Professional Documents
Culture Documents
Spectrum MP 2 em
Uploaded by
All Bgm MixOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Spectrum MP 2 em
Uploaded by
All Bgm MixCopyright:
Available Formats
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
SSC PUBLIC EXAMINATIONS..JULY 2020
SPECTRUM
2 MODEL PAPER 2
GENERAL SCIENCE
(Refraction at plane surfaces &
Structure of atom )
Class: X [Max.Marks: 100] Time 3-15 hrs
1. This is revision practice paper only.
2. Prepared for two lessons only
3. You can watch video on this model paper.
PART A
(PHYSICAL SCIENCE-46M)
(Refraction at plane surfaces &
Structure of atom )
SECTION I
NOTE: 1. Answer ALL the questions in ONE word
2.Each question carries 1 mark
6x1m=6 M
1. Write Snell’s law.
2. Statement 1:- n12 = V2/V1
Statement 2:- sin C =1/n21
Which statement is correct?
A) 1 only B)2only C) both D) none
3. What is spectrum?
4. Match the following and select correct option.
a)Maximum number of electrons in a sub shell 1) 2n2
b)Maximum number of electrons in any shell 2) 2l+1
c)Maximum number of de generated orbitals for l 3) 2(2l+1)
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
A)a-1;B-2;C-3 B)a-3;B-1;C-2 C)a-1;B-3;C-2 D)a-3;B-2;C-1
5. What is the shape of a orbital if l=1?
6. The wave length of a radio wave is 1 m.Fibd its frequency.
SECTION II
NOTE: 1. Answer ALL the questions in ONE or TWO sentences.
2. Each question carries 2 marks 4X 2= 8M
7. Rithvik saw a mirage on a road and some doubts were arose in his
mind. Write two doubts.
8. Guess,what will happen if an electron gains energy?
9. Complete the given diagram.
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
10. What colours do you observe when strontium chloride and cupric
chloride are burn in a non luminous flame?
SECTION III
NOTE: 1. Answer ALL the questions in TWO or FOUR sentences. 4 X 4= 16M
2. Each question carries 4 mark
11. Write postulates of Bohr atomic model.
12. Write two applications of total internal reflection.
13. Answer the following questions
s.no substance Refractive index
1 A 0.31
2 B 1.5
3 C 1.0003
4 Diamond x
1)Write the the substances,A,B and C in the ascending order of
their densities.
2) What is x ?
3) What is the speed of light in substance B?
4) Write the common nature of the given substances.
14. Fill in the table.
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
n l ml subshells No.of subshells
1 0 0 (4Q)? 1
2 0 (1Q)? 2s 1
1 -1,0,+1 2p (6Q)?
3 0 0 3s (7Q)?
1 (2Q)? 3p 3
2 (3Q)? (5Q)? (8Q)?
SECTION IV
NOTE: 1. Answer ALL the questions in 8 to 10 sentences.
2. There is internal choice for each question.
3. Each question carries 8 marks 2X 8 = 16 M
15. Explain
a) Hund’s rule b) Aufbau principle
(OR) Write about four Quantum numbers.
16. How do youfind the refractive index of a glass slab?
(OR)How do you verify experimentally that sin i/sin r is a constant?
PREPARED BY RAMMOHAN,VZM
ANSWERS
1. n1 sin i= n2 sin r
2. A
3. A group of wave lengths.
4.B
5.Dumbel
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
6. freqyency= speed of light/wave length
=3X10 8m/s / 1m =3X10 8 Hz
7.Electron jumps from lower orbit to higher orbit and re back after
some time by emmising energy.
8. 1Q:-Which phenomenon is involved in the formation mirage?
2Q:-What are the conditions to form a mirage?
3Q:-Why does it occur on sunny days only?
4Q:- Which one looks like a fake water?
9. 1)Strontium chloride produces a crimson colour flame.2) Cupric
chloride produces a green colour flame.
