Professional Documents
Culture Documents
CH 4 Reading Guide
Uploaded by
Kapil NathanOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CH 4 Reading Guide
Uploaded by
Kapil NathanCopyright:
Available Formats
Chapter 4: Carbon and the Molecular Diversity of Life
Chapter 4: Carbon and the Molecular Diversity of Life
4.1 Explain what organic compounds are, and how they might have arisen on Earth.
4.2 Use examples to demonstrate how carbon’s atomic structure results in a wide range of
molecular structures.
4.3 Identify the key chemical groups that affect the function of biological molecules.
This chapter prepares you for understanding organic compounds. Functional groups determine
the properties and activity of various molecules. Note how Stanley Miller’s experiment provides
an important link to evolution. Before starting this chapter, check the opening page to appreciate
the intent of the Study Tip and then take a minute to review the unique abilities of carbon.
Study Tip: As you begin this chapter, note that the intent of the text’s Study Tip is for you to be
able to identify chemical groups on different molecules. Keep that in mind as you work through
the chapter. Also, since carbon is the basis for all biological molecules, take a minute to review
the unique properties of carbon illustrated in the opening figure.
Concept 4.1 Organic chemistry is key to the origin of life
LO 4.1: Explain what organic compounds are, and how they might have arisen on Earth.
1. Study this figure of Stanley Miller’s experiment to simulate conditions thought at the time
of the experiment to have existed on early Earth. Explain the elements of this experiment,
using arrows to indicate what occurs in various parts of the apparatus.
2. What was collected in the sample for chemical analysis? What was concluded from the re-
sults of this experiment?
Copyright © 2021 Pearson Education, Inc. -1-
Chapter 4: Carbon and the Molecular Diversity of Life
Concept 4.2 Carbon atoms can form diverse molecules by bonding to four other atoms
LO 4.2: Use examples to demonstrate how carbon's atomic structure results in a wide range of
molecular structures.
3. Make an electron distribution diagram of carbon.
a. How many valence electrons does carbon have? _________________________
b. How many bonds can carbon form? ________________________
c. What type of bonds does it form with other elements? __________________________
4. Carbon chains form skeletons of organic molecules. How can the carbon skeletons differ?
5. What is a hydrocarbon? Name two. Are hydrocarbons hydrophobic or hydrophilic?
6. In Chapter 2 you learned about isotopes. Because students often confuse this word with iso-
mer, define each term here and give an example.
Definition Example
Isotope
Isomer
7. Variation in the architecture of molecules can cause variable biological effects. Give an ex-
ample of how isomers can vary in their pharmacological effects.
-2- Copyright © 2021 Pearson Education, Inc.
Chapter 4: Carbon and the Molecular Diversity of Life
Concept 4.3 A few chemical groups are key to molecular function
LO 4.3: Identify the key chemical groups that affect the function of biological molecules.
8. Define functional group.
9. Here is an idea that will recur throughout your study of the function of molecules: a change
in structure will change the function. You see this in enantiomers, you will see it in proteins
and enzymes, and now we are going to look at testosterone (a male sex hormone) and estra-
diol (a female sex hormone). Despite the similarities between these two molecules, you
know what a vastly different effect each has. Label each functional group in the following
sketch and circle the differences.
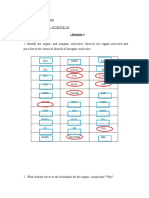
10. There are seven chemical groups important in biological processes that you should know.
Using Figure 4.9 in your text, complete the following chart.
Hydroxyl Carbonyl Carboxyl Amino Sulfhydryl Phosphate Methyl
Structure
Group
properties
Example
11. Using the chemical groups above, see if you can answer the following prompts:
a. –NH2:
b. Can form covalent cross-links that stabilize protein structure:
c. Key component of ATP:
d. Can affect gene expression:
e. –CH3:
Copyright © 2021 Pearson Education, Inc. -3-
Chapter 4: Carbon and the Molecular Diversity of Life
f. Is always polar:
g. Determines the two groups of sugars:
h. Has acidic properties:
i. COOH:
j. Acts as a base:
k. Circle and identify three functional groups in the molecule shown above.
11. ATP is an important molecule in the cell because it releases energy that can be used for cell
work. Draw and label the overall reaction of ATP being converted to ADP with the release
of energy.
Test Your Understanding, p. 65
Now you should be ready to test your knowledge. Place your answers here:
1. ______
2. ______
3. ______
4. ______
5. ______
6. ______
7. ______
-4- Copyright © 2021 Pearson Education, Inc.
You might also like
- C9e Answers Active Reading 04Document4 pagesC9e Answers Active Reading 04Jordan Camina75% (8)
- Applied Chem Module Week 1Document6 pagesApplied Chem Module Week 1Unibelle Joy Lachica100% (2)
- Biochemistry QsDocument4 pagesBiochemistry QsValine Cysteine MethionineNo ratings yet
- AP Bio Chapter 2 Active Reading GuideDocument10 pagesAP Bio Chapter 2 Active Reading Guidesam quo yay100% (1)
- Chapter 4 Carbon and Molecular DiversityDocument3 pagesChapter 4 Carbon and Molecular DiversityZoe AposNo ratings yet
- AP Chapter 4Document3 pagesAP Chapter 4jessicaNo ratings yet
- Chapter 4: Carbon and The Molecular Diversity of LifeDocument1 pageChapter 4: Carbon and The Molecular Diversity of LifeMohammadNo ratings yet
- AP Bio Ch4-5reading GuiedesDocument17 pagesAP Bio Ch4-5reading GuiedesAstrii LyNo ratings yet
- Biomolecules Exam RevisionDocument3 pagesBiomolecules Exam RevisionCarmen CheahNo ratings yet
- Chapter 1 & Chapter 2 - Chemistry and LifeDocument25 pagesChapter 1 & Chapter 2 - Chemistry and Lifeprehealthhelp100% (6)
- ADVANCED CHEMISTRY Q3 Module Jan 2021 PDFDocument48 pagesADVANCED CHEMISTRY Q3 Module Jan 2021 PDFLouis C. GutierrezNo ratings yet
- General Chemistry 1 Quarter 2 - MELC 11 Week 6: Activity SheetDocument9 pagesGeneral Chemistry 1 Quarter 2 - MELC 11 Week 6: Activity SheetJoshua De La Vega0% (1)
- Consumer Chem. Q1 For Week 5 Riza Laxamana Version 3Document15 pagesConsumer Chem. Q1 For Week 5 Riza Laxamana Version 3Ces Michaela Cadivida100% (1)
- SAS Module 1 NewDocument11 pagesSAS Module 1 NewKyutieNo ratings yet
- Themes in The Study of LifeDocument4 pagesThemes in The Study of Lifeapi-233187566No ratings yet
- Study Guide Chapter 2: Life Chemistry and Energy: 0. ApplicationDocument19 pagesStudy Guide Chapter 2: Life Chemistry and Energy: 0. ApplicationKelly WayneNo ratings yet
- Fuel MoleculesDocument3 pagesFuel MoleculesPhillip CookNo ratings yet
- Study Questions For Test 1 BioDocument4 pagesStudy Questions For Test 1 BiokadooieNo ratings yet
- Drawing Organic Molecular Structures: 02 FEBUARY 2024Document8 pagesDrawing Organic Molecular Structures: 02 FEBUARY 2024jhezieljansNo ratings yet
- ALM BiochemistryDocument4 pagesALM BiochemistryBrian BradyNo ratings yet
- Science: The Carbon Compounds and Chemical BondsDocument16 pagesScience: The Carbon Compounds and Chemical BondsCelline Isabelle ReyesNo ratings yet
- OC STR 1 WB - Intro & AlkanesDocument16 pagesOC STR 1 WB - Intro & Alkaneswayne.ilearnacadhkNo ratings yet
- Organic and Inorganic Compounds Identification and FunctionsDocument9 pagesOrganic and Inorganic Compounds Identification and FunctionsMark Brian FloresNo ratings yet
- Science 9 - Q2 - Mod4 - CARBON ATOM A UNIQUE ONE - VerFinalDocument8 pagesScience 9 - Q2 - Mod4 - CARBON ATOM A UNIQUE ONE - VerFinalHerdie Anne LedesmaNo ratings yet
- AllChapterQuestions PDFDocument23 pagesAllChapterQuestions PDFKim Na NaNo ratings yet
- SMILE Science 9 Q2W4 5 EditedDocument8 pagesSMILE Science 9 Q2W4 5 EditedRaven Third-partyAccNo ratings yet
- Physical ScienceDocument22 pagesPhysical ScienceMary Joyce Nicolas ClementeNo ratings yet
- Lesson 22 Why C Is An Important ElementDocument6 pagesLesson 22 Why C Is An Important ElementJoycee DhNo ratings yet
- Chapter 2Document10 pagesChapter 2AnonymousNo ratings yet
- Dwnload Full Microbiology Principles and Explorations 9th Edition Black Solutions Manual PDFDocument35 pagesDwnload Full Microbiology Principles and Explorations 9th Edition Black Solutions Manual PDFcamisuglilyfizsm100% (9)
- Microbiology Principles and Explorations 9th Edition Black Solutions ManualDocument35 pagesMicrobiology Principles and Explorations 9th Edition Black Solutions Manualduongnujl33q100% (15)
- Science: The Carbon Compounds and Chemical BondsDocument19 pagesScience: The Carbon Compounds and Chemical BondsAnnie Bagalacsa Cepe-TeodoroNo ratings yet
- Quarter-2 General-Chemistry-1 M12 V2Document17 pagesQuarter-2 General-Chemistry-1 M12 V2Raquel LicudineNo ratings yet
- Science 9 Q2module 4Document8 pagesScience 9 Q2module 4alexablisssNo ratings yet
- Biomacromolecules JigsawDocument3 pagesBiomacromolecules JigsawCarmen CheahNo ratings yet
- Grade-9-Science Q2 Wk4 GLAKDocument16 pagesGrade-9-Science Q2 Wk4 GLAKMorana TuNo ratings yet
- Science: Quarter 4 - Module 2: BiomoleculesDocument31 pagesScience: Quarter 4 - Module 2: BiomoleculesMerrie Anne Pascual Bagsic75% (4)
- CH 4 Carbon and Molecular Diversity of LifeDocument3 pagesCH 4 Carbon and Molecular Diversity of Lifewil7ver100% (1)
- Anatomy Physiology Course InfoDocument5 pagesAnatomy Physiology Course InfoJessa ChanNo ratings yet
- Lesson 1 in Organic Chemistry (MBS 524)Document29 pagesLesson 1 in Organic Chemistry (MBS 524)id.villegas.sciencenorthNo ratings yet
- E.Sci9 - Q2 - Week 5Document7 pagesE.Sci9 - Q2 - Week 5HersheyNo ratings yet
- ORGANIC CHEM Individual Activity jANUARY 8Document1 pageORGANIC CHEM Individual Activity jANUARY 8Shantelle LomogbadNo ratings yet
- Macromolecule Pattern Matching Activity SosnowskiDocument26 pagesMacromolecule Pattern Matching Activity SosnowskidkisNo ratings yet
- (Q1) MODULE 4 - Chemical and Structural Formulas PDFDocument18 pages(Q1) MODULE 4 - Chemical and Structural Formulas PDFJewel SantiagoNo ratings yet
- Chemistry Worksheet Summer AssignmentDocument10 pagesChemistry Worksheet Summer AssignmentJohn Joseph CambaNo ratings yet
- Stereochemistry Lab: Isomers & Molecular ModelsDocument19 pagesStereochemistry Lab: Isomers & Molecular Modelssophia del rosarioNo ratings yet
- Microbiology Principles and Explorations 9th Edition Black Solutions ManualDocument25 pagesMicrobiology Principles and Explorations 9th Edition Black Solutions ManualRobertFordicwr100% (50)
- NTSE 10th Chem Part 2Document94 pagesNTSE 10th Chem Part 2Rishabh BhartiNo ratings yet
- Topic 10 Alkanes Remote Learning JCAFDocument22 pagesTopic 10 Alkanes Remote Learning JCAFEllson LinNo ratings yet
- COES110B Chemistry For Engineers Lab 4 - MOLECULE SHAPESDocument8 pagesCOES110B Chemistry For Engineers Lab 4 - MOLECULE SHAPESCJ MangasepNo ratings yet
- Organic Chemistry Module Notes and LinksDocument14 pagesOrganic Chemistry Module Notes and Linkshernys NietoNo ratings yet
- BIO 101 Course Objectives Sp23Document6 pagesBIO 101 Course Objectives Sp23begush015No ratings yet
- Assessment Questions in General Biology 1Document1 pageAssessment Questions in General Biology 1yuihatesuNo ratings yet
- Leaning Packet 6 Engg Chem 1Document42 pagesLeaning Packet 6 Engg Chem 1Ritchel Conde BoholNo ratings yet
- Genchem5&6sheesh OgDocument5 pagesGenchem5&6sheesh Ogwencylle casilNo ratings yet
- General-Chemistry1 Quarter1 Week2Document24 pagesGeneral-Chemistry1 Quarter1 Week2Rose RepuestoNo ratings yet
- Chapter 2 Active Reading GuideDocument10 pagesChapter 2 Active Reading GuideAnonymous y0j9r8UNo ratings yet
- Report 1 Learning Software - Benjamin CruzDocument12 pagesReport 1 Learning Software - Benjamin Cruzapi-674986426No ratings yet
- G9 Ste Conchem Q1 WK1Document20 pagesG9 Ste Conchem Q1 WK1Breeza Marie VeralloNo ratings yet
- CH 5 Reading GuideDocument18 pagesCH 5 Reading GuideKapil NathanNo ratings yet
- CH 6 Reading GuideDocument13 pagesCH 6 Reading GuideKapil NathanNo ratings yet
- CH 12 Reading GuideDocument9 pagesCH 12 Reading GuideKapil NathanNo ratings yet
- CH 8 Reading GuideDocument9 pagesCH 8 Reading GuideKapil NathanNo ratings yet