Professional Documents
Culture Documents
Dermatological Reviews - 2022 - Honigman - Differential Diagnosis of Melasma and Hyperpigmentation - Removed
Uploaded by
meisya 102018090Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Dermatological Reviews - 2022 - Honigman - Differential Diagnosis of Melasma and Hyperpigmentation - Removed
Uploaded by
meisya 102018090Copyright:
Available Formats
Received: 20 January 2022 | Revised: 26 June 2022 | Accepted: 29 June 2022
DOI: 10.1002/der2.144
INVITED REVIEW
Differential diagnosis of melasma and hyperpigmentation
Anthony Honigman1 | Michelle Rodrigues2,3
1
Dermatology Department, The Royal
Melbourne Hospital, Melbourne, Australia Abstract
2
Chroma Dermatology, Pigment and Skin of Background: Melasma is a common disorder of acquired hyperpigmentation
Colour Centre, Victoria, Australia
affecting millions of people worldwide, predominantly women. Despite the
3
Department of Dermatology and Department
of Paediatrics, Royal Children's Hospital, incidence, there are numerous hyperpigmentary conditions which may clinically
Melbourne University, Melbourne, Victoria, resemble melasma. The differential diagnosis of melasma is therefore wide and may
Australia
prove to be a diagnostic challenge for clinicians. This can delay the appropriate
Correspondence treatment, adversely impacting sufferers. Our review aims to provide clinicians with
Michelle Rodrigues, Ground Floor, suite
an understanding of and a comprehensive approach to assessing, diagnosing and
15/202 Jells Rd, Victoria 3150, Australia.
Email: dr.rodrigues@gmail.com treating melasma and other disorders of hyperpigmentation.
KEYWORDS
hyperpigmentation, melanogenesis, melasma, pigmentary disorders
1 | INTRODUCTION 2.1 | Presentations and clinical classifications
Melasma is an acquired hyperpigmentation disorder and is charac- Melasma presents clinically as brown macules or patches and is
terized by light‐to‐dark brown‐colored irregular macules or patches generally a clinical diagnosis. It is classified by both location and
on sun‐exposed areas of skin, usually the face.1 It is the most depth of involvement.
common cause of facial pigmentation and is a cutaneous disorder
affecting all races with particular prominence in darker‐skinned
individuals.2 Despite being a common condition with strong 2.1.1 | Location
therapeutic demand, diagnosis may prove challenging for physi-
cians as there are numerous conditions that can present similarly There are three types of melasma as classified clinically according to
to melasma. It is, thus, imperative for dermatologists to their facial distribution, and these include centrofacial, malar, and
appreciate and consider common differential diagnoses when mandibular patterns (see Table 1).3,4 The most common clinical
assessing patients with melasma. pattern (in 50%–80% of cases) is the centrofacial pattern, where lesions
are predominant in the center of the face – affecting the forehead,
cheeks, nose, upper lip, or chin (Figure 1A). The malar pattern is restricted
2 | M E L A SM A to the malar cheeks on the face and can include the nose (Figure 1B),
while mandibular melasma is present on the ramus of the mandible
Melasma is an acquired hyperpigmentation disorder that is seen (jawline) and may include the chin.5,6 The mandibular pattern of melasma
most typically on the face. It is a common disorder of tends to be noted in postmenopausal women.4,7
hyperpigmentation affecting millions worldwide and predomi- Variants of melasma have been reported on other sun‐exposed
nantly affecting women (90% of cases) and those with Fitzpatrick areas of the body, and a newer classification termed “extra‐facial”
skin type III and IV.2 melasma has emerged. This classification pattern encompasses
This is an open access article under the terms of the Creative Commons Attribution‐NonCommercial‐NoDerivs License, which permits use and distribution in any
medium, provided the original work is properly cited, the use is non‐commercial and no modifications or adaptations are made.
© 2022 The Authors. Dermatological Reviews published by John Wiley & Sons Ltd.
30 | wileyonlinelibrary.com/journal/der2 Dermatological Reviews. 2023;4:30–37.