10. drawing
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
11. 1)Niels Bohr proposed that electrons in an atom occupy
stationary orbits of fixed energy at different distances from the
nucleus.
2) When an electron jumps from a lower to higher energy state it
absorbs energy or emits its energy when such a jump from a
higher energy state.
3) The energy of an electron in an atom can have also certain
values E1, E2, E3... that is the energy is quantized. soda:-
12. Application of total internal reflection
1)Mirage:-
It is an optical illusion where it appears that water is collected on
the road at a distance, but when we go there the road is dry.
2)Brilliance of diamond
3)Optical fibres:- optical fibre is very thin fibre made of glass or
plastic. They communicate signals.
4) A metal ball covered with black carbon dust is appears shiny
underwater.
13.
1)C<A<B
2) x=2.42
3) 2X108m/s
4) all are transparent
14.
1)0 2) -1;0:+1 3) -2;-1;0:+1,+2 4) 1s
5) 3d 6) 3 7) 1 8) 5
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
15)
1) Aufbau principal:- Orbitals are filled in the order of increasing
energy.
or Less energy orbitals fill first.
2) Two general rules helps to predict electron configuration.
3)Electrons are assigned to orbitals in order of increasing the value
of( n + l) value.
4) For subshell with the same value of n + l electrons are assigned
first to the subshell with lower n.
5) Ascending order of energies where is atomic orbitals is given
below.
1s<2s<2p<3s<3p<4s<3d<5s....
eg:- 4s is filled first and 3d next.
because 4s<3d
Hund's rule :- 1)a)According to the rule electron pairing in orbitals
starts only when all available empty orbitals of the same energy are
singly occupide.
example:- The electron configuration of carbon atom is 1S2 2S2 2P2
2) The first four electrons go into 1S and 2s orbitals.
3) The next two electrons go into 2px and 2Py orbitals,but they do not
pair into 2PX orbital.
OR
15) Each electron in an atom is described by a set of four Quantum
numbers.
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
1) Principal quantum number (n):-
The principal quantum number is used to describe the size and energy
of the main shell.
It is denoted by n and n has positive integers values of 1,2,3,....
It is used to know the number of orbitals and electrons in an Orbit. if n
increases that orbit is farther from the nucleus and the energy
increases.
2) Angular momentum quantum number (l).
l has integer values from 0 to n -1 for each value of n.
Each l value represents one subshell.
It is used to describe the shape of an Orbital.
if l =0 means s orbital.if l =1 means p orbital.if l =2 means d orbital.
3)Magnetic quantum number (ml): The magnetic quantum number ml
has integer values between -l and +l including 0.
If l =0 the possible ml value is 1.
if l =1 the possible ml values are - 1, 0 ,+1
4) spin quantum number (ms)
It refers two possible orientations of the spin of electrons.
One clockwise and other one anticlockwise spin
These are represented by + 1/ 2 and -1 / 2.
16)
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
Aim:- identifying relation between angle of incidence and angle of
refraction. or sini/sin r = constant.
Procedure:-
1) Prepare a pro circle as shown in the figure. Draw the lines MM
horizontally and NN vertically on the pro circle.
2)Place a semicircular glass
disk so that its diameter coincides with the interface line MM and its
Centre coincides with the point O.
3) Take a laser light and send it along NN in such a way that the laser
propagates from air to glass through the interface at point O and
observe the way of laser light coming from other side of the disc.
4)There is no deviation.
5)Send the laser light along a line which makes 15 ° with NN and the
see that it must pass through point O.
6) Measure the corresponding angle of refraction and repeat the
experiment with angle of incidence of 20° 30° 40° 40° and 60° and not
the corresponding angles of refraction.
8) Find sin i and sin r for every i and r.
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
9) Note on the values in the table.
10) If i increases r also increases.
conclusion:- sin i/ sin r = const.
OR
16)
1) Measure the thickness of a glass slab and in your notebook.
2) Take white chart and fix it on the table.
3) Take the slab and place it in the middle of
the chart.
