Professional Documents
Culture Documents
Org Chem Reviewer
Uploaded by
LAGUERTA, JOHN MICHAEL A.0 ratings0% found this document useful (0 votes)
10 views2 pagesThis document contains 30 multiple choice or true/false questions related to organic chemistry concepts such as types of ethers, alcohol synthesis reactions, properties of alkanes and alkenes, oxidation reactions of alcohols, substitution reactions of alkanes and arenes, tests for detecting unsaturation, and properties of alkyl halides, aldehydes, ketones, carboxylic acids, esters, amines, and aromatic compounds.
Original Description:
REVIEWER FOR ORG Chem
Original Title
ORG CHEM REVIEWER
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains 30 multiple choice or true/false questions related to organic chemistry concepts such as types of ethers, alcohol synthesis reactions, properties of alkanes and alkenes, oxidation reactions of alcohols, substitution reactions of alkanes and arenes, tests for detecting unsaturation, and properties of alkyl halides, aldehydes, ketones, carboxylic acids, esters, amines, and aromatic compounds.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
10 views2 pagesOrg Chem Reviewer
Uploaded by
LAGUERTA, JOHN MICHAEL A.This document contains 30 multiple choice or true/false questions related to organic chemistry concepts such as types of ethers, alcohol synthesis reactions, properties of alkanes and alkenes, oxidation reactions of alcohols, substitution reactions of alkanes and arenes, tests for detecting unsaturation, and properties of alkyl halides, aldehydes, ketones, carboxylic acids, esters, amines, and aromatic compounds.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 2
1.
Anesthetic Ether – Ethyl ether
2. A method of alkane synthesis by heating two molecules of alkyl halide with Sodium metal in a dry ether
– Wurtz synthesis reaction
3. Typical alcohol present in beverages and is formed from fermentation of carbohydrates – Ethanol
4. Ether being used for treating dry cough – Methoxy ether
5. The process of producing an alkane by obtaining alkyl halide with Magnesium in dry ether and treating it
with H2O – Hydrolysis of Grignard reagent
6. Preparation of symmetrical ethers by dehydration of two primary alcohols using H2SO4 – Williamson
synthesis
7. Chemical reagent prepared by reacting alkyl halides with Lithium in an ether solvent – Organolithium
reagent
8. An oxidizing reagent which can oxidize some primary alcohols up to aldehydes only – Pyridinium
chlorochromate
9. Smaller alkanes are gases at room temperature. Larger alkanes are solids at room temperature –
Statement 1 only is correct
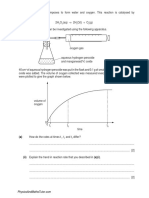
10. A sample of Hexene is added with Bromine water. Based on the principle of Bromine test for
unsaturation, what will be the result? – Brown of Bromine disappears
Note: Bromine – if added to unsaturated hydrocarbon the brown color will disappears, but if added to
saturated hydrocarbon the brown color will not change or disappear.
11. Methanol – Formaldehyde – Formic acid
Note: Oxidation of methanol will produce Formaldehyde, and oxidation of Formaldehyde will produce
Formic acid
12. The ff. statements about boiling point and melting point of hydrocarbon are correct – More pi bonds
mean higher boiling and melting point
13. Major component of rubbing alcohol used for sterilization and sanitation – Isopropyl alcohol
14. What is epoxide? – 3-carbon cyclic ether
15. Common substitution reactions of alkane include – Halogenation and Combustion
16. The chemical indicator used in Baeyer’s test for unsaturation – Potassium permanganate
Note: KMnO4 is a pink sol. When it is added to unsaturated hydrocarbon it will contain brown
coloration, if added to saturated hydrocarbons the color will not change and remains pink
17. Predicted product of halogenation of alkenes? – Alkyl halide & Hydrogen halide
18. Electrophilic substitution of phenols take place in what position – Ortho and Para
19. The ff. tests are used to detect the presence or absence of unsaturation in hydrocarbon sample –
KMnO4 test
Note: Test for unsaturation – Baeyer’s Test and Bromine Test
20. Chemical reaction for alcohol synthesis includes – Hydration of Alkenes, Reduction of aldehydes and
ketones, Acid-catalyzed cleavage of Epoxides by H2O
21. An acid that can dehydrate alcohol – Sulfuric acid
22. Oxidation of secondary alcohols will produce – Ketone
23. Benzene does not undergo addition reaction. It undergoes electrophilic substitution reaction – True
24. Oxidation of primary alcohol will produce – Aldehyde & Carboxylic acid
25. The ff. statements correctly describe Alkyl halides, except – Undergo electrophilic substitution reaction
26. The conversion of phenol into salicylic acid – Kolbe reaction
27. The aldehyde formation via treating phenol with chloroform and aqueous hydroxide – Reimer-Tiemann
reaction
28. A 17-Carbon polycyclic aromatic compound which serves as parent compound of steroidal hormones –
Cyclopentanoperhydrophenanthrene
29. An environmental pollutant that is also a carcinogen with several Benzene rings – Benzopyrene
30. Which of the ff. is arranged in decreasing of boiling and melting point? – Propyne-Propene-Propane
You might also like
- Lab Report Act.6Document6 pagesLab Report Act.6Pattrick Lintag100% (2)
- Synthesis and Characterization of Alkane, Alkene and AlkyneDocument9 pagesSynthesis and Characterization of Alkane, Alkene and Alkynesapphirerk100% (3)
- 2013 A Level H2 Chem Paper 2 Suggested Solutions and Comments PDFDocument8 pages2013 A Level H2 Chem Paper 2 Suggested Solutions and Comments PDFImagreenbucklegirl SGNo ratings yet
- Amines, Amide and Derivatives: Edit and Compiled By: Hafiz Abdul Rafay Latif KayaniDocument26 pagesAmines, Amide and Derivatives: Edit and Compiled By: Hafiz Abdul Rafay Latif KayaniPalwasha KardarNo ratings yet
- Alcohols and Phenols: Based On Mcmurry'S Organic Chemistry, 9 EditionDocument47 pagesAlcohols and Phenols: Based On Mcmurry'S Organic Chemistry, 9 Edition劉靖騰No ratings yet
- M Sc-1Document12 pagesM Sc-1Shreyas BhandaryNo ratings yet
- Organic Chemistry - Notes On Alkanes To Esters-StudentDocument12 pagesOrganic Chemistry - Notes On Alkanes To Esters-Studentjasmineramkissoon786No ratings yet
- Alcohols Phenols and EthersDocument81 pagesAlcohols Phenols and Ethersjjprakash82chemNo ratings yet
- 17 - Alcohols, Esters and Carboxylic AcidsDocument46 pages17 - Alcohols, Esters and Carboxylic AcidsenderothNo ratings yet
- Notes On "ORGANIC CHEMISTRY" CBSE Class XIIDocument52 pagesNotes On "ORGANIC CHEMISTRY" CBSE Class XIIMahesh AdhikariNo ratings yet
- Carbonyl Compounds Aldehydes KetonesDocument58 pagesCarbonyl Compounds Aldehydes KetonesNur Aliyah Abdul RazakNo ratings yet
- Named Reaction TestDocument5 pagesNamed Reaction Testaleena'No ratings yet
- Carbonyl Compound-2Document20 pagesCarbonyl Compound-2fishindasea00No ratings yet
- Important Chemical Reactions For Class 12 Chemistry With MechanismDocument9 pagesImportant Chemical Reactions For Class 12 Chemistry With MechanismSoma SahaNo ratings yet
- Reasioning and Name RXDocument7 pagesReasioning and Name RXPavankumar SNo ratings yet
- Alcohols, Phenols and EthersDocument11 pagesAlcohols, Phenols and EthersSandeepNo ratings yet
- TEsts For UnsaturationDocument16 pagesTEsts For UnsaturationMahrishiShuklaNo ratings yet
- Chem Activity 1Document16 pagesChem Activity 1Jarvis StarkNo ratings yet
- Chemistry All Important ReactionsDocument14 pagesChemistry All Important Reactionsaayushnair2506100% (1)
- Organic Chemistry Revision Questions-1Document3 pagesOrganic Chemistry Revision Questions-1jatin2006gamil.comNo ratings yet
- Revision Booket-4 (Organic Chemistry) (18 Marks) : A Complete Revision Material For Class XII As Per New Syllabus of NCERTDocument14 pagesRevision Booket-4 (Organic Chemistry) (18 Marks) : A Complete Revision Material For Class XII As Per New Syllabus of NCERTabiNo ratings yet
- Hydroxy CompoundsDocument9 pagesHydroxy Compoundschong56No ratings yet
- Organic Chemistry Name ReactionDocument13 pagesOrganic Chemistry Name ReactionDeep GuptaNo ratings yet
- Organic Name ReactionsDocument8 pagesOrganic Name Reactionstiwari_anunay1689No ratings yet
- Aldol CondensationDocument4 pagesAldol CondensationSreeja SatheeshNo ratings yet
- 11 Alcohols Phenols and EthersDocument6 pages11 Alcohols Phenols and EthersVansh VaibhavNo ratings yet
- Important Chemical Reactions For Class 12 ChemistryDocument10 pagesImportant Chemical Reactions For Class 12 ChemistryRitishNo ratings yet
- 13 AminesDocument8 pages13 AminesShesha krishnaNo ratings yet
- Aldehydes and Ketones: ResultsDocument7 pagesAldehydes and Ketones: ResultsStephanie Joy EscalaNo ratings yet
- Chem 5-1st Post Lab DiscussionDocument41 pagesChem 5-1st Post Lab DiscussionJesselie SalayaNo ratings yet
- Discussion Exp 1Document6 pagesDiscussion Exp 1Dhirah Yuhans67% (3)
- Updated Grade 12 Organic Name Reactions-Units 10,11,12,13Document10 pagesUpdated Grade 12 Organic Name Reactions-Units 10,11,12,13Tanmay SharmaNo ratings yet
- Name Reactions 1Document81 pagesName Reactions 1Anjish PatelNo ratings yet
- Alcohols, Diols, TriolsDocument32 pagesAlcohols, Diols, TriolsShivam GuptaNo ratings yet
- Alcohols Phenols & EtherDocument10 pagesAlcohols Phenols & EtherVipin AroraNo ratings yet
- Name ReacitonsDocument6 pagesName ReacitonspzohmingthangaNo ratings yet
- BaeyerDocument1 pageBaeyerLivaashini NadarajanNo ratings yet
- Reactions of Amines and AmidesDocument7 pagesReactions of Amines and AmidesClechecyn Ella AgpadNo ratings yet
- 2 Name ReactionsDocument10 pages2 Name ReactionsArvindKumar100% (1)
- Hydrocarbon NotesDocument7 pagesHydrocarbon Notesl8627352No ratings yet
- Formulae For: AL Dehydes, Ketones & CarboxylicDocument16 pagesFormulae For: AL Dehydes, Ketones & CarboxylicSâmïr Kumar MundariNo ratings yet
- Iumportant ReactionsDocument9 pagesIumportant ReactionsAakash ShuklaNo ratings yet
- Organic Named File SarwarDocument8 pagesOrganic Named File SarwarShohom DeNo ratings yet
- Name RXNS: 1. Sandmeyer ReactionDocument10 pagesName RXNS: 1. Sandmeyer ReactionnitsNo ratings yet
- Class12 - Organichemistry - Named ReactionsDocument6 pagesClass12 - Organichemistry - Named ReactionsMᴀïᴢᴍɛɛŋ AŋꜱᴀʀïNo ratings yet
- Porg Lab Finals 1Document3 pagesPorg Lab Finals 1esther samonteNo ratings yet
- Alcohol and PhenolDocument13 pagesAlcohol and PhenolLia Yuli KusumaNo ratings yet
- Oxidation ReactionDocument21 pagesOxidation ReactionNor AzilaNo ratings yet
- Organic Chemistry-Named ReactionsDocument10 pagesOrganic Chemistry-Named Reactionsatharvbaghel4444No ratings yet
- STD XII Class Note Chapter 12 13 2021 22Document16 pagesSTD XII Class Note Chapter 12 13 2021 22Saurabh ShekharNo ratings yet
- Aldehydes and KetonesDocument8 pagesAldehydes and KetonesApple Bottom JeansNo ratings yet
- تقرير العضويه العملي الكحول PDFDocument6 pagesتقرير العضويه العملي الكحول PDFزينب هانيNo ratings yet
- Alcohols: Methods of PreparationDocument15 pagesAlcohols: Methods of PreparationKarthik SharmaNo ratings yet
- Alcohols: Methods of PreparationDocument15 pagesAlcohols: Methods of PreparationKarthik SharmaNo ratings yet
- Important Chemical Reactions For Class 12 Chemistry: Classes Competitive Exams Buy A Course +919243500460Document10 pagesImportant Chemical Reactions For Class 12 Chemistry: Classes Competitive Exams Buy A Course +919243500460sssNo ratings yet
- Chemistry HSC Board Important Reactions PDFDocument47 pagesChemistry HSC Board Important Reactions PDFNutzie YNo ratings yet
- Class 12 Organic Chemistry Important Topics: - Sandmeyer ReactionDocument7 pagesClass 12 Organic Chemistry Important Topics: - Sandmeyer ReactionSPCET.FY.24No ratings yet
- Alkohol Dari Senyawa KarbonilDocument31 pagesAlkohol Dari Senyawa KarbonilAshfie MarwaNo ratings yet
- Carbonyl Compounds: A2 Chemistry Unit 4Document45 pagesCarbonyl Compounds: A2 Chemistry Unit 4Faddy Oraha100% (1)
- Name ReactionsDocument10 pagesName ReactionsMUKUL SINGHNo ratings yet
- Hazardous - Resistance List KL10K Coil Coating v1.2Document6 pagesHazardous - Resistance List KL10K Coil Coating v1.2ajajsainNo ratings yet
- Classification of DyesDocument29 pagesClassification of DyesarafathosainNo ratings yet
- Researc H Article: Kidapawan City DivisionDocument10 pagesResearc H Article: Kidapawan City Divisionneil licatanNo ratings yet
- Assertion Reason Aldehydes KetonesDocument3 pagesAssertion Reason Aldehydes KetonessuryaisonemailNo ratings yet
- Alkyl HalidesDocument19 pagesAlkyl HalidesSaeed AnwarNo ratings yet
- Examination Details: A-Level German 2020/JUNE-OCTDocument27 pagesExamination Details: A-Level German 2020/JUNE-OCTNorhafiza RoslanNo ratings yet
- Application of Enzymes As Food Antioxidants: ReviewDocument5 pagesApplication of Enzymes As Food Antioxidants: ReviewNguyễn QuangNo ratings yet
- 1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byDocument9 pages1 Hydrogen Peroxide Decomposes To Form Water and Oxygen. This Reaction Is Catalysed byKelvin DenhereNo ratings yet
- Sikalastic®-726 Balcony One Shot Part B: Safety Data SheetDocument11 pagesSikalastic®-726 Balcony One Shot Part B: Safety Data Sheetcphammond83No ratings yet
- Emkarate Rl220h Msds v1.5Document11 pagesEmkarate Rl220h Msds v1.5luanacompany954No ratings yet
- Antidotes For Chemical ExposureDocument2 pagesAntidotes For Chemical ExposureSeshadri VasuNo ratings yet
- ORGHEM LAB HydrocarbonsDocument11 pagesORGHEM LAB HydrocarbonsJasmine CatanaNo ratings yet
- Echavia Concept-PaperDocument3 pagesEchavia Concept-PaperJefrey M. ButilNo ratings yet
- Oil and Gas 1Document9 pagesOil and Gas 1Africana RoyalNo ratings yet
- HND Polymer Note Part OneDocument46 pagesHND Polymer Note Part OnemuhammadmaihadisiNo ratings yet
- Two-Dimensional Metal-Organic Framework MaterialsDocument141 pagesTwo-Dimensional Metal-Organic Framework Materialsnavneetkaur77No ratings yet
- GreenChemistry ReviewMagneticcatalystsDocument30 pagesGreenChemistry ReviewMagneticcatalystsDave SallaoNo ratings yet
- Ptical Isomerism in Lactic AcidDocument10 pagesPtical Isomerism in Lactic AcidJay SoniNo ratings yet
- Bleaching Effect On Palm OilDocument239 pagesBleaching Effect On Palm Oilde eagle100% (6)
- A Blend of Skimmed Milk and Vegetable Fat in Powdered FormDocument8 pagesA Blend of Skimmed Milk and Vegetable Fat in Powdered FormAnuradha MarapanaNo ratings yet
- Neet Code C Question Paper PDFDocument41 pagesNeet Code C Question Paper PDFMagnifestoNo ratings yet
- Methyl Methacrylate MSDSDocument81 pagesMethyl Methacrylate MSDSJeEJyZaNo ratings yet
- PVC: ChemistryDocument55 pagesPVC: ChemistryAnjumol Salim100% (2)
- Essential Organic Chemistry 2nd Edition Bruice Test Bank Full Chapter PDFDocument41 pagesEssential Organic Chemistry 2nd Edition Bruice Test Bank Full Chapter PDFbasilthoatuis6100% (13)
- 1 (2 Bromoethoxy) 2 MethoxybenzeneDocument1 page1 (2 Bromoethoxy) 2 MethoxybenzeneSwasti ChemexNo ratings yet
- Expt 7 Classification Tests For HydrocarbonsDocument7 pagesExpt 7 Classification Tests For HydrocarbonsRizzalaine Caringal87% (30)
- A Review of Production and Processing of KiwifruitDocument7 pagesA Review of Production and Processing of KiwifruitadNo ratings yet
- CSEC Biology - AssimilationDocument1 pageCSEC Biology - AssimilationRaveena SinghNo ratings yet
- This Study Resource Was: Calculating Saponification Numbers Definition: The Saponification ReactionDocument3 pagesThis Study Resource Was: Calculating Saponification Numbers Definition: The Saponification ReactionJirapat ThonglekpechNo ratings yet