Components
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 8 | FIXATION OF TISSUE SAMPLE
shrinkage of tissues
Preserves fats, glycogen and A soft fixative and does not
Types of Fixatives: According to Action mucin harden some cytoplasmic
structures adequately
A. Microanatomical Fixatives
enough for paraffin
• Permit general microscopic study of tissue structures embedding.
1. 10% Formol Saline Allows tissue enzymes to be
2. 10% Neutral Buffered Formalin studies because it does not
3. Heidenhein’s susa – preservation of the tumor precipitate proteins
specifically skin Recommended for nervous
4. Formol sublimate (formol corrosive) tissue preservation
5. Zenker’s solution Allows natural tissue colors
6. Zenker’s formol (Helly’s solution) to be restored: recommended
7. Bouin’s solution – preservation of embryo for colored tissues
8. Brasil solution photography
B. Cytological Fixatives Tolerant fixative used for
• Preserve specific parts mailing specimen
o Nuclear Fixative
- Preserve nuclear structure (chromosomes). 10% Formol Saline
Contain glacial acetic acid, due to its affinity
to nuclear chromatin. • Microanatomical fixative
- pH 4.6 or less • Recommended for fixation of central nervous tissues
- Examples: and general postmortem tissues for histochemical
1. Flemming’s Fluid examination
2. Carnoy’s Fluid – fastest type of fixatives • Fixation time: 24 hours at 35°C / 95°F
3. Bouin’s Fluid • 48 hours at 20-25°C / 65-77°F
4. Newcorner’s Fluid • Preserves enzymes and nucleoproteins
5. Heidenhain susa • Large specimens may be fixed for a long time
o Cytoplasmic • Slow fixative
- Cytoplasmic structure, no glacial acetic acid,
(destroy mitochondria and Golgi bodies of the 10% Neutral buffered formalin or Phosphate-buffered
cytoplasm) formalin (pH 7)
- pH >4.6
• Recommended for preservation and storage of surgical,
- Examples:
post-mortem and research specimen
1. Flemming’s Fluid without acetic acid
2. Helly’s Fluid • Fixation time: 4-24 hours
3. Formalin with “post-chroming” • Prevents precipitation of acid formalin pigments on
4. Regaud’s Fluid (Muller’s fluid) post-mortem tissues
5. Orth’s Fluid – preservation of Rickettsiae • Best fixative for tissues containing iron pigments and
C. Histochemical Fixatives for elastic fibers
• Preserve the chemical constituents of cells and tissues • Longer to prepare; time consuming; reduce reactivity of
1. Formal Saline 10% myelin to Weigfert’s iron hematoxylin stain; inert
2. Absolute Ethyl Alcohol towards lipids
3. Acetone – diagnosis of rabies for brain specimen Formol – Corrosive or Formol – Sublimate
4. Newcomer’s Fluid
• Formol-mercuric chloride solution is recommended for
Aldehyde Fixatives
routine post-mortem tissues
Formaldehyde (Formalin) • Fixation time: 3-24 hours
• Penetrates small pieces of tissues rapidly
• 10% formalin – most widely used • Excellent for many staining procedures including silver
• A gas produced by the oxidation of methyl alcohol reticulum methods.
• Pure stock solution of 40% - unsatisfactory for routine • Slow penetration; form a mercuric chloride deposits;
fixation inhibits determination of the extent of tissue
• Usual fixation time – 24 hours decalcification
• Buffered to pH 7 with phosphate buffer
Alcoholic Formalin or Gendre’s Fixative
Advantages Disadvantages
Cheap, readily available, May cause sinusitis, allergic • Fixation is faster
easy to prepare, relatively rhinitis, excessive • For rapid diagnosis because it fixes and dehydrates at
stable lacrimation or allergic the same time
dermatitis
Compatible with most stains May produce considerable
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 8 | FIXATION OF TISSUE SAMPLE
• Good for preservation of glycogen and for micro- • Will form precipitate on standing but this is of no
incineration technique - (good for demonstration of consequence.
minerals)
Chromate Fixative
• Used to fix sputum since it coagulates mucus
• Produces gross hardening of tissues Chromic Acid
• Causes partial lysis of RBCs
• Preservation of iron-containing pigments is poor • Used in 1-2% aqueous solution
• Precipitates all proteins and adequately preserves
Glutaraldehyde carbohydrates
• A strong oxidizing agent
• Made up of 2 formaldehyde residues, linked by 3
• Not used because it is hazardous
carbon chains
• For routine light microscopic work Potassium Dichromate
• Buffered glutaraldehyde, followed by secondary
fixation in osmium tetroxide is satisfactory for electron • Used in 3% aqueous solution
microscopy • Fixes but does not precipitate cytoplasmic structures
• Fixation time: ½ hour to 2 hours • Preserves lipids
• Preserves plasma proteins • Preserves mitochondria
• Advantages: Produce less tissue shrinkage
Regaud’s Fluid or Muller’s Fluid
• Disadvantages: Reduces PAS positivity of reactive
mucin. • Fixation time: 12-48 hours
• Penetrates tissues well
Metallic Fixatives
• Hardens tissues better and more rapidly than Orth’s
Mercuric Chloride fluid
• Recommended for demonstration of chromatin,
• Most common metallic fixative. mitochondria, mitotic figures, Golgi bodies, RBC and
• Nuclear components are shown in fine detail. colloid-containing tissues. GMRC
• Routine fixative of choice for preservation of cell detail
in tissue photography Orth’s Fluid
• Trichrome staining is excellent. Permits brilliant
• Fixation time: 36-72 hours
metachromatic staining of cells.
• Recommended for study of early degenerative
• Excellent for trichrome staining (collagen)
processes and tissue necrosis
• Recommended for renal tissue, fibrin, connective
• Demonstrates Rickettsiae and other bacteria
tissues and muscles.
• Preserves myelin better than buffered formalin
• Avoid using metal caps, jewelries.
Lead Fixative
Zenker-formol (Helly’s Solution)
• Used in 4% aqueous solution of basic lead acetate
• Fixation time: 12-24 hours
• Recommended for acid mucopolysaccharides
• Excellent microanatomic fixative for pituitary gland,
• Fixes connective tissue mucin
bone marrow, and blood containing organs such as
• Takes up carbon dioxide to form insoluble carbonate
spleen and liver
especially on prolonged standing
• Penetrates and foxes tissues as well
• Nuclear fixation and staining is better than Zenker’s Picric Aid Fixative
fluid
• Normally used in strong saturated aqueous solution
Heidenhain’s Susa Solution (1%)
• Excellent fixative for glycogen demonstration
• Recommended mainly for tumor biopsies especially of
• Penetrates tissues well and fixes small tissues rapidly
the skin
• Also dyes the tissues. Allows brilliant staining with the
• Excellent cytologic fixative
Trichrome method.
• Fixation time: 3-12 hours
• Bouin’s Solution
• Penetrates and fixes tissues rapidly and evenly
o Recommended for fixation of embryos and
• Produces brilliant results with sharp nuclear and
pituitary biopsies. Excellent fixative for preserving
cytoplasmic details
soft and delicate structures
• After fixation, tissue should be transferred directly to
o Fixation time: 6-24 hours
high grade alcohol to avoid swelling.
o Produces minimal distortion of microanatomical
B-5 Fixative structures
o Permits brilliant staining of tissues
• Commonly used for bone marrow biopsies o Preserves glycogen
• Rapid fixation can be achieved in 1 ½-2 hours o Does not need washing out
• Brasil Alcoholic Picroformol Fixative
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 8 | FIXATION OF TISSUE SAMPLE
o Better and less messy than Bouin’s solution o Recommended for cytoplasmic structures
o Excellent fixative for glycogen particularly the mitochondria
o Fixation time: 24-48 hours / 1-2 days
Alcohol Fixatives
Trichloroacetic Acid
• Rapidly denatures and precipitates proteins by
destroying hydrogen and other bonds • Precipitates proteins
• Must be used in concentrations raging from 70% - • Can be used as a weak decalcifying agent and a fixative
100% because less concentrated solution will produce at the same time
lysis of cells. • Its softening effect on dense fibrous tissues facilitates
• Absolute Alcohol preparation of such sections
o Used to fix and preserve glycogen, pigments, • Disadvantage: Poor penetrating agent
blood, tissue films and smears. • Suitable only for small pieces of tissues or bones
o The color of the specimen can be preserved for
photographic works using 80% alcohol Acetone
o It is ideal for small tissue fragments
• Used at ice cold temperature ranging from -5°C to 4°C
o Excellent for glycogen preservation
• Recommended for the study of water diffusible
o Preserves nuclear stains
enzymes especially phosphatases and lipases
• Methyl Alcohol
• Used in fixing brain tissues for diagnosis of rabies
o Excellent for fixing dry and wet smears, blood
• Used as a solvent for certain metallic salts to be used in
smears and bone marrow tissues
freeze substitution techniques for tissues blocks
o Fixes and dehydrates at the same time
o Penetration is slow • It dissolves fat and preserves glycogen poorly
o Tissues may be overhardened and difficult to cut if • Evaporates rapidly
left for more than 48 hours. Heat Fixation
• 95% Isopropyl Alcohol
o Used for fixing touch preparations • Involves thermal coagulation of tissue protein for rapid
• Ethyl Alcohol diagnosis
o Used at concentrations of 70% to 100%
Microwave Technique
o Used as simple fixative
o Fixation time: 18-24 hours • Works as physical agent to increase the movement of
o Preserves but does not fix glycogen molecules and accelerates fixation.
o Preserves nucleoproteins and nucleic acid, hence • Used to accelerate staining, decalcification,
used for histochemistry and enzyme studies. immunohistochemistry and electron microscopy.
• Carnoy’s Fluid
o Recommended for fixing chromosomes. Lymph Secondary Fixation
glands and urgent biopsies
o Used to fix brain tissues for the diagnosis of rabies • Is the process of placing an already fixed tissue in a
o Fixation time: 1-3 hours second fixative.
o Considered as the most rapid fixative Post-Chromatization
o Fixes and dehydrates at the same time
o Permits good nuclear staining and differentiation • Form of secondary fixation
• 2.5-3% potassium dichromate for 24 hours to acts as
Osmium Tetroxide mordant for better staining and aid in cytologic
• Fixes conjugated fats and lipids permanently by making preservation of tissues.
them insoluble Washing Out
• Preserves cytoplasmic structures well such as Golgi
bodies and mitochondria • The process of removing excess fixative from the tissue
• Fixes myelin and peripheral nerves, it is used after fixation
extensively for neurological tissues • Solution used for Washing Out
• Produces brilliant nuclear staining with safranin 1. Tap water used to remove:
• It adequately fixes materials for ultrathin sectioning in ❖ Hellys, Zenkers Flemming
electron microscopy. ❖ Formalin
• Flemming’s Solution ❖ Osmic acid
o Most common chrome-osmium acetic acid fixative 2. 50-70% alcohol
o Fixation time: 24-48 hours ❖ Picric acid (Bouin’s)
o Excellent fixative for nuclear structures 3. Alcoholic iodine
o Permanently fixes fat ❖ Mercuric fixative
• Flemming’s Solution Without Acetic Acid
o Made up of only chromic acid and osmic acid
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 8 | FIXATION OF TISSUE SAMPLE
Factors that Affect Fixation of Tissues
• Retarded by:
o Size and thickness of the tissue specimen
❖ Larger tissues require more fixatives and
longer fixation time
o Presence of mucus
❖ Tissue chain contain mucus are fixed slowly
and poorly
o Presence of fat
❖ Fatty tissues should be cut in thin sections and
fixed longer
o Presence of blood
❖ Tissues containing blood large amount of
blood should be flushed out with saline before
fixing
o Cold temperature
❖ Inactivates enzymes
• Enhanced by:
o Size and Thickness of Tissue
❖ Smaller and thinner tissues require less fixative
and shorter fixation time
o Agitation
❖ Fixation is accelerated when automatic or
mechanical tissue processing is used.
o Moderate heat (35-56°C)
❖ Accelerates fixation but hastens autolytic
changes and enzymes destruction
Fixation Artefacts
• Formalin Pigment
o Known artefact produced under acid conditions
o Reduced by fixation in phenol-formalin
o Color black/brown stain under the microscope
• Crush Artefact
o Found in surgical specimen (liver biopsies)
o Associated with an intense eosinophilic staining
o Due to partial coagulation of protein by ethanol
o Incomplete wax impregnation
Difficulties Encountered Due to Improper Fixation
Difficulties Causes
Failure to arrest early Failure to fix immediately
autolysis of cells Insufficient fixative
Removal of substances Wrong choice of fixative
soluble in fixing agent
Loss or inactivation of
enzymes needed for study
Presence of artefact Incomplete washing of
pigments on tissue sections fixative
Tissues are soft and feather- Incomplete fixation
like in consistency
Shrinkage and swelling of Over fixation
cells and tissue structures
Tissue blocks are brittle and Prolonged fixation
hard
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 9 | DECALCIFICATION
Decalcification • To prevent obscuring the microanatomic detail of
sections.
• Decalcification is the procedure whereby calcium or
lime salts are removed from tissues following fixation. Characteristics of a Good Decalcifying Agent
• The purpose is to remove calcium ions and lime salts.
• Must be capable of removing calcium salts from tissue
• It should be done after fixation and before impregnation
completely.
to ensure and facilitate the normal cutting of sections.
• Must not cause any destruction to cells and tissue
• Decalcification is an optional process in the tissue
component and structural integrity of the specimen.
processing.
• Has a good staining capacity. (it should be mordant or
• Decalcification adjusts the hard substance of bones to
accentuator)
the softness of paraffin embedding medium.
• Bones are the main object of decalcification in a Calcium may be Removed by any of the Following
surgical pathology laboratory. Agents
o Others: Teeth, calcified tumors and heart valves.
• Decalcification enables the histotechnologist to cut soft • Acids
sections of the bone using the microtome, so that they • Chelating agents
can be processes like any other soft tissue of the body. • Ion exchange resins
• Decalcification is a lengthy procedure, as bone pieces • Electrophoresis
have to be left in the decalcifying agent for several days
Acid Decalcifying Agents
or even weeks, depending on the size of the tissue.
• With the advancement of the histopathology laboratory • Most widely used agents for routine decalcification.
we can cut it for 30 minutes or 1 hour. Stable, easily available and relatively inexpensive.
• The principle of decalcification is fairly simple. Strong • The ration of the tissue to the decalcifying agent is 1:20
mineral acids, such as 10% hydrogen chloride (HCl), or • 10% Aqueous Nitric Acid Solution
weak organic acids, such as 5-10% formic acid o Most common and fastest decalcifying agent used
(HCOOH), form soluble calcium salts in an ion so far
exchange that moves calcium into the decalcifying o Recommended concentrations would be 5%-10%
solution. o Rapid in action
• Make sure that the tissue has been adequately fixed and o Produces minimum distortion of tissues and good
rinsed well to prevent any undesired reaction with the nuclear staining
decalcifying agent. Buffered formalin is a satisfactory o Decalcification Time: 12-24 hours
fixative for bone but where the preservation of bone • Formol-Nitric Acid
marrow is important, some laboratories use alternatives o Decalcification Time: 1-3 days
such as zinc formalin mixtures. B-5, formol-acetic o Rapid acting recommended for urgent biopsies
alcohol (Davidson’s fixative), or Bouin’s solution. o Nuclear staining is relatively good
• In order to protect the cellular and fibrous elements of o Produces less tissue destruction than 10% aqueous
bone from damage caused by the acids used as nitric acid.
decalcifying agents, it is particularly important to • Perenyi’s Fluid
thoroughly fix these specimens prior to decalcification. o Decalcification time: 2-7 days
• Decalcification should be done after fixation and before o Recommended for routine purposes
impregnation, to ensure and facilitate the normal cutting o Decalcifies and softens tissue at the same time
of sections and to prevent obscuring the microanatomic o Nuclear and cytoplasmic staining is good
detail of such sections by bone dust and other cellular o Maceration is avoided due to the presence of
debris. chromic acid and alcohol.
• Inadequate decalcification may result in poor cutting of • Phloroglucin – Nitric Acid
hard tissues and damage to the knife edge during o Decalcification time: 12-24 hours
sectioning. o Most rapid decalcifying agent so far
• Poorly-fixed specimens become macerated during o Recommended for urgent works
decalcification and stain poorly afterwards. This is very o Nuclear staining is poor
noticeable in areas containing bone marrow. It is o Prolonged decalcification produces extreme tissue
therefore common practice for laboratories to extend distortion
fixation times for bone specimens before commencing o Complete decalcification cannot be determined by
decalcification. chemical means
• High-quality fine tooth saws should be used to prepare • Hydrochloric Acid
bone slices. Coarse saws can cause considerable o Inferior to nitric acid in its role as a decalcifying
mechanical damage and force bone fragments into the agent because of its slower action and greater
soft tissues present in the specimen. distortion of tissues
o Produces good nuclear staining
Purpose of Decalcification
• Von Ebner’s Fluid
• To ensure and facilitate the normal cutting of sections o Permits relatively good cytologic staining
o Moderately rapid decalcifying agent
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 9 | DECALCIFICATION
o Does not require washing out before dehydration • A layer of ion exchange resin (1/2 thick) is used over
o Recommended for teeth and small pieces of bone the bottom of the container and the specimen is placed
• Formic Acid on top of it. Then the decalcifying agent is added.
o Moderate acting decalcifying agent which produces • The tissue may stay for 1-14 days. The degree of
better nuclear staining with less tissue distortion. decalcification may be measured by physical or X-ray
May be used as a fixative and decalcifying agent method.
o Safer to handle than nitric acid or hydrochloric acid
o Recommended for routine decalcification of Electrophoresis
postmortem research tissues
• A process whereby positively charged calcium ions are
o Decalcification time: 2-7 days
attracted to a negative electrode and subsequently
o Recommended for small pieces of bones and teeth removed from the decalcifying solution.
o Suitable for routine surgical specimens, when
• This method is satisfactory for small bone fragments,
immunohistochemical staining is needed.
processing only a limited number of specimens at a
o Relatively slow, not suitable for urgent works
time.
o Requires neutralization with 5% sodium sulfate and
• Good cytologic and histologic details are not always
washing out.
preserved.
• Formic Acid – Sodium Citrate Solution
o Decalcification time: 3-14 days Factors Influencing Rate of Decalcification
o Permits better nuclear staining than nitric acid
method • Structure
o Recommended for autopsy materials, bone marrow, o High concentration and greater amount of fluid will
cartilage and tissues studied for research purposes. increase the speed of the process
• Trichloroacetic Acid o The concentration and the amount of
o Decalcification time: 4-8 days decalcification is directly proportional to the rate
o Permits good nuclear staining of decalcification.
o Does not require washing out • Temperature
o Suitable only for small spicules of bone o 37°C impaired nuclear staining of Van Gieson’s
• Sulfurous Acid stain for collagen fibers
o Very weak decalcifying agent suitable only for o 55°C tissue will undergo complete digestion within
minute pieces of bone 24-48 hours
• Chromic Acid (Flemming’s Fluid) o RT (room temperature) range of 18-30°C
o May be used as a fixative and a decalcifying agent • Volume
o Used to decalcify minute bone spicules o Ratio 20:1
o Nuclear staining with hematoxylin is inhibited • Time
o Forms precipitates at the bottom, which requires o 1-2 days/24-48 hours ideal time for decalcification
frequent changes of solution o Dense bone tissue – 14 days longer
o Degree of decalcification cannot be measure by
Measuring the Extent of Decalcification
routine chemical test.
• Physical or Mechanical Test
Chelating Agent
o Done by touching with the fingers to determine the
• Ethylene Diamine Tetraacetic Acid (EDTA) consistency of tissue.
o Combines with calcium ions and other salts to form o Bending, needling or by use of scalpel if it bends
weakly dissociated complexes and facilitate easily that means decalcification is complete.
removal of calcium salts. o Pricking, causes damage and distortion of tissue.
o Commercial name: Versene • X-Ray or Radiological Method
o Recommended for detailed microscopic studies o Best method for determining complete
o It is a very slow decalcifying agents decalcification
o For small specimen, 1-3 weeks ▪ *not recommended on issue fixed mercuric
o For dense cortical bone, it will take 6-8 weeks to chloride, (radio opacity).
decalcify • Chemical Method (Calcium Oxalate Test)
o pH must be 6.5 – 7.4 o Simple, reliable and convenient method for routine
o EDTA is used for immunohistochemical, enzyme purposes
staining and electron microscopy o Detect Ca in the decalcifying solution by
o EDTA inactivates alkaline phosphatase activity precipitation of insoluble calcium hydroxide or
calcium oxalates.
Ion Exchange Resin
Post-Decalcification
• Ammonium form of polystyrene resin that hastens
decalcification by removing calcium ions from formic • The removal of acid from tissue or neutralized
acid containing decalcifying solutions. chemically by immersing the specimen either saturated
• Not recommended for fluids containing mineral acids lithium carbonate solution or 5-10% aqueous sodium
such as nitric acid or hydrochloric acid. bicarbonate solution for several hours.
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 9 | DECALCIFICATION
• Simply rinse the decalcified specimens with running tap
water.
Tissue Softeners
• Unduly hard tissues which are liable to damage the
microtome knives may require tissue softeners, aside
from decalcification.
• Perenyi’s fluid
• Moliflex
• 2% HCl
• 1% HCl in 70% Alcohol
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 10 | DEHYDRATION AND CLEARING
Dehydration • Prolong storage of lower concentration (<70% alcohol)
it will tend to macerate the tissue.
• The aim of the dehydration is to remove fixative and
• Alcohol temperature also place a role in dehydration.
water from the tissue and replacing them with
• 37°C it will hasten the dehydration time
dehydrating fluid in preparation for impregnation.
• Ethyl alcohol
• This fixative and water were accumulated during the
o Recommended for routine dehydration.
process of fixation and washing out.
o Clear, colorless flammable fluid.
• Dehydrating fluids are generally used in increasing
o Considered to be the best dehydrating agent
strengths.
because it is fast-acting.
• Increasing strengths = all the aqueous tissue fluids are
• Methyl alcohol
removed but with little disruption to the tissue due to
o This is toxic dehydrating agent.
diffusion currents.
o Used for blood and tissue films for smear
• Drying. The removal of the water by evaporation. In preparations.
tissue processing in histopathology, we never allow the o When you ingest/drink 10mL of alcohol it will be
sample to be air dried. We used the process metabolize to become formic acid. The formic acid
dehydration. In dehydration it involves slow may cause blindness.
substitution of the water in the tissue with the used of
• Buthyl alcohol
the organic solvent.
o Utilized in plant and animal micro-techniques;
• Organic solvents are the dehydrating fluid. slow dehydrating agent, produces less shrinkage ad
• Removal of intracellular and extracellular water from hardening.
the tissue following fixation and prior to wax o Recommended for tissues who do not require rapid
impregnation. It is important to keep the dehydration processing.
times as brief as possible to minimize the risk of
extracting cellular constituents. General Schedule for Alcohol Dehydration
• The amount of each stage should not be less than 10x
• 70% - alcohol – 6 hours
the volume of the tissue in order to ensure complete
penetration of the tissue by the dehydrating agent. • 90% - alcohol – 12 hours
• Volume of the ratio to the dehydrating agents is 1:10. • 100% - 2 hours
• For delicate tissues, particularly embryonic and animal • 100% - 1 hour
tissues, it is recommended to start processing with 30% • 100% - 1 hour
ethyl alcohol. Acetone
Characteristics of an Ideal Dehydrating Solution
• Both fixative and dehydrating agent.
• It must dehydrate rapidly • Cheap, rapid acting dehydrating agent. Dehydrates in ½
• Not evaporate very fast (.5) to 2 hours.
• Be able to dehydrate fatty tissues • Clear, colorless highly flammable and extremely
• Should not harden your tissues volatile fluid.
• It should not remove stains • Rapid in action but penetrates tissues poorly and causes
brittleness in tissues that are prolonged dehydrated.
• Should not the toxic
• Produces considerable tissue shrinkage
• Should not be a fire hazard
• Not recommended for routine dehydration purposes.
Commonly Used Dehydrating Agents
Dioxane (Diethylene Dioxide)
• Alcohol (Ethyl alcohol, methyl alcohol)
• This is an excellent dehydrating and clearing agent
• Acetone
• Produces less tissue shrinkage
• Dioxane
• Tissues can be left for long periods of time without
• Cellosolve
affecting the consistency or staining properties of the
• Triethyl Phosphate
specimen
• Tetrahydrofuran
• Tissue sections dehydrated with dioxane tend to ribbon
Alcohol poorly.
• Expensive and extremely dangerous
• Tissue is passed through a series of progressively • Produces poor ribbon during microtomy
increasing concentrations of alcohol.
• 85%-95% - liable to produce considerable shrinkage Cellusolve (Ethylene Glycol Monoethyl Ether)
and hardening of tissues leading to distortion.
• Both dehydrating and clearing agent.
• 95% or absolute alcohol – it may tend to harden only
• Dehydrates rapidly
the surface of the tissue while the deeper parts are not
completely penetrated; it will cause excessive • The tissue may be transferred from water or normal
hardening of the outer portion of the tissue; it will not saline directly to cellusolve and stored in it for months
penetrate properly without producing hardening or distortion.
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 10 | DEHYDRATION AND CLEARING
• Ethylene glycol ether is combustible at 110°F to 120°F o Should not dissolve out aniline dyes
and is toxic. o Should not evaporate quickly in a water bath
• Propylene based glycol ether should be used instead. o Should make tissues transparent
• Common Clearing Agents Used
Triethyl Phosphate o Xylene
• It removes water very readily and produces very little ▪ It is colorless, clearing agent that is most
commonly used in the laboratory
distortion and hardening of tissue.
▪ It is most rapid clearing agent suitable for
• It is used to dehydrate sections and smears following
agent biopsy.
certain stains and produces minimum shrinkage.
▪ Clearing time: .5-1 hour
Tetrahydrofuran ▪ It makes the issue transparent
▪ Does not extract aniline dye
• It is both a dehydrating agent and clearing agent since it ▪ Can be used for celloidin sections because it
is miscible in both water and paraffin. does not dissolve celloidin
• It may be used for demixing, clearing and dehydrating ▪ It is cheap
paraffin sections before and after staining. ▪ Disadvantages:
• It causes less shrinkage and easier cutting of sections Highly flammable
with fewer artefacts. If used longer than 3 hours, it will make
• It does not dissolve aniline dyes the tissues excessively hard and brittle
• It is toxic if ingested or inhaled Not suitable for nervous tissues and lymph
nodes (causes considerable hardening and
Additives to Dehydrating Agents shrinkage of tissues)
4% phenol + each 95% ETOH baths Xylene becomes milky when an
incomplete dehydrated tissue is immerse
• Acts as a tissue softener for hard tissues such as in it.
tendons, nails, or dense fibrous tissues. o Toluene
▪ May be used as a substitute for xylene or
Anhydrous Copper Sulfate benzene
• It acts both as a dehydrating agent and as an indicator of ▪ Clearing time: 1-2 hours
water content of the last bath. ▪ Acts fairly rapidly and is recommended for
routine purposes
• To insure complete dehydration, a layer of anhydrous
▪ Tissues do not become excessively hard and
copper sulfate, about ¼ inch deep is placed in the
brittle if left for 24 hours
bottom of the container and covered with filter paper.
▪ It is not carcinogenic
This will accelerate dehydration by removing water
▪ Disadvantages:
from the dehydrating fluid. A bloodiest coloration of
Relatively slower than xylene and benzene
copper sulfate crystals will indicate full saturation of
Tends to acidify in a partially filled vessel
dehydrating fluids with water. Alcohol is then discarded
More expensive
and changed with a fresh solution.
o Benzene
• Anhydrous copper sulfate color blue discoloration.
▪ It is preferred as clearing agent in embedding
Clearing (De-alcoholization) process of tissues because it penetrates and
clears tissues rapidly.
• The process whereby alcohol or a dehydrating agent is ▪ Clearing time: 15-60 minutes
removed from the tissue and replaced with a substance ▪ Volatizes rapidly in paraffin oven, easily
that will dissolve the wax with which the tissue is to be removed in the tissue
impregnated. ▪ Does not make tissues hard and brittle but it
• Process of replacing the dehydrating fluid with a fluid causes minimum shrinkage
that is miscible with both dehydrating fluid, ▪ It makes tissues transparent.
impregnating or embedding medium. ▪ Disadvantages:
• Most commonly used clearing agents are xylene, It is highly flammable
dioxane, chloroform, and cedarwood oil. If section is let in benzene for a long time,
• The clearing agent will make microscopic tissue considerable tissue shrinkage may be
preparations transparent due to their high index of observed.
refraction. Excessive exposure to this reagent it is
• Characteristics of a Good Clearing Agent very toxic and carcinogenic to human.
o Miscible with alcohol to promote rapid removal of It may caused or damaged the bone
the dehydrating agent marrow and it may result to aplastic
o Should be miscible with and easily removed by anemia.
melted paraffin wax. o Chloroform
o Should not produce excessive shrinkage, hardening ▪ Slower in action than xylene but causes less
or damage of tissue. brittleness
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 10 | DEHYDRATION AND CLEARING
▪ Suitable for large tissue specimens. Thicker ▪ It is non-toxic but has offensive odor and
tissue blocks (up to 1 cm) are can be should be used in a well-ventilated room.
processed. o Methyl Benzoate and Methyl Salicylate
▪ Clearing time: 6-24 hours ▪ Slow-acting clearing agents that can be used
▪ It is recommended for tough tissues, nervous when double embedding techniques are
tissues, lymph nodes, and embryos required.
▪ Not flammable o Glycerin and Gum Syrup
▪ Disadvantage: ▪ Are used when the tissue is to be cleared
Relatively toxic to the liver after directly from water, as in a frozen section.
prolonged inhalation
Wax impregnation after chloroform
clearing is relatively slow
Does not make tissues transparent
Difficult to remove from paraffin sections
because it is not very volatile
Complete clearing is difficult to evaluate
o Cedarwood oil
▪ It is used to clear both paraffin and celloidin
sections during embedding process
▪ It is recommended for central nervous tissue
and cytological studies
▪ Clearing time: 2-3 days
▪ Very penetrating clearing agent
▪ Clears celloidin in 5-6 days
▪ Does not dissolve aniline dyes
▪ Makes tissues transparent
▪ Disadvantage:
Extremely slow clearing agent, not
recommended for routine purposes
Becomes milky upon prolonged storage
and should not be filtered before use
Very expensive
o Aniline oil
▪ Not normally utilized as a routine clearing
agent
▪ Recommended for clearing embryos, insects,
and very delicates specimens due to its ability
to clear 70% alcohol without excessive tissues
shrinkage and hardening.
o Clove Oil
▪ Causes minimum shrinkage of tissues
▪ Its quality is not guaranteed due to its tendency
to become adulterated
▪ Wax impregnation after clearing with clove oil
is slow and difficult
▪ Tissues become brittle, aniline dyes are
removed and celloidin is dissolved
▪ Expensive solution – unsuitable for routine
clearing purposes
o Carbon Tetrachloride
▪ Its properties are similar to chloroform
although it is relatively cheaper
▪ Same disadvantage of chloroform
▪ It produces considerable tissue hardening and
dangerous to inhale on prolonged exposure due
to its highly toxic effects.
o Tetrahydrofuran*
▪ Tetrahydrofuran is superior to ordinary
dehydrating and clearing agents due to its
ability to perform two processes at the same
time thereby shortening the total processing
time and allowing more time for fixation.
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 11 | IMPREGNATION, EMBEDDING, AND TRIMMING
Impregnation ▪ For rapid diagnosis with less technicality
▪ 2-3 changes of wax is required
• Also known as infiltration. • Vacuum Embedding
• Process whereby the clearing agent is completely o Involves the wax impregnation under negative
removed from the tissue & replaced by a medium that atmospheric pressure (400-500mmHg) inside an
will completely fill all the tissue cavities, thereby giving embedding oven.
a firm consistency to the specimen o Gives the fastest results
• Process of replacing the clearing agent with the ▪ To hasten removal of air bubbles and clearing
infiltrating medium. agent
• The medium used to infiltrate the tissue is usually the ▪ Promote rapid wax penetration
same medium used for embedding. ▪ Time reduce from 25-75% of the normal time
require.
Four Types of Tissue Impregnation and Embedding
Media Factors Affecting Paraffin Wax Impregnation
1) Paraffin Wax Impregnation • Nature and size of the tissues to be processed
• The man who introduced paraffin wax • Type of clearing agents to be used
embedding: Butschlii
• Simplest, most common and the best Precautions Observed in Paraffin Wax Impregnation
infiltrating/embedding medium.
• Tissue should not be left for longer periods of time
• Process is very rapid, allowing sections to be prepared
within 24 hrs. • Maintained a temperature 2 to 5°C above the melting
point
• Disadvantage:
o Overheated paraffin makes the specimen brittle • Paraffin wax must be pure
o Prolonging will cause excessive tissue shrinkage • Fresh wax should be filtered before use
and hardening • When using automatic tissue processing machine, wax
o Is not recommended for fatty tissues (the usually becomes admixed with the clearing agent,
dehydrants and clearing agents used in the process especially in the first beaker
dissolve and remove fat from the tissues). • For fixed knife microtomes, a relatively hard wax with
• After clearing, tissue is submerged in 2 or more changes a higher melting point is recommended
of melted paraffin wax. Substitutes for Paraffin Wax
• Temperature of paraffin oven = it should be 55-60°C
(Paraffin oven must be maintained at a temperature 2- • Paraplast
5°C above the MP of the paraffin wax to be used.) o Mixture of highly purified paraffin and synthetic
• Melting point (common Waxes) plastic polymers
• 45, 52, 56 58°C o Melting point of 56-57°C
• Wax with melting point = 56°C is normally used for ▪ Embeddol – 56-58°C
routine work. ▪ Bioloid – semisynthetic type of wax that is
• Lab temp: 20-24°C PW melting pt. 54-58°C recommended for embedding the eyes
• Lab temp: 15-18°C Melting point of wax 50 and ▪ Tissue mat – is a product of paraffin
54°C • Ester Wax
• Hard tissue require wax with higher melting point o Has lower melting point 46-48°C
• When wax has been reused, some water is mixed with o Harder than paraffin
it. o Not soluble in water but soluble in 95% ethyl
• If excessive water accumulates, this may impair the alcohol
impregnating capacity of the medium. • Water Soluble Waxes
• To remove excess water = heat the wax to 100-105°C o Mostly polyethylene glycols
• Paraffin wax may be used twice. o Melting point 38-42°C or 45-56°C
o Carbowax - most common (hydroscopic)
Three ways by which Paraffin Wax Impregnation ▪ 4 changes of carbowax for routine processing
and Embedding May be Performed: ▪ 70%, 90%, 100% (2x)
▪ 56°C at 30 minutes
• Manual Processing ▪ 45 minutes and 1 hour (with agitation)
Fixation Time
10% Buffered Formalin 24 hours 2) Celloidin (Collodion) Impregnation
70% Alcohol 6 hours • Purified form of nitrocellulose soluble in many solvents
95% Alcohol 12 hours • Suitable for specimen with hollow cavities
100% Alcohol 2 hours • Recommended for processing neurological tissues
100% Alcohol 1 hour • Avoiding the crumbling of tissues during sectioning
100% Alcohol 1 hour • Does not require heat as compared to paraffin wax
Clearing 1 hour (2x) • Disadvantage:
Impregnation: Paraffin wax 15 minutes (4x) o Very slow (lasting for several days or weeks
Embedding: Paraffin wax 3 hours o Very thin section difficult to cut
• Automatic Processing o Photomicrographs are difficult to obtain
o With the use of auto Technicon. o Very volatile
▪ Fixes, dehydrates, clears and infiltrates tissues
MIDTERMS | HPCT311 LECTURE |K.BAGANG
WEEK 11 | IMPREGNATION, EMBEDDING, AND TRIMMING
Two Methods for Celloidin Impregnation • Used to facilitate cutting of large block of dense
firm tissue like the brain.
1. Wet Celloidin – recommended for bones, teeth, large
brain sections and whole organs. Types of Blocking – Out Embedding Molds
2. Dry Celloidin – preferred for processing of whole eye
sections. A. Leuckhart’s Embedding Mold
• Consist of two L-shaped strips of heavy brass of metal
Nitrocelllulose Method arranged on a flat metal plate and which can be moved
to adjust the size of the mold to the size of the specimen
• Low Viscosity Nitrocellulose (L.V.N.) is another form
of celloidin soluble in equal concentration of ether and
alcohol, with lower viscosity, allowing it to be used in
higher concentration and still penetrate tissue rapidly
• It forms a harder tissue block and makes cutting of
thinner sections possible
• The tendency to tissues to crack may be prevented by
adding plasticizers (oleum ricini or castor oil) when
embedding chrome-mordanted tissues.
• L.V.N. is more explosive than celloidin.
3) Gelatin Impregnation B. Compound Embedding Unit
• Rarely used except when dehydration is to be avoided • Made up of a series of interlocking plates resting on a
and when tissue are subjected to histochemical and flat metal base, forming several compartments
enzyme studies
• Used as an embedding medium for delicate specimens
and frozen tissue sections.
• It is water soluble, does not require dehydration and
clearing, although fixatives should still washed out by
running water.
• Tissue is placed in 10% gelatin with 1% phenol for 24
hours, transferred to 20% gelatin with 1% phenol for
the next 12 hours, Finally to another fresh solution of
20% gelatin with 1% phenol which is then allowed to
cool in a refrigerator until impregnation and embedding C. Plastic Embedding Rings & Base Mold
are completed. • Consist of a special stainless steel base mold fitted with
• Tissue should not be more than 23 mm. thick a plastic embedding ring, which later serves as the
• The volume of the impregnating medium should be at block holder during cutting.
least 25x the volume of the tissues.
Embedding
• Process by which the impregnated tissue is placed into a
precisely arranged position in a mold containing a
medium which is then allowed to solidify.
Orientation D. Disposable Embedding Molds
• Peel-away
• Process by which the tissue is arrange in precise • Plastic Ice trays
position in the mold during embedding, on the • Paper boats
microtome before cutting and on the slide before
staining. Trimming of Sections
4) Plastic (Resin)
• Is the process of removing the excess wax by cutting off
• It has provided superior results for light microscopic
from the block to expose the tissue surface in
studies, particularly in when using hard tissues
preparation for sectioning.
• Tissue sections must be 4-6um, such in renal biopsies
• Once the wax has solidified,
and bone marrow biopsies.
o The wax block is removed from the mold, the
• Plastic are classified into epoxy, polyester, or acrylic,
identification number is noted & the excess wax is
based on their chemical composition.
cut off from the block to expose the tissue surface
Other Embedding Methods in preparation for cutting.
1. Celloidin or Nitrocellulose Method
• Recommended for embedding hard tissues
2. Double Embedding Method
• Process in which tissues are first infiltrated
with celloidin and
• Subsequently embedded in paraffin mass.
MIDTERMS | HPCT311 LECTURE |K.BAGANG
You might also like
- Fresh Tissue Examination - HistopathDocument4 pagesFresh Tissue Examination - HistopathIrish De VeraNo ratings yet
- Mod 2.1Document7 pagesMod 2.1Pauline Louise S. DURANNo ratings yet
- Fresh Tissue ExaminationDocument6 pagesFresh Tissue ExaminationHenessey Palisoc ResuelloNo ratings yet
- HistotechniquesDocument47 pagesHistotechniquesJean VipinosaNo ratings yet
- Trans Histopath 3Document5 pagesTrans Histopath 33A PEÑA AndreaNo ratings yet
- Routine Histotechniques - Lecture 1Document47 pagesRoutine Histotechniques - Lecture 1Gerald John Paz100% (1)
- Preparation of Fresh TissueDocument3 pagesPreparation of Fresh TissueMaster ChiefNo ratings yet
- HPCT Lec MidtermsDocument25 pagesHPCT Lec MidtermsKRISTERLEX RUIZNo ratings yet
- Histopath Lec MidetermsDocument48 pagesHistopath Lec MidetermsMark jay LlanoNo ratings yet
- Lesson 1Document3 pagesLesson 1Krizza UrmazaNo ratings yet
- Fresh Tissue Examination: Mark Lester B. Cauan, RMTDocument22 pagesFresh Tissue Examination: Mark Lester B. Cauan, RMTMarissa CordovaNo ratings yet
- Nursing Implications For Diagnostic Tests: Bone Scan and Gallium ScanDocument1 pageNursing Implications For Diagnostic Tests: Bone Scan and Gallium ScanAnisa JamitoNo ratings yet
- GiggzzDocument11 pagesGiggzzBrahmvansh GujralNo ratings yet
- Fresh Tissue ExaminationDocument23 pagesFresh Tissue ExaminationMarissa CordovaNo ratings yet
- Proper Processing of Fresh Tissue - SPC MLS 2B - Histopath LabDocument4 pagesProper Processing of Fresh Tissue - SPC MLS 2B - Histopath LabMa. Erikah CancioNo ratings yet
- Health DigestDocument14 pagesHealth DigestKristina SabuNo ratings yet
- Gregorios-Histopathologic-Techniques Ch3 and 29Document78 pagesGregorios-Histopathologic-Techniques Ch3 and 29EllaineSonejaNo ratings yet
- The Role of Ioc in Sarcoma SurgeryDocument31 pagesThe Role of Ioc in Sarcoma SurgeryLILA RUSTIKANo ratings yet
- Cytological TechniquesDocument4 pagesCytological TechniquesJamie100% (1)
- BIOPSY Oral SurgeryDocument34 pagesBIOPSY Oral SurgeryNisha ChoudharyNo ratings yet
- LaSala Rocco-SCACM 2017 Workshop-Cyto Histo IntroDocument36 pagesLaSala Rocco-SCACM 2017 Workshop-Cyto Histo Introrut4soloNo ratings yet
- Bacteria, Virus, Chlamydia, Rickettsia, Mycoplasma, Parasite, Toxin, PoisonsDocument5 pagesBacteria, Virus, Chlamydia, Rickettsia, Mycoplasma, Parasite, Toxin, PoisonsNerdyPotatoNo ratings yet
- HistopathDocument6 pagesHistopathRichell VillacarlosNo ratings yet
- TissuesDocument27 pagesTissuesAngela Nicole Udan100% (1)
- Histopath Review 2017 (3.0 Hrs Lecture) Notes PDFDocument111 pagesHistopath Review 2017 (3.0 Hrs Lecture) Notes PDFPerlieCaguete100% (1)
- Bone Marrow Sampling: Bone Marrow Cytology (Aspiration) Vs Histology (Core Biopsy)Document5 pagesBone Marrow Sampling: Bone Marrow Cytology (Aspiration) Vs Histology (Core Biopsy)Thamil ArasanNo ratings yet
- 1 Introduction To Tissue ProcessingDocument7 pages1 Introduction To Tissue ProcessingAngel RamosNo ratings yet
- Fresh Tissue ExaminationDocument6 pagesFresh Tissue ExaminationChiizu iraNo ratings yet
- His To ChemistryDocument61 pagesHis To Chemistrycutereedah62No ratings yet
- LAB-Histopath Midterms 01Document5 pagesLAB-Histopath Midterms 01Jashmine May TadinaNo ratings yet
- BIOPSYDocument52 pagesBIOPSYAyyagari Kameswar RaoNo ratings yet
- Basic HistologyDocument6 pagesBasic HistologycelecosibNo ratings yet
- Intraoperative CytologyDocument86 pagesIntraoperative Cytologypreeti sharmaNo ratings yet
- Histopathology: 1. Routine Histopathologic ExaminationDocument2 pagesHistopathology: 1. Routine Histopathologic ExaminationPat DazaNo ratings yet
- 02 HP Lab - Fresh Tissue ExaminationDocument30 pages02 HP Lab - Fresh Tissue ExaminationLaiza Fathma AbilosaNo ratings yet
- His To PathologyDocument196 pagesHis To PathologyKelvssNo ratings yet
- Hstcyt1 Lcc1 Short Term NotesDocument33 pagesHstcyt1 Lcc1 Short Term NotesMHEKAELLA SAMSONNo ratings yet
- Chapter 17 Cytology SpecimensDocument2 pagesChapter 17 Cytology SpecimensHalidah Rahawarin100% (1)
- 1) BiopsyDocument30 pages1) BiopsySosa GeorgeNo ratings yet
- HISTOPATHOLOGIC AND CYTOLOGIC TECHNIQUES LEC Module 2Document14 pagesHISTOPATHOLOGIC AND CYTOLOGIC TECHNIQUES LEC Module 2Clair TugnaNo ratings yet
- Introduction To Histopathology and CytologyDocument25 pagesIntroduction To Histopathology and CytologyVinayNo ratings yet
- Histopathology Laboratory OverviewDocument31 pagesHistopathology Laboratory OverviewaliNo ratings yet
- Cytology Sample Collection and Preparation For Veterinary PractitionersDocument8 pagesCytology Sample Collection and Preparation For Veterinary PractitionersKmo mastnNo ratings yet
- CMCRP Rle ReviewerDocument13 pagesCMCRP Rle ReviewerAyang 00No ratings yet
- Lesson-21 Exfoliative Cytology PDFDocument4 pagesLesson-21 Exfoliative Cytology PDFSasa AbassNo ratings yet
- Histopathology PrelimsDocument2 pagesHistopathology PrelimscassseeeyyyNo ratings yet
- 2098 Sample Preparation OpticalDocument27 pages2098 Sample Preparation OpticalRitesh ThakurNo ratings yet
- Exfoliative CytologyDocument9 pagesExfoliative Cytologymiguel gaquitNo ratings yet
- Methods Used in Histology: Tissue Preparation, Histochemistry, CytochemistryDocument50 pagesMethods Used in Histology: Tissue Preparation, Histochemistry, Cytochemistrynav_malhiNo ratings yet
- Animal Cytology (Compatibility Mode)Document13 pagesAnimal Cytology (Compatibility Mode)AdarshBijapurNo ratings yet
- Tissue Handling and GrossingDocument48 pagesTissue Handling and GrossingCAMILLE MAGNO100% (3)
- Biopsy CytologyDocument9 pagesBiopsy CytologyX-ManNo ratings yet
- Lab Diagnosis of Cancer: Staging and PrognosisDocument35 pagesLab Diagnosis of Cancer: Staging and PrognosisAuri ArlidenNo ratings yet
- HP - 2 - Gross Examination, Fixation, Decalcification, Dehydration Version 3Document7 pagesHP - 2 - Gross Examination, Fixation, Decalcification, Dehydration Version 3Klieden LobrigasNo ratings yet
- Tissue ProcessingDocument4 pagesTissue ProcessingSeon u 'No ratings yet
- BIOPSYDocument8 pagesBIOPSYASHLEY DAWN BUENAFENo ratings yet
- Lec 1. Introduction To Histology - AABDocument14 pagesLec 1. Introduction To Histology - AABejekorangNo ratings yet
- Pap Smear: Dr. Monika NemaDocument114 pagesPap Smear: Dr. Monika NemafadoNo ratings yet
- Histopathology and CytologyDocument26 pagesHistopathology and CytologyKirsten ValenzuelaNo ratings yet
- 307-01 Automatic Transmission 10 Speed - Description and Operation - DescriptionDocument12 pages307-01 Automatic Transmission 10 Speed - Description and Operation - DescriptionCARLOS LIMADANo ratings yet
- Cheng-Yi Cheng - Yi: KBU 10A/15A/25A/35A SERIESDocument2 pagesCheng-Yi Cheng - Yi: KBU 10A/15A/25A/35A SERIESThomas ThomasNo ratings yet
- Illiquidity and Stock Returns - Cross-Section and Time-Series Effects - Yakov AmihudDocument50 pagesIlliquidity and Stock Returns - Cross-Section and Time-Series Effects - Yakov AmihudKim PhượngNo ratings yet
- Nihonto Part IDocument38 pagesNihonto Part IGergő VidaNo ratings yet
- Chapter 7 Analysis of Stress and StrainDocument20 pagesChapter 7 Analysis of Stress and StrainLong Nguyễn HoàngNo ratings yet
- 12th Maths EM Second Mid Term Exam 2023 Question Paper Thenkasi District English Medium PDF DownloadDocument2 pages12th Maths EM Second Mid Term Exam 2023 Question Paper Thenkasi District English Medium PDF Downloadharishsuriya1986No ratings yet
- Same Virtus: Alarm ListDocument23 pagesSame Virtus: Alarm ListLacatusu Mircea100% (1)
- Solved - Which $1,000 Bond Has The Higher Yield To Maturity, A T...Document4 pagesSolved - Which $1,000 Bond Has The Higher Yield To Maturity, A T...Sanjna ChimnaniNo ratings yet
- Crack Width Design - IS456-2000Document1 pageCrack Width Design - IS456-2000Nitesh SinghNo ratings yet
- Nursing Research Lecture 4aDocument26 pagesNursing Research Lecture 4asyamsul anwarNo ratings yet
- Vertical Ow Constructed Wetland Planted With Heliconia Psittacorum Used As Decentralized Post-Treatment of Anaerobic Ef Uent in Southern BrazilDocument10 pagesVertical Ow Constructed Wetland Planted With Heliconia Psittacorum Used As Decentralized Post-Treatment of Anaerobic Ef Uent in Southern BrazilAlfonso Ruiz PérezNo ratings yet
- Sample Chapter - Oil and Gas Well Drilling Technology PDFDocument19 pagesSample Chapter - Oil and Gas Well Drilling Technology PDFDavid John100% (1)
- Solenoid ValveDocument76 pagesSolenoid ValveazlanNo ratings yet
- Meteorology Konu Konu Ayrılmış SorularDocument278 pagesMeteorology Konu Konu Ayrılmış Sorularjames100% (1)
- Acuvim II Profibus Modules Users Manual v1.10Document36 pagesAcuvim II Profibus Modules Users Manual v1.10kamran719No ratings yet
- MD Manu en Megaflex WebDocument58 pagesMD Manu en Megaflex WebPhu, Le HuuNo ratings yet
- Murata Data Caps ESR ESLDocument6 pagesMurata Data Caps ESR ESLecl_manNo ratings yet
- Attention Gated Encoder-Decoder For Ultrasonic Signal DenoisingDocument9 pagesAttention Gated Encoder-Decoder For Ultrasonic Signal DenoisingIAES IJAINo ratings yet
- Teaching Tactics and Teaching Strategy: Arthur W. Foshay'Document4 pagesTeaching Tactics and Teaching Strategy: Arthur W. Foshay'Ahmed DaibecheNo ratings yet
- Silicon Controlled RectifierDocument38 pagesSilicon Controlled RectifierPaoNo ratings yet
- Engine Control System Circuit Diagram: Without This Message by Purchasing NovapdfDocument3 pagesEngine Control System Circuit Diagram: Without This Message by Purchasing NovapdfJose Luis Gutierrez TamayoNo ratings yet
- Fiber SyllabusDocument1 pageFiber SyllabusPaurav NayakNo ratings yet
- Faculty: Geology Exploration Specialty: Geology Engineering Group: 123.6 Student: Asef Sadiqov Teacher: Afet Israfilova Theme: The EarthDocument16 pagesFaculty: Geology Exploration Specialty: Geology Engineering Group: 123.6 Student: Asef Sadiqov Teacher: Afet Israfilova Theme: The EarthKenan RehmanNo ratings yet
- Student - The Passive Voice Without AnswersDocument5 pagesStudent - The Passive Voice Without AnswersMichelleNo ratings yet
- From Assessment To Purchase - A Three-Stage ModelDocument15 pagesFrom Assessment To Purchase - A Three-Stage ModelRONAL EMERSON NOA ORTEGANo ratings yet
- Plagiarism - ReportDocument6 pagesPlagiarism - ReportDipesh NagpalNo ratings yet
- K46 ManualDocument8 pagesK46 ManualDavid KasaiNo ratings yet
- I C Engine LabDocument3 pagesI C Engine LabDevNo ratings yet
- Shaping Plastic Forming1Document24 pagesShaping Plastic Forming1Himan JitNo ratings yet
- Chapter 3Document23 pagesChapter 3pganoelNo ratings yet
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincFrom EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincRating: 3.5 out of 5 stars3.5/5 (137)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 5 out of 5 stars5/5 (4)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeRating: 4 out of 5 stars4/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactFrom EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactRating: 5 out of 5 stars5/5 (5)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideFrom EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNo ratings yet
- A Perfect Red: Empire, Espionage, and the Quest for the Color of DesireFrom EverandA Perfect Red: Empire, Espionage, and the Quest for the Color of DesireRating: 4 out of 5 stars4/5 (129)
- Water-Based Paint Formulations, Vol. 3From EverandWater-Based Paint Formulations, Vol. 3Rating: 4.5 out of 5 stars4.5/5 (6)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Bioplastics: A Home Inventors HandbookFrom EverandBioplastics: A Home Inventors HandbookRating: 4 out of 5 stars4/5 (2)
- Chemistry: a QuickStudy Laminated Reference GuideFrom EverandChemistry: a QuickStudy Laminated Reference GuideRating: 5 out of 5 stars5/5 (1)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsFrom EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNo ratings yet
- Guidelines for Integrating Process Safety into Engineering ProjectsFrom EverandGuidelines for Integrating Process Safety into Engineering ProjectsNo ratings yet
- The Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Alkali Metals, Alkaline Earth Metals and Transition Metals | Children's Chemistry BookNo ratings yet
- The Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookFrom EverandThe Periodic Table of Elements - Post-Transition Metals, Metalloids and Nonmetals | Children's Chemistry BookNo ratings yet