4) Draw a line around it. Remove the slab.
Name the vertices of the rectangle so
formed as A B Cand D.
5)Draw a perpendicular to the longest line
AB of the rectangle at any point on it.
6) Place the slab again in the rectangle
ABCD.
7) take a needle and place it at a point P in such a way that its length
is parallel to AB on the perpendicular line at the distance of 20 cm
from the Slab.
8)Now take another needle and by seeing the first needle from the
other side of the slab try to keep the middle so that it forms straight
line with the first needle.
RAMMOHAN MODEL PAPER ….2
Spectrum model papers 10 TH CLASS PHYSICS JULY 2020
9) Remove the slab and observe the positions of the needles. They
are in the same line.
10)Draw a perpendicular line from the second Needle to the line on
which first needle is placed. Name the intersection point as Q.
11) Measure the distance between P and Q.
The distance is called vertical shift of the glass slab.
12) Do the same activity for various instances of the nail paint from
the slab. We will get the same vertical shift.
13) refractive index = thickness of the glass slab/ (thickness of
the class - vertical shift)
…….by RAMMOHAN…
Please subscribe” HIGH SCHOOL PHYSICS by RAMMOHAN” you
tube channel for explanation on this paper..
RAMMOHAN MODEL PAPER ….2
You might also like
- An Introduction to Synchrotron Radiation: Techniques and ApplicationsFrom EverandAn Introduction to Synchrotron Radiation: Techniques and ApplicationsNo ratings yet
- Tom Mboya University College Exam Focuses on Inorganic Chemistry ConceptsDocument4 pagesTom Mboya University College Exam Focuses on Inorganic Chemistry ConceptsEZEKIEL IGOGONo ratings yet
- Ap X Pre Final-Iii Physical Science QPDocument4 pagesAp X Pre Final-Iii Physical Science QPGiyuurengu rochiiNo ratings yet
- ALL QB's PDFDocument36 pagesALL QB's PDFanimesh0gargNo ratings yet
- AP-Physical Science Sample Paper - Class 10 Question PaperDocument5 pagesAP-Physical Science Sample Paper - Class 10 Question PaperFirdosh KhanNo ratings yet
- S.S.C. Public Examinations - 2024 General Science - Model: PAPER-I (Physical Science) PaperDocument4 pagesS.S.C. Public Examinations - 2024 General Science - Model: PAPER-I (Physical Science) PaperSri Lakshmi DuvviNo ratings yet
- Delhi Public School: General InstructionsDocument16 pagesDelhi Public School: General InstructionsAyush jhaNo ratings yet
- Std X Unit Test Science Physics Chemistry BiologyDocument1 pageStd X Unit Test Science Physics Chemistry BiologyLekshmy BNo ratings yet
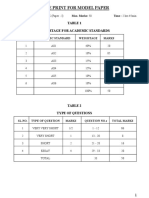
- Blue Print For Model Paper: Table 1 Weightage For Academic StandardsDocument5 pagesBlue Print For Model Paper: Table 1 Weightage For Academic StandardsKrishna VeniNo ratings yet
- Previous Hse Questions and Answers of The Chapter "Structure of Atom"Document12 pagesPrevious Hse Questions and Answers of The Chapter "Structure of Atom"YADUKRISHNAN K NAIRNo ratings yet
- Vidyamandir Classes: Innovating For Your SuccessDocument13 pagesVidyamandir Classes: Innovating For Your SuccessSuhana SinghNo ratings yet
- 05 Physics Model-2Document4 pages05 Physics Model-2Kgmghs ChiralaNo ratings yet