26377489, 2023, 1, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/der2.144 by Nat Prov Indonesia, Wiley Online Library on [31/07/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
HONIGMAN AND RODRIGUES | 33
TABLE 2 Summary of common pigmentation disorders
Disorder Clinical appearance Location of the lesion(s) Age of onset
Melasma Symmetric, light to dark brown patches Centrofacial 20–40 years
Solar lentigines Well‐demarcated tan to dark brown) Sun exposed sites Any age
round/oval macules
Ephelides Well‐demarcated, sharply defined macules Sun exposed sites Typically childhood
a
Café‐au‐lait macules Tan to brown patches Commonly on trunk Birth or early
childhood
Post‐inflammatory Hyperpigmentation ± erythema and/or scaling Any site Any age
hyperpigmentation
Drug‐induced Greyish hyperpigmentation Face ± involvement of other special sites Any age
hyperpigmentation
Exogenous ochronosis Blue–black confluent hyperpigmentation Sites of hydroquinone application Any age
Acquired dermal macular Gray–brown to brown macules and patches Mainly the face but may occur on the trunk Middle age (typically
hyperpigmentation over 30 years
(ADMH) of age)
Acanthosis nigricansa Symmetric, hyperpigmented, velvety plaques Intertriginous areas – neck, groin, and axillae Usually less than
40 years of age
Dermatosis papulosis Pigmented papules Bilateral malar eminences and forehead During adolescence
nigra (DPN)
Nevus of Otaa Blue–gray confluence of individual macules Distribution of the first two branches of the Infancy or puberty
trigeminal nerve
Hori's nevus Blue–gray to gray–brown macules Bilateral zygomatic areas 20–70 years
a
Entities with possible systemic involvement.
early in life (Table 2). They are asymptomatic and present as light‐to‐ The location of excess pigment within the layers of the skin will
dark brown well‐circumscribed, pigmented macules or patches determine its color. Epidermal hypermelanosis will appear tan, brown, or
usually greater than 20 mm in size and often located on the trunk. dark brown and may take months to years to resolve without
They are epidermal in origin, representing an increase in melanin in treatment.1 Hyperpigmentation within the dermis has a blue–gray
melanocytes and basal keratinocytes. Multiple CALMs are often appearance and may either be permanent or resolve over a protracted
associated with genetic syndromes, with more than six CALMs (5 mm period of time if left untreated.19,20 PIH should be considered when
or larger, prepubertal; 15 mm or larger postpubertal) raising suspicion patients provide a history of any type of inflammation or injury before
for disorders such as neurofibromatosis (most commonly NF1), its onset, for example, acne, eczema, psoriasis, or trauma. Patients will
tuberous sclerosis, Albright syndrome, Legius syndrome, or Fanconi often present with hyperpigmented macules and patches of varying size
anemia.18 CALMs are diagnosed clinically and a skin biopsy is usually in the same distribution as the initial inflammatory process. Although
not required to confirm the diagnosis. usually a clinical diagnosis, more difficult cases can be aided with a
biopsy for histopathological evaluation.
3.2 | Postinflammatory hyperpigmentation
3.3 | Drug‐induced hyperpigmentation
Postinflammatory hyperpigmentation (PIH) is skin darkening occur-
ring after cutaneous injury or inflammation that arises in all skin A number of different medications have been associated with skin
types (Table 2), but more frequently affects the skin of color pigmentation, including neurological medications (chlorpromazine,
patients (Fitzpatrick skin type IV–VI). Often, as the skin in darker amitriptyline), cytotoxic agents (bleomycin, anthracycline), analgesics,
patients recovers from an acute inflammatory cutaneous disease anticoagulants, antimicrobials (chloroquine, minocycline), antiretro-
or injury, it may become hyperpigmented or hypopigmented virals, metals, and antiarrhythmics (amiodarone).21
(postinflammatory hypopigmentation). PIH can occur in both the Drug‐induced hyperpigmentation can occur at any age and often
epidermis and dermis and is a result of melanocytes responding to presents as a slate‐gray coloration affecting the face and other areas
a cutaneous insult which causes increased production and irregular of the body.1 Melasma typically lacks this deep, slate‐gray appear-
2,19
redistribution of melanin. ance and does not commonly involve other body sites (upper limbs,
26377489, 2023, 1, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/der2.144 by Nat Prov Indonesia, Wiley Online Library on [31/07/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
34 | HONIGMAN AND RODRIGUES
shins, scar sites) assisting clinicians in their diagnosis (Table 2).
Pathophysiology of medication‐induced pigmentation occurs through
several different mechanisms depending on the drug itself. Some
medications trigger an accumulation of melanin (antimalarials) which
is often found to be worse in sun‐exposed areas, while other
medications accumulate within dermal macrophages and cause
pigmentation itself. Some medications cause the synthesis of new
pigment (lipofuscin) or the accumulation of iron and lysis of red blood
cells (minocycline).21,22 Diagnosing drug‐induced hyperpigmentation
can prove difficult and clinicians should be vigilant in examining a
patient's complete medical history to determine the specific culprit
and rule out other conditions that may be leading to skin findings.
3.4 | Exogenous ochronosis
Exogenous ochronosis (EO) is a rare, blue–black pigmentation of the F I G U R E 2 Acanthosis nigricans on the malar region of a male of
Sri Lankan background. Image source: Dr. Michelle Rodrigues,
face, lateral and posterior neck, and extensor surfaces and is caused by
Chroma Dermatology.
the deposition of microscopic ocher‐colored pigment in the dermis
(Table 2). The term ochronosis is derived from the word “ocher” in Greek
language, which refers to yellow discoloration.23 The etiology of EO is not autosomal dominant trait due to mutations in fibroblast growth factor
fully understood; however, it has been linked with the use of skin‐care receptor 3.28 Malignant acanthosis nigricans is associated with gastro-
products containing hydroquinone, resorcinol, phenol, mercury, and picric intestinal adenocarcinomas and genitourinary cancer such as prostate,
acid as well as unprotected prolonged sun exposure.2,23,24 breast, and ovarian. This subtype of acanthosis nigricans is typically rapid
It is thought that hyperpigmentation associated with EO is due to in onset and may precede, accompany or follow the onset of internal
local competitive inhibition of the enzyme homogentisic oxidase by malignancy.29 Multiple medications have also been linked to acanthosis
hydroquinone, which leads to local accumulation of homogentisic nigricans, these include nicotinic acid, systemic glucocorticoids, diethyl-
acid and its metabolic products that polymerizes to form the typical stilboestrol, combined oral contraceptive pill, estrogen, growth hormone
“ochronotic” pigment in the papillary dermis.23,25 EO is clinically and therapy, protease inhibitors, niacin, and injected insulin.7,30,31 The
histologically similar to its endogenous counterpart – endogenous pathogenesis of acanthosis nigricans is thought to be related to growth
ochronosis, also known as alkaptonuria, although it does not exhibit factor levels and insulin‐mediated activation of insulin‐like growth factors
systemic effects and is not an inherited disorder. on keratinocytes and increased growth factor levels. This activation
Clinically, it manifests as hyperpigmentation in photoexposed triggers the proliferation of fibroblasts and the enhanced stimulation of
regions, often affecting the zygomatic regions in a symmetrical epidermal keratinocytes.32 In patients with malignant acanthosis nigricans,
pattern. The lesions are typically blue–black macules usually the most probable stimulating factors are secreted by cancer cells – likely
with pinpoint and caviar‐like papules23,26 and are often confused transforming growth factor or epidermal growth factor, as both levels are
with melasma, PIH, and Riehl's melanosis. Diagnosis is confirmed on high in those with gastric adenocarcinoma. Histology reveals papilloma-
histology, which remains the gold standard of EO diagnosis. tosis, hyperkeratosis with minimal hyperpigmentation. There is thinning of
The pathognomic histopathological feature is the presence of the epidermis and an upward projection of the dermal papillae.27
23
ocher‐colored, banana‐shaped fibers within the dermis.
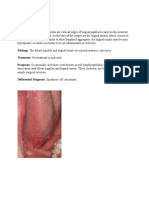
3.6 | Dermatosis papulosis nigra
3.5 | Acanthosis nigricans
Dermatosis papulose nigra (DPN) is a benign epidermal growth that
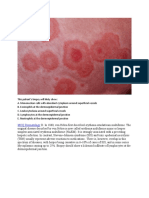
Acanthosis nigricans is characterized by velvety hyperpigmented presents as hyperpigmented or skin‐colored papules that develop on
macules and patches that progress to symmetrical palpable plaques the face and neck.
(Figure 2). Although most commonly appearing in intertriginous areas of The lesions initially resemble freckles but may gradually become
the body, including the axilla, groin, and neck, acanthosis nigricans can papular and increase in both size and number with age. The lesions
occur in almost any anatomical location including the face (Table 2).27 range in size from 1 to 5 mm and commonly appear symmetrically on
Acanthosis nigricans typically occurs in individuals younger than 40 the malar cheeks, temples, and forehead (Table 2).
years of age and is associated with obesity, hypothyroidism, acromegaly, Although it is seen in other races, it is a common manifestation
polycystic ovary disease, insulin‐resistant diabetes, Cushing's disease, and diagnosed primarily in African–American, Afro‐Caribbean, sub‐Saharan
Addison disease. Familial acanthosis nigricans arises as a result of an African, and Asian patients. The reported incidences in these populations
26377489, 2023, 1, Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/der2.144 by Nat Prov Indonesia, Wiley Online Library on [31/07/2023]. See the Terms and Conditions (https://onlinelibrary.wiley.com/terms-and-conditions) on Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License
36 | HONIGMAN AND RODRIGUES
conditions often negatively impact physical and emotional wellbeing. 17. Fathman EM, Habif TP. Skin Disease: Diagnosis and Treatment. 1st ed.
Therefore, clinicians must have comprehensive knowledge and a Mosby; 2001.
18. Davis EC, Callender VD. Post‐inflammatory hyperpigmentation: a
systematic approach to melasma and its common differential
review of the epidemiology, clinical features, and treatment options
diagnoses. in skin of color. J Clin Aesthet Dermatol. 2010;Jul 3(7):20‐31.
19. Chang MW. Disorders of hyperpigmentation. In: Bolognia JL,
A C KN O W L E D G M E N T Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Elsevier Mosby;
2009:333‐389.
Open access publishing facilitated by The University of Melbourne, as
20. Hassan S, Zhou X. Drug induced pigmentation. StatPearls [Internet].
part of the Wiley ‐ The University of Melbourne agreement via the StatPearls Publishing; 2021.
Council of Australian University Librarians. 21. Bell AT, Roman JW, Gratrix ML, Brzezniak CE. Minocycline‐
induced hyperpigmentation in a patient treated with erlotinib for
non‐small cell lung adenocarcinoma. Case Rep Oncol. 2017;10(1):
CO NFL I CT OF INTERES T
156‐160.
The authors declare no conflict of interest.
22. Daadaa N, Ben Tanfous A. Riehl melanosis. StatPearls [Internet].
StatPearls Publishing; 2021.
D A TA A V A I L A B I L I T Y S T A T E M E N T 23. Levin CY, Maibach H. Exogenous ochronosis. an update on clinical
There will be no data availability statement for the article. features, causative agents and treatment options. Am J Clin
Dermatol. 2001;2:213‐217.
24. Penneys NS. Ochronosis‐like pigmentation from hydroquinone
ORCID bleaching creams. Arch Dermatol. 1985;121:1239‐1240.
Anthony Honigman http://orcid.org/0000-0002-4872-6340 25. Findlay GH, Morrison JG, Simson IW. Exogenous ochronosis and
Michelle Rodrigues http://orcid.org/0000-0002-9747-1882 pigmented colloid milium from hydroquinone bleaching creams. Br
J Dermatol. 1975;93:613‐622.
26. Ghosh A, Coondoo A. Lichen planus pigmentosus: the controversial
REFERENCES consensus. Indian J Dermatol. 2016;61(5):482‐486.
1. Rodrigues M, Pandya AG. Melasma: clinical diagnosis and manage- 27. Fukuchi K, Tatsuno K, Matsushita K, Kubo A, Ito T, Tokura Y. Familial
ment options. Australas J Dermatol. 2015;56:151‐163. acanthosis nigricans with p.K650T FGFR3 mutation. J Dermatol.
2. Vashi NA, Kundu RV. Facial hyperpigmentation: causes and 2018;45(2):207‐210.
treatment. Br J Dermatol. 2013;169(suppl 3):41‐56. 28. Yu Q, Li XL, Ji G, et al. Malignant acanthosis nigricans: an early
3. Lee AY. Recent progress in melasma pathogenesis. Pigment Cell diagnostic clue for gastric adenocarcinoma. World J Surg Oncol.
Melanoma Res. 2015;28:648‐660. 2017;15(1):208.
4. Mandry Pagan R, Sanchez JL. Mandibular melasma. P R Health Sci. J. 29. Stals H, Vercammen C, Peeters C, Morren MA. Acanthosis nigricans
2000;19:231‐234. caused by nicotinic acid: case report and review of the literature.
5. Tamega Ade A, Miot LD, Bonfietti C, Gige TC, Marques ME, Dermatology. 1994;189(2):203‐206.
Miot HA. Clinical patterns and epidemiological characteristics of 30. Mellor‐Pita S, Yebra‐Bango M, Alfaro‐Martínez J, Suárez E.
facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. Acanthosis nigricans: a new manifestation of insulin resistance in
2013;27(2):151‐156. patients receiving treatment with protease inhibitors. Clin Infect Dis.
6. Ogbechie‐Godec OA, Elbuluk N. Melasma: an up‐to‐date compre- 2002;34(5):716‐717.
hensive review. Dermatol Ther. 2017;7(7):305‐318. 31. Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations
7. Fleming MG, Simon SI. Cutaneous insulin reaction resembling of endocrine disorders. Transl Pediatr. 2017;6(4):300‐312.
acanthosis nigricans. Arch Dermatol. 1986;122(9):1054‐1056. 32. Metin SA, Lee BW, Lambert WC, Parish LC. Dermatosis papulosa
8. Handel AC, Miot LDB, Miot Ha. Melasma: a clinical and epidemio- nigra: a clinically and histopathologically distinct entity. Clin
logical review. Bras Dermatol. 2014;89(5):771‐782. Dermatol. 2017;35(5):491‐496.
9. O'Brien TJ, Dyall‐Smith D, Hall AP. Melasma of the forearms. 33. Bhat RM, Patrao N, Monteiro R, Sukumar D. A clinical, dermoscopic,
Australas J Dermatol. 1997;38:35‐37. and histopathological study of dermatosis papulosa nigra (DPN)—an
10. Grimes PE, Yamada N, Bhawan J. Light microscopic, immuno- Indian perspective. Int J Dermatol. 2017;56(9):957‐960.
histochemical, and ultrastructural alterations in patients with 34. Hafner C, Landthaler M, Mentzel T, Vogt T. FGFR3 and PIK3CA
melasma. Am J Dermatopathol. 2005;27(2):96‐101. mutations in stucco keratosis and dermatosis papulosa nigra. Br
11. Cayce KA, Feldman SR, McMichael AJ. Hyperpigmenation: a review J Dermatol. 2010;162(3):508‐512.
of common treatment options. J Drugs Dermatol. 2004;3:668‐673. 35. Agarwal P, Patel BC. Nevus of Ota and Ito. StatPearls [Internet].
12. Hori Y, Kawashima M, Oohara K, Kukita A. Acquired, bilateral nevus StatPearls Publishing; 2021.
of Ota‐like macules. J Am Acad Dermatol. 1984;10:961‐996. 36. Sheth VM, Pandya AG. Melasma: a comprehensive update: part I.
13. Mishra SN, Dhurat RS, Deshpande DJ, Nayak CS. Diagnostic utility J Am Acad Dermatol. 2011;65:689‐697.
of dermatoscopy in hydroquinone‐induced exogenous ochronosis. 37. Vinay K, Bishnoi A, Kamat D, Chatterjee D, Kumaran MS, Parsad D.
Int J Dermatol. 2013;52(4):413‐417. Acquired dermal macular hyperpigmentation: an update. Indian
14. Kang HY, Bahadoran P, Suzuki I, et al. In vivo reflectance confocal Dermatol Online J. 2021;12(5):663‐673.
microscopy detects pigmentary changes in melasma at a cellular 38. Wang L, Xu AE. Four views of Riehl's melanosis: clinical appearance,
level resolution. Exp Dermatol. 2010;19(8):e228‐e233. dermoscopy, confocal microscopy and histopathology. J Eur Acad
15. Rhodes AR, Harrist TJ, Momtaz TK. The PUVA‐induced pigmented Dermatol Venereol. 2014;28(9):1199‐1206.
macule: a lentiginous proliferation of large, sometimes cytologically 39. Kanwar AJ, Dogra S, Handa S, Parsad D, Radotra BD. A study of 124
atypical, melanocytes. J Am Acad Dermatol. 1983;9:47‐58. Indian patients with lichen planus pigmentosus. Clin Exp Dermatol.
16. Stern JB, Peck GL, Haupt HM, Hollingsworth HC, Beckerman T. 2003;28(5):481‐485.
Malignant melanoma in xeroderma pigmentosum: search for a 40. Brady MF, Rawla P. Acanthosis nigricans. StatPearls [Internet].
precursor lesion. J Am Acad Dermatol. 1993;28(4):591‐594. StatPearls Publishing; 2021.
You might also like
- Case Summary Pediatrics32 5-Year-ODocument12 pagesCase Summary Pediatrics32 5-Year-OMaryam FadahNo ratings yet
- (Krobotova, 2012) PDFDocument3 pages(Krobotova, 2012) PDFrosaNo ratings yet
- Welcome To The Autistic CommunityDocument325 pagesWelcome To The Autistic Communitybrooke100% (3)
- Disorders of PigmentationDocument4 pagesDisorders of Pigmentationعبدالعزيز احمد علي عتشNo ratings yet
- 08-Skin TumoursDocument8 pages08-Skin TumoursChris Tan100% (1)
- Dermnet NZ: MelasmaDocument5 pagesDermnet NZ: MelasmaWin TheinNo ratings yet
- Hyperpigmented DisorderDocument49 pagesHyperpigmented Disorderضبيان فرحانNo ratings yet
- Conditions of The Integumentary SystemDocument10 pagesConditions of The Integumentary SystemNICOLE RAFAEL TACDERASNo ratings yet
- Hyperpigmentation and Melasma: D Rigopoulos, MD, PHD, S Gregoriou, MD, & A Katsambas, MD, PHDDocument8 pagesHyperpigmentation and Melasma: D Rigopoulos, MD, PHD, S Gregoriou, MD, & A Katsambas, MD, PHDGÜLSÜM GÜNEŞNo ratings yet
- Dr. Tandiono Hermanda, SP - KKDocument6 pagesDr. Tandiono Hermanda, SP - KKAchmad Fahri BaharsyahNo ratings yet
- Conditions of The Integumentary System 2Document12 pagesConditions of The Integumentary System 2NICOLE RAFAEL TACDERASNo ratings yet
- Leg, Arm, and Chest Papules: ERM ASEDocument9 pagesLeg, Arm, and Chest Papules: ERM ASEdeenutz93No ratings yet
- Dermotologic - BoardsDocument10 pagesDermotologic - BoardsSoojung NamNo ratings yet
- Skin Tumors: Dr. Ihsan Al-Turfy Consultant Dermatologist College of Medicine/Baghdad MBCHB, DDV, Ficms, CabdDocument149 pagesSkin Tumors: Dr. Ihsan Al-Turfy Consultant Dermatologist College of Medicine/Baghdad MBCHB, DDV, Ficms, CabdRomi WijiantoNo ratings yet
- MelasmDocument22 pagesMelasmLeonardo Alves F AlmeidaNo ratings yet
- 3.skin PathologyDocument38 pages3.skin PathologyFaisal MehboobNo ratings yet
- 6 - Disorders of MelanocytesDocument5 pages6 - Disorders of MelanocytesAbdul FatahNo ratings yet
- Benign Skin TumoursDocument7 pagesBenign Skin TumoursMan LorNo ratings yet
- An Overview On Melasma 2376 0427 1000216Document18 pagesAn Overview On Melasma 2376 0427 1000216dhilaidrisNo ratings yet
- Case 17: 28-Year-Old With A Rash - Mr. MoellerDocument19 pagesCase 17: 28-Year-Old With A Rash - Mr. MoellerJDNo ratings yet
- AcneDocument14 pagesAcneIbrahim MansarayNo ratings yet
- وسام عوض Benign Skin Tumor-5 (Muhadharaty)Document6 pagesوسام عوض Benign Skin Tumor-5 (Muhadharaty)Alaa AhmedNo ratings yet
- SHS.317.LEC-04 Skin Tumours, Burns PDFDocument36 pagesSHS.317.LEC-04 Skin Tumours, Burns PDFHashir AliNo ratings yet
- Anatomi Dan Fisiologi KuliitDocument40 pagesAnatomi Dan Fisiologi KuliitZulfani AdhