Professional Documents
Culture Documents
SAS Module 1 New
Uploaded by
KyutieOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
SAS Module 1 New
Uploaded by
KyutieCopyright:
Available Formats
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
Lesson title: Introduction to Organic Chemistry Materials:
Pen and SAS
Lesson Objectives: At the end of this lesson, you should be able to:
1. differentiate organic and inorganic compounds. References:
2. explain the different types of formula. Exploring General Organic,
3. understand and be able to draw structural, and Biological Chemistry
condensed and line formulas by: H. Stephen Stoker
Productivity Tip:
Try doing a Picture Walk before starting this module. Take a quick look at the captions, activities, picutures,
etc. This is to give your brain an idea of what’s coming-it’s like watching a trailer of a movie. Doing this for a
minute will help your brain organize your thoughts before studying.
A. LESSON PREVIEW/REVIEW
Introduction (2 mins)
Organic chemistry is the chemistry of the compounds of carbons. It is a chemistry associated with all living
matter in both plants and animals. As you study organic chemistry you will see that organic compounds are
everywhere around us. They are in our foods, flavours, and fragrances; in medicines, toiletries, and cosmetics;
in our plastics, films, fibers, and resin; in our paints, varnishes and glues; and of course in our bodies and the
bodies of all other living organisms (2). Carbohydrates, fats, proteins, vitamins, hormones, and enzymes are
organic compounds.
Activity 1: What I Know Chart, part 1 (5 mins)
Instructions: In this chart, reflect on what you know. Do not worry if you are sure or not sure of your answers.
This activity simply serves to get you started on thinking about our topic. Answer only the first column, ‘What I
Know”. Leave the third column “What I Learned “blank at this time.
What I Know Questions: What I Learned (Activity 4)
1what is inorganic chemistry?
2. What is organic chemistry?
3 What are the different types of
formula?
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
B.MAIN LESSON
Activity 2: Content Notes (30 mins.)
During the later part of the eighteenth century and the early part of the nineteenth century, chemist
began to categorize compounds into two types: ORGANIC and INORGANIC COMPOUNDS.
ORGANIC COMPOUNDS are compounds obtained from living organisms. INORGANIC COMPOUNDS are
compounds obtained from mineral constituents of the Earth.
During this early period, chemists believed that a special “vital force” supplied by a living organism was
necessary for the formation of an organic compound. This concept was proved incorrect in 1828 by a German
Chemist Friedrick Wohler, Wohler heated an aqueous solution of two inorganic compounds, ammonium
chloride and silver cyanate, and obtained urea ( a component of urea)
Other chemists had successfully synthesized organic compounds from inorganic starting materials, As
a result, the vital-force theory was completely abandoned, but the definitions of these terms no longer reflect
their historical origins.
ORGANIC CHEMISTRY Is the study of hydrocarbons (compounds of carbon and hydrogen) and their
derivatives.
INORGANIC CHEMISTRY Is the study of all substances other than hydrocarbons and their derivatives?
COMPARISON OF ORGANIC AND INORGANIC COMPOUNDS
COMPARISON OF PROPERTIES OF MOST ORGANIC AND INORGANIC COMPOUNDS
PROPERTY ORGANIC INORGANIC
Flammable Yes No
Melting point Low High
Boiling point Low High
Solubility in water No(for most) Yes
Solubility in nonpolar liquids Yes No
Types of bonding Covalent Ionic
Reactions occur between Molecules Ions
Atoms per molecule Many Few
Structure Complex Simpler
Electrolyte No Yes
th
Reference: Chemistry for Health Sciences 8 edition by: George I. Sackeim, and Deniis D. Lehman
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
BONDING CHRACTERISITICS OF THE CARBON ATOM
Organic compounds
Compounds of carbon
Held together by covalent bonds.
Covalent bonds are formed by sharing of electrons.
In organic chemistry the term bond is used to designate a sharred pair of electrons.
Carbon has four electrons; this means that carbon can form a maximum of four covalent bonds.
Bonds are usually represented by a short, straight line connecting the atoms, with each bond representing a
shared pair of electrons .
Carbon atoms
Have the unique ability to bond to each other in a wide variety of ways that involve long chains of
carbon atoms or cyclic arrangements (rings) of carbon atoms.
Carbon can meet to this four-bond requirements in three different ways:
o By bonding to four other atoms. This situation requires the presence of four single bonds.
⸺C⸺
Four single bond
o By bonding to three other atoms. This situation requires the presence of two single bonds and
one double bond.
Two single bonds and one double bond
By bonding to two other atoms. This situation requires the presence of either two double bonds
or a triple bond and a single bond.
Two One triple bond
Double bond One single bond
TYPES OF FORMULAS:
1. STRUCTURAL FORMULAS
In organic compounds are often written using a structural rather than a molecular formula.
Structural formula shows the exact way in which the atoms are connected to each other but a
molecular formula does not.
Is a two-dimensional structural representation that shows how the various atoms in a molecule
are bonded to each other.
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
Two types of Structural Formula:
1.1 Expanded Structural Formula
Is a structural formula that shows all atoms in a molecule and all bonds connecting the atoms.
1.2. Condensed Structural Formula
Is a structural formula that uses groupings of atoms, in which central atoms and the atoms connected to
them are written as a group, to convey molecular structural information.
EXPANDED AND STRUCTURAL FORMULA OF: METHANE, ETHANE AND PROPANE
Methane Ethane Propane
H H H H H H
Expanded structural
formula H⸺C⸺H H⸺C⸺C⸺H H⸺C⸺C⸺C⸺H
H H H H H H
Condensed structural CH4 CH3⸺CH3 CH3⸺CH2⸺CH3
formula
2. SKELETAL STRUCTURAL FORMULA
Is a structural formula that shows the arrangement and bonding of carbon atoms present in an organic
molecule but does not show the hydrogen atoms attached to the carbon atoms.
C⸺C⸺C⸺C⸺C means the same as CH3⸺CH2⸺CH2⸺CH2⸺CH3
Skeletal structural formula condensed structural formula
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
3. BOND LINE-FORMULAS (Bond-line structure, skeletal formula)
In which carbon atoms are implied at the corners and ends of lines, and each carbon atom is
understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. A
representation of molecular structure in which covalent bonds are represented with one line for each
level of bond order. The most common and the fastest to draw. Bond line formulas are easy to draw for
molecules with multiple bonds (4). Bond is used to designate a shared pair of electrons.
For example, we can represent pentane (CH3CH2CH2CH2CH3) and isopentane [(CH3)2CHCH2CH3] as
follows(4):
Below is an example of a more complicated molecule
The following are the suggested steps for drawing a lin-angle structure: (4)
1. Each line represents a bond
2. Remove all hydrogens bonded to carbon
3. Remove all carbons
4. Because the carbons on the left are drawn straight across, we cannot see corners easily, so bend your
lines ( zig-zag) so that the corners are apparent.
Examples:
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
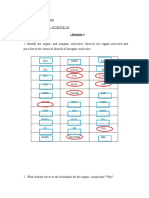
Activity 3: Skill-building Activities (with answer key) (30 mins + 5 mins checking)
Instructions: Consider the differences between organic and inorganic compounds as you answer each
of the following questions: Check your answers against the Keys to Correction at the last pages of this
SAS. Write your Score.
1. Which compounds make good electrolytes?
2. Which compound exhibit ionic bonding?
3. Which compounds have lower melting point?
4. Which compounds are more likely to be soluble in water?
5. Which compounds are flammable?
Instructions: Convert the following structural formulas into: Condensed structural formula, and Line formulas.
STRUCTURAL FORMULA CONDENSED FORMULA LINE FORMULAS
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
Activity 4: What I Know Chart, part 2 (3 mins)
Instruction: To review what was learned from this session, please go back to Activity 1 and answer the “What I
Learned” column. Notice and reflect any changes in your answers.
Activity 5: Check for Understanding (35 mins)
Instructions: Some of the following structural formulas are incorrect (that is, they do not represent real
compounds) because they have atoms with an incorrect number of bonds. Which structural formulas are
incorrect, and which atoms in them have an incorrect number of bonds?
H H H H
1. H-C ≡ C-C-H 2. H-C=C-H
H H H H
HH H H
3. H-N-C-C-O-H 4. H-C- C=O-H
H H H H H
H HO
║
5. H-C-C-C-H
H H
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
Instructions: Complete the following structural formulas by adding enough hydrogen to complete the
teravalence of each carbon. Then write the molecular formula for each compound.
1. C-C=C-C-C
2. C-C-C-OH
3. C-C-C-C-NH2
OH
4. C-C-C-C-C
5. C=C-C-OH
C. LESSON WRAP-UP
Activity 6: Thinking about Learning (5 mins)
A. Work Tracker: You are done with this session! Let’s track your progress. Shade the session
number you just completed.
Reminder: Instructor/facilitator. Please direct the students to mark their place in the work tracker
which is simply a visual to help students track how much work they have accomplished and how
much work there is left to do.
P1 P2 P3
1 2 3 4 5 6 7 8 9 10
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
B. Think about your Learning: What part of the module that you think i need to elaborate further
and why?
FAQs
1. What is urrea?
Ans. Urea is the simplest naturally occurring amide, it is a water soluble white solid in the human body
from carbon dioxide and ammonia through a complex series of metabolic reactions.
2. Where do we obtain Organic Compounds?
Ans. Chemists obtain organic compounds either by isolation from plant and animal sources or by
synthesis in the laboratory.
KEY TO CORRECTIONS for Activity # 3:
Instructions: Consider the differences between organic and inorganic compounds as you answer each of the
following questions:
1. Which compounds make good electrolytes?
Ans. Inorganic Compounds
2. Which compound exhibit ionic bonding?
Ans. Organic Compounds
3. Which compounds have lower melting point?
Ans. Organic Compounds
4. Which compounds are more likely to be soluble in water?
Ans. Inorganic Compounds
5. Which compounds are flammable?
Ans. Organic Compounds
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
Instructions: Convert the following structural formulas into: Condensed structural formula, and Line formulas.
STRUCTURAL FORMULA CONDENSED FORMULA LINE FORMULAS
CH3CH2CH2CH3
CH3CHCH3
CH3
CH3CH2CH2CH2CH3
CH3CH2CH2CH2CH2CH2CH2CH3
This document is the property of PHINMA EDUCATION
Course Code: CHE-005 Lec.
SAS Module 1
Name: Class number: _______
Section: ____________ Schedule: _______________________________________ Date: _______________
Citations:
1. General, Organic and Biochemistry (5th ed.)by: Katherine J. Denniston, Joseph J. Topping,
Robert L. Caret
2. Introduction to General, Organic and Biocehmsitry (8th ed.) by: Frederick A. Bettelhem,
William H. Brown, Mary K. Campbell, Shawn O. Farrel
3. Chemistry for Health Sciences (8th) by: George L. Sackeim, Dennis D Lehman
4. https://chem.libretexts.org/Courses/University_of_South_Carolina__Upstate/USC_Upstate%3A_CHEM_U109_-
_Chemistry_of_Living_Things_(Mueller)/11%3A_Organic_Chemistry/11.03_Condensed_Structural_and_Line-
Angle_Formulas
This document is the property of PHINMA EDUCATION
You might also like
- 79Document49 pages79khalilNo ratings yet
- Lesson 1 in Organic Chemistry (MBS 524)Document29 pagesLesson 1 in Organic Chemistry (MBS 524)id.villegas.sciencenorthNo ratings yet
- Consumer Chem. Q1 For Week 5 Riza Laxamana Version 3Document15 pagesConsumer Chem. Q1 For Week 5 Riza Laxamana Version 3Ces Michaela Cadivida100% (1)
- NTSE 10th Chem Part 2Document94 pagesNTSE 10th Chem Part 2Rishabh BhartiNo ratings yet
- Biology Concepts and Investigations 3rd Edition Hoefnagels Solutions ManualDocument36 pagesBiology Concepts and Investigations 3rd Edition Hoefnagels Solutions Manualcatmammotham9t4100% (27)
- ADVANCED CHEMISTRY Q3 Module Jan 2021 PDFDocument48 pagesADVANCED CHEMISTRY Q3 Module Jan 2021 PDFLouis C. GutierrezNo ratings yet
- Dwnload Full Biology Concepts and Investigations 3rd Edition Hoefnagels Solutions Manual PDFDocument36 pagesDwnload Full Biology Concepts and Investigations 3rd Edition Hoefnagels Solutions Manual PDFjunemojarrazqmxj100% (17)
- College of Education: Objective: To Recall Basic Chemistry and Chemical Bonding Concepts. Reading LinkDocument3 pagesCollege of Education: Objective: To Recall Basic Chemistry and Chemical Bonding Concepts. Reading Linkjohn alester cuetoNo ratings yet
- Organic Chemistry: Catalan (Cat) Carlos - Jaime@uab - Cat Carles Jaime CardielDocument6 pagesOrganic Chemistry: Catalan (Cat) Carlos - Jaime@uab - Cat Carles Jaime CardielNeils ArenósNo ratings yet
- m1 Organic ChemDocument29 pagesm1 Organic ChemJelaica EspinuevaNo ratings yet
- Microbiology Principles and Explorations 9th Edition Black Solutions ManualDocument25 pagesMicrobiology Principles and Explorations 9th Edition Black Solutions ManualRobertFordicwr100% (50)
- Science G9 Q2 W4 ModDocument8 pagesScience G9 Q2 W4 ModRhia Mae AjocNo ratings yet
- CH 4 Reading GuideDocument4 pagesCH 4 Reading GuideKapil NathanNo ratings yet
- Dwnload Full Microbiology Principles and Explorations 9th Edition Black Solutions Manual PDFDocument35 pagesDwnload Full Microbiology Principles and Explorations 9th Edition Black Solutions Manual PDFcamisuglilyfizsm100% (9)
- MODULE 1 - Overview of Organic Chemistry (20200810)Document22 pagesMODULE 1 - Overview of Organic Chemistry (20200810)Mark SeverinoNo ratings yet
- Microbiology Principles and Explorations 9th Edition Black Solutions ManualDocument35 pagesMicrobiology Principles and Explorations 9th Edition Black Solutions Manualduongnujl33q100% (15)
- CONCHEM-9 Q1 W3 Mod3Document33 pagesCONCHEM-9 Q1 W3 Mod3kayedecena29No ratings yet
- EM Agravante Module 1 Organic ChemDocument31 pagesEM Agravante Module 1 Organic ChemCarlyne LaneteNo ratings yet
- Consumer Chemistry-Q1 - Module6 - Functional-Groups-Landingin-v3Document15 pagesConsumer Chemistry-Q1 - Module6 - Functional-Groups-Landingin-v3Ces Michaela Cadivida100% (1)
- Identify The Organic and Inorganic Molecules. Encircle The Organic Molecules and Put A Box To The Chemical Formula of Inorganic MoleculesDocument9 pagesIdentify The Organic and Inorganic Molecules. Encircle The Organic Molecules and Put A Box To The Chemical Formula of Inorganic MoleculesMark Brian FloresNo ratings yet
- E.Sci9 - Q2 - Week 5Document7 pagesE.Sci9 - Q2 - Week 5HersheyNo ratings yet
- BIO 1401 Module Prof. C. Katongo-1Document79 pagesBIO 1401 Module Prof. C. Katongo-1OliverNo ratings yet
- Chapter 2 Chemical Context of LifeDocument5 pagesChapter 2 Chemical Context of LifeZoe AposNo ratings yet
- General Chemistry: Southern Leyte State University Hinunangan CampusDocument33 pagesGeneral Chemistry: Southern Leyte State University Hinunangan CampusLlyNo ratings yet
- Biology Notes Co1Document32 pagesBiology Notes Co1Rohit AgrawalNo ratings yet
- Dwnload Full Biology 3rd Edition Brooker Solutions Manual PDFDocument35 pagesDwnload Full Biology 3rd Edition Brooker Solutions Manual PDFhudsonlosjames100% (14)
- Quarter-2 General-Chemistry-1 M12 V2Document17 pagesQuarter-2 General-Chemistry-1 M12 V2Raquel LicudineNo ratings yet
- Full Download Biology 3rd Edition Brooker Solutions ManualDocument18 pagesFull Download Biology 3rd Edition Brooker Solutions Manualmaurineheckathorneus100% (37)
- G9 Ste Conchem Q1 WK1Document20 pagesG9 Ste Conchem Q1 WK1Breeza Marie VeralloNo ratings yet
- Review: Step Step From Organic Biochem Istry: Chemistry ToDocument57 pagesReview: Step Step From Organic Biochem Istry: Chemistry ToBarnali DuttaNo ratings yet
- OC STR 1 WB - Intro & AlkanesDocument16 pagesOC STR 1 WB - Intro & Alkaneswayne.ilearnacadhkNo ratings yet
- Intro To BiologyDocument14 pagesIntro To BiologyDawar Aziz MemonNo ratings yet
- Module - General Chemistry OrganicDocument126 pagesModule - General Chemistry Organicjames.caones11No ratings yet
- Chemistry Worksheet Summer AssignmentDocument10 pagesChemistry Worksheet Summer AssignmentJohn Joseph CambaNo ratings yet
- MHR - Unit 1 Atoms, Elements, and CompoundsDocument10 pagesMHR - Unit 1 Atoms, Elements, and CompoundsJudyNo ratings yet
- Dwnload Full Visual Anatomy and Physiology 2nd Edition Martini Solutions Manual PDFDocument36 pagesDwnload Full Visual Anatomy and Physiology 2nd Edition Martini Solutions Manual PDFalluviumopuntialjvoh100% (9)
- Week 2 - Learning PacketDocument20 pagesWeek 2 - Learning PacketMichael TayagNo ratings yet
- Dwnload Full Fundamentals of Anatomy and Physiology 10th Edition Martini Solutions Manual PDFDocument36 pagesDwnload Full Fundamentals of Anatomy and Physiology 10th Edition Martini Solutions Manual PDFwaterycobstone.yg9zl100% (9)
- The New Chemist Company Publications- Accessible Organic Chemistry: The New Chemist CompanyFrom EverandThe New Chemist Company Publications- Accessible Organic Chemistry: The New Chemist CompanyNo ratings yet
- Lecture 1 - Introduction To Biological Macro MoleculesDocument34 pagesLecture 1 - Introduction To Biological Macro Moleculescurlicue100% (1)
- General Chemistry 1 Quarter 2 - MELC 11 Week 6: Activity SheetDocument9 pagesGeneral Chemistry 1 Quarter 2 - MELC 11 Week 6: Activity SheetJoshua De La Vega0% (1)
- Technical Sciences - Organic MoleculesDocument110 pagesTechnical Sciences - Organic MoleculesLondekaNo ratings yet
- SMILE Science 9 Q2W4 5 EditedDocument8 pagesSMILE Science 9 Q2W4 5 EditedRaven Third-partyAccNo ratings yet
- Week 1: Organic Chemistry: Biochemistry /V1.0By: Engr. Gina E. DiocosDocument6 pagesWeek 1: Organic Chemistry: Biochemistry /V1.0By: Engr. Gina E. DiocosMa RieNo ratings yet
- Study Guides 2.1-2.3Document8 pagesStudy Guides 2.1-2.3MA. ASUNCION BeroNo ratings yet
- Organic Chemistry For StudentsDocument54 pagesOrganic Chemistry For StudentsSean Gabriel LacambraNo ratings yet
- A L D e H y D e S: Elicit (Review)Document5 pagesA L D e H y D e S: Elicit (Review)Ma. Leny LacsonNo ratings yet
- Science 9 - Q2 - Week 6-M17-M18Document18 pagesScience 9 - Q2 - Week 6-M17-M18Rhyan Zero-four BaluyutNo ratings yet
- Module 3 OrgchemDocument7 pagesModule 3 OrgchemJHUNNTY LOZANONo ratings yet
- Chapter - 2 ShortDocument16 pagesChapter - 2 ShortNadeem ArainNo ratings yet
- Synthesis of Oil of WintergreenDocument16 pagesSynthesis of Oil of WintergreenvarunNo ratings yet
- Organic Chemistry Kimberly CarterDocument175 pagesOrganic Chemistry Kimberly CarterPinaki MandalNo ratings yet
- General Chemistry 1: Diffun CampusDocument4 pagesGeneral Chemistry 1: Diffun CampushaydeeNo ratings yet
- Study Guide Chapter 2: Life Chemistry and Energy: 0. ApplicationDocument19 pagesStudy Guide Chapter 2: Life Chemistry and Energy: 0. ApplicationKelly WayneNo ratings yet
- Sed 222Document115 pagesSed 222agboanthonyokpeNo ratings yet
- Grade-9-Science Q2 Wk4 GLAKDocument16 pagesGrade-9-Science Q2 Wk4 GLAKMorana TuNo ratings yet
- Chapter 4 Carbon and Molecular DiversityDocument3 pagesChapter 4 Carbon and Molecular DiversityZoe AposNo ratings yet
- KHKKKDocument39 pagesKHKKKdaney67299No ratings yet
- BIO024 Session-1 IGDocument6 pagesBIO024 Session-1 IGKenny McCormickNo ratings yet
- NCM 131 Unit IIIC Interaction Oriented Nursing TheoriesDocument138 pagesNCM 131 Unit IIIC Interaction Oriented Nursing TheoriesKyutieNo ratings yet
- NCM 131 Unit IIIB. The System Oriented TheoriesDocument130 pagesNCM 131 Unit IIIB. The System Oriented TheoriesKyutieNo ratings yet
- NCM 131 Unit I. Evolution of NursingDocument91 pagesNCM 131 Unit I. Evolution of NursingKyutieNo ratings yet
- NCM 131 Unit IIIA. The Needs TheoriesDocument112 pagesNCM 131 Unit IIIA. The Needs TheoriesKyutieNo ratings yet
- NCM 131 Unit II. Introductory Concepts of Nursing Theory PDFDocument30 pagesNCM 131 Unit II. Introductory Concepts of Nursing Theory PDFKyutieNo ratings yet
- Organic Chemistry: An Introduction ToDocument45 pagesOrganic Chemistry: An Introduction ToTechnology Developer ChannelNo ratings yet
- ChemistryDocument166 pagesChemistryjakesidhuNo ratings yet
- Ebooks doTERRA Essential Oil Chemistry HandbookDocument82 pagesEbooks doTERRA Essential Oil Chemistry HandbookJozsef Kunder100% (3)
- AGUILLON-AERL JAY-T..-Lesson-1.6-1.17Document2 pagesAGUILLON-AERL JAY-T..-Lesson-1.6-1.17AERLJAY TVNo ratings yet
- Carbon and The Molecular Diversity of Life: For Campbell Biology, Ninth EditionDocument65 pagesCarbon and The Molecular Diversity of Life: For Campbell Biology, Ninth EditionlisaNo ratings yet
- Chemistry A1 OrganicDocument72 pagesChemistry A1 OrganicNoor MuhammadNo ratings yet
- 22.2 - Alkanes, Cycloalkanes, Alkenes, Alkynes, and Aromatics - Chemistry LibreTextsDocument1 page22.2 - Alkanes, Cycloalkanes, Alkenes, Alkynes, and Aromatics - Chemistry LibreTextsbrettNo ratings yet
- 13.1 Formulae, Functional Groups and The Name of Organic CompoundsDocument17 pages13.1 Formulae, Functional Groups and The Name of Organic Compoundssafiya_91No ratings yet
- 9701 Chemistry Syllabus 0101Document93 pages9701 Chemistry Syllabus 0101Hanish GaonjurNo ratings yet
- Lesson 1 Carbon CompoundsDocument27 pagesLesson 1 Carbon CompoundsMARY JOY MARQUEZNo ratings yet
- Unit 7 - Introduction To Organic Chemistry Student VersionDocument35 pagesUnit 7 - Introduction To Organic Chemistry Student VersionKetia OssombaNo ratings yet
- CH1001 2010 (Language2) NotesDocument18 pagesCH1001 2010 (Language2) Notesbav92No ratings yet
- GOC-jeemain GuruDocument108 pagesGOC-jeemain GuruncomdpNo ratings yet
- 12 Intro To OrganicDocument129 pages12 Intro To OrganicSyamil AdzmanNo ratings yet
- Chemical Composition of PetroleumDocument111 pagesChemical Composition of PetroleumAnonymous iCFJ73OMpDNo ratings yet
- Closo 52 PDFDocument64 pagesCloso 52 PDFKartik RanaNo ratings yet
- IB2 - Topic 10.1 - Fundamentals of Organic ChemistryDocument21 pagesIB2 - Topic 10.1 - Fundamentals of Organic ChemistryIqra NadeemNo ratings yet
- CHM 234: Worksheet #1 Due: Tuesday, August 30 in Class A. Line Angle/Skeletal StructuresDocument7 pagesCHM 234: Worksheet #1 Due: Tuesday, August 30 in Class A. Line Angle/Skeletal StructuresJean OlbesNo ratings yet
- Introduction To Ogranic ChemistryDocument33 pagesIntroduction To Ogranic ChemistrySuryani JumatNo ratings yet
- Lecture 1.1 Organic Chemistry - MKDocument59 pagesLecture 1.1 Organic Chemistry - MKqurrelNo ratings yet
- Introduction To Organic ChemistryDocument10 pagesIntroduction To Organic ChemistryKit GabrielNo ratings yet
- Introduction To Organic Chemistry: Structural Formulae Section - 1Document108 pagesIntroduction To Organic Chemistry: Structural Formulae Section - 1SUKH RAM VAISHNAVNo ratings yet
- Chapter 10 PDFDocument82 pagesChapter 10 PDFJm GarciaNo ratings yet
- Organic Chemistry - Some Basic Principles and Techniques Shobhit NirwanDocument43 pagesOrganic Chemistry - Some Basic Principles and Techniques Shobhit NirwanTanmoy GuptaNo ratings yet
- Organic Compounds and The Atomic Properties of CarbonDocument110 pagesOrganic Compounds and The Atomic Properties of Carbonmahbobullah rahmaniNo ratings yet
- Alkana-1Document61 pagesAlkana-1ayundhaNo ratings yet
- Applied Chem Week 1-3Document32 pagesApplied Chem Week 1-3Zenly AlleraNo ratings yet
- 6 - Organic ChemistryDocument27 pages6 - Organic ChemistryAlvaro CatalaNo ratings yet
- STEM Gen Chem 1 Q1 M2Document20 pagesSTEM Gen Chem 1 Q1 M2Roland AgraNo ratings yet
- Chapter 1 - Introduction of Organic Chemistry Structure and BondingDocument41 pagesChapter 1 - Introduction of Organic Chemistry Structure and BondingClinton NdhlovuNo ratings yet