- SSLC Pre Model Examination - 2023: ChemistryDocument2 pagesSSLC Pre Model Examination - 2023: ChemistryhadiyxxNo ratings yet
- SSC PS em Dceb VZMDocument38 pagesSSC PS em Dceb VZMV HemanthNo ratings yet
- Tenth class Physical Science Model paper solutionsDocument4 pagesTenth class Physical Science Model paper solutionskatta swathiNo ratings yet
- Phy Science (Em)Document4 pagesPhy Science (Em)WarHead SupremeNo ratings yet
- SHCC 1718 Phy Mock 2 PDFDocument12 pagesSHCC 1718 Phy Mock 2 PDFVincent haNo ratings yet
- Chemistry Paper Set 2017 SA 1Document4 pagesChemistry Paper Set 2017 SA 1Daulot SarmaNo ratings yet
- 18.physical Science English Medium Modal Paper - 2Document4 pages18.physical Science English Medium Modal Paper - 2Naga BushanNo ratings yet
- Chem Wa1Document2 pagesChem Wa1Balarama RajuNo ratings yet
- Structure of The Atom - ExamDocument4 pagesStructure of The Atom - Examzehra giyoriNo ratings yet
- Section-A: Physics Marks: 12: Science (Class-X) Major Test # 02Document2 pagesSection-A: Physics Marks: 12: Science (Class-X) Major Test # 02YASHVI MODINo ratings yet
- Pen and Paper Parent Proctored Test: InstructionsDocument2 pagesPen and Paper Parent Proctored Test: InstructionsshantanuNo ratings yet
- State University of Zanzibar Solid State Physics ExamDocument4 pagesState University of Zanzibar Solid State Physics ExamHossam Abdalla SalehNo ratings yet
- CHAPTERWISETEST - D09 Dec 2022Document4 pagesCHAPTERWISETEST - D09 Dec 2022Atharva SisodiyaNo ratings yet
- Instruction For CandidatesDocument4 pagesInstruction For CandidatesAmit PokhariaNo ratings yet
- (2nd Sem) - Engineering-Physics-2-Eas-201-2011-12Document3 pages(2nd Sem) - Engineering-Physics-2-Eas-201-2011-12Mahima FamousNo ratings yet
- BS109 Sem-1 Feb 2022Document4 pagesBS109 Sem-1 Feb 2022Mohammad NadirNo ratings yet
- Structure of The Atom - Exam - Part1Document1 pageStructure of The Atom - Exam - Part1zehra giyoriNo ratings yet
- CHM1011 S1,2011 PDFDocument28 pagesCHM1011 S1,2011 PDFSasuke AhmedNo ratings yet
- Structure of Atom-hsslive-AnilDocument4 pagesStructure of Atom-hsslive-AnilDhana Aryal100% (1)
- PHT 100 Engineering Physics ADocument10 pagesPHT 100 Engineering Physics AAdarsh QclwNo ratings yet
- ChemistryDocument2 pagesChemistryshriya shettyNo ratings yet
- Vtu Be 1st Year Physics Question PaperDocument4 pagesVtu Be 1st Year Physics Question PapermidhunmathewNo ratings yet
- Engineering Physics B NotesDocument10 pagesEngineering Physics B NotesBalagopal VNo ratings yet
- HW_Chap 1_231Document3 pagesHW_Chap 1_231Vĩ NguyễnNo ratings yet
- SSLC Science em 5 SetDocument29 pagesSSLC Science em 5 Setmchandhu875No ratings yet
- PHY5 B07 Quantum MechanicsDocument2 pagesPHY5 B07 Quantum MechanicsLakshmi E. SNo ratings yet
- Physics-Xii QP PhyDocument6 pagesPhysics-Xii QP PhyermaharajanNo ratings yet
- X Class Ps em Set 1Document4 pagesX Class Ps em Set 1RajuNo ratings yet
- Engg Physics June 2012 NewDocument4 pagesEngg Physics June 2012 NewPrasad C MNo ratings yet
- PHYSICS-THEORY-AND-PRACT-S5 by NESA 2023 EXAMDocument30 pagesPHYSICS-THEORY-AND-PRACT-S5 by NESA 2023 EXAMsingeniyoemmanuel15No ratings yet
- SCH 200 Atomic Structure and Chemical BondingDocument4 pagesSCH 200 Atomic Structure and Chemical BondingPst Kaka ClaranceNo ratings yet
- Time Allowed: 20mins Section-A (Marks 12) Roll NumberDocument3 pagesTime Allowed: 20mins Section-A (Marks 12) Roll NumberHaiderNo ratings yet
- Nanostructure and Technology Exam QuestionsDocument3 pagesNanostructure and Technology Exam QuestionsSnehardraNo ratings yet
- CHEMISTRYDocument2 pagesCHEMISTRYtanayjadhav70.2No ratings yet
- Narayana 05 05 2022 SR IIT OUTGOING LT IIT Jee Main GTM QPDocument19 pagesNarayana 05 05 2022 SR IIT OUTGOING LT IIT Jee Main GTM QPZeusNo ratings yet
- One Mark QuestionsDocument4 pagesOne Mark Questionshari95No ratings yet
- Chem Q.bank Xi 2022Document16 pagesChem Q.bank Xi 2022rishikaa.saxenaNo ratings yet
- Multiple Choice Questions (Type-1) : NCERT Exemplar Solutions of Class 11 Chemistry Chapter 2 Structure of AtomDocument13 pagesMultiple Choice Questions (Type-1) : NCERT Exemplar Solutions of Class 11 Chemistry Chapter 2 Structure of AtomGagan PhadkeNo ratings yet
- Gem Chem MidsemDocument2 pagesGem Chem MidsemRohan TiwariNo ratings yet
- f2 Endterm 1 Series 2Document70 pagesf2 Endterm 1 Series 2abu326274No ratings yet
- Section ADocument7 pagesSection AitsmepragyanvermaNo ratings yet
- Solid State Physics Spectroscopy and Laser Physics 2017Document3 pagesSolid State Physics Spectroscopy and Laser Physics 2017SnehardraNo ratings yet
- Structure of Atom Worksheet SolutionsDocument3 pagesStructure of Atom Worksheet SolutionsMariaNo ratings yet
- DPS Bangalore North chemistry weekly testDocument3 pagesDPS Bangalore North chemistry weekly testDaksh PathakNo ratings yet
- Affan Telek - Chemistry Unit Test 1 Ver - 1Document14 pagesAffan Telek - Chemistry Unit Test 1 Ver - 1Affan TelekNo ratings yet
- Delhi Public School Bangalore North Academic Session 2022-23 Worksheet-Answer KeyDocument6 pagesDelhi Public School Bangalore North Academic Session 2022-23 Worksheet-Answer KeyShashwatNo ratings yet
- AssddgfghhfDocument9 pagesAssddgfghhfbahasismanogluNo ratings yet
- Mid ADocument3 pagesMid AFaiza AkterNo ratings yet
- Women EmpowermentDocument14 pagesWomen EmpowermentProfessor HappyNo ratings yet
- Evolution - The Dissent of DarwinDocument7 pagesEvolution - The Dissent of DarwinluminitalupuliasaNo ratings yet
- Project Review: Arun III Hydroelectric Project: Background Location and AccessibilityDocument2 pagesProject Review: Arun III Hydroelectric Project: Background Location and AccessibilitySachin ChakradharNo ratings yet
- Iec 61724-1 2017 Version-Selection of Pyranometers v2008Document4 pagesIec 61724-1 2017 Version-Selection of Pyranometers v2008wwahib2100% (1)
- Modern CV Template StyleDocument2 pagesModern CV Template StyleRedi Joel Córdova ArbietoNo ratings yet
- Fiber Optic Temperature Laser RadarDocument7 pagesFiber Optic Temperature Laser RadarLesther RivasNo ratings yet
- Axisymm TutorialDocument15 pagesAxisymm Tutorialmudur6No ratings yet
- Module 3Document2 pagesModule 3Julius CabinganNo ratings yet
- Project Time Management and Budget PlanningDocument68 pagesProject Time Management and Budget PlanningLindelani ndalaNo ratings yet
- Analisis Unsur-Unsur Intrinsik Cerpen "Senyum Karyamin" Karya Ahmad TohariDocument8 pagesAnalisis Unsur-Unsur Intrinsik Cerpen "Senyum Karyamin" Karya Ahmad TohariP4T GamingNo ratings yet
- Products Profile Unitech Ikkcomunitech Ikkcomsfspsfsp Profile 6 SFSPDocument46 pagesProducts Profile Unitech Ikkcomunitech Ikkcomsfspsfsp Profile 6 SFSPSaad AkramNo ratings yet
- Guru Nanak Dev University, Amritsar: Notification No. B.Tech. (Mech. Engg.) (CBES) /-8th Semester/May-2019/1Document1 pageGuru Nanak Dev University, Amritsar: Notification No. B.Tech. (Mech. Engg.) (CBES) /-8th Semester/May-2019/1Nehal GuptaNo ratings yet
- Quick Start GuideDocument3 pagesQuick Start Guideswornavidhya.mahadevanNo ratings yet
- 03 Preliminary PagesDocument12 pages03 Preliminary PagesBillie Jan Louie JardinNo ratings yet
- Eh TW5825Document2 pagesEh TW5825Lim Hendra Kurniawan Halim (Hendra)No ratings yet
- 3014 LED PLW3014CA Series: Product DatasheetDocument14 pages3014 LED PLW3014CA Series: Product DatasheetfaberjetNo ratings yet
- Coursebook Analysis Jeremy Harmer How To Teach English Pearson 2007Document1 pageCoursebook Analysis Jeremy Harmer How To Teach English Pearson 2007Jamie Leigh McGeorgeNo ratings yet
- Enumerators (x50) - Job Search MalawiDocument4 pagesEnumerators (x50) - Job Search MalawicliftonkacheremNo ratings yet
- C#Brain TeasersDocument5 pagesC#Brain TeasersNishant.kumaridNo ratings yet
- SWIFTgpi Newsflash August Application Providers FinalDocument7 pagesSWIFTgpi Newsflash August Application Providers FinalSushma V KumarNo ratings yet
- Install Notes VTK5 VC2010 Win64Document5 pagesInstall Notes VTK5 VC2010 Win640raeNo ratings yet
- Jongka:the Traditional Korean Family: Exploring Jongka Food in The Context of Korean Food CategoriesDocument14 pagesJongka:the Traditional Korean Family: Exploring Jongka Food in The Context of Korean Food CategoriesSözen BayraktarNo ratings yet
- Year 4 Geography Field Trip BookletDocument4 pagesYear 4 Geography Field Trip Bookletapi-357287502No ratings yet
- Business Aptitude Test: BAT™ Module I - Academic AptitudeDocument18 pagesBusiness Aptitude Test: BAT™ Module I - Academic AptitudePriyam SaraogiNo ratings yet
- Communication Education For AdultsDocument7 pagesCommunication Education For AdultsHaechan MarkNo ratings yet
- Method of Statement - Testing and CommissioningDocument1 pageMethod of Statement - Testing and CommissioningTiam Yee YongNo ratings yet
- Designing Test Suites: Design A Test Suite For The Following ProblemDocument2 pagesDesigning Test Suites: Design A Test Suite For The Following ProblemAndroid Sourvivors sNo ratings yet
- Writing Task 1 - Integrated Question (Test #12) : Your Guide For TOEFL Writing 24+Document3 pagesWriting Task 1 - Integrated Question (Test #12) : Your Guide For TOEFL Writing 24+AstridBarraNo ratings yet
- Skyline Skylites - Unit SkylightsDocument2 pagesSkyline Skylites - Unit SkylightskdpmansiNo ratings yet
- Joanne Mitchell 2223rtkl0155 Email Orig - RedactedDocument29 pagesJoanne Mitchell 2223rtkl0155 Email Orig - RedactedmegankshannonNo ratings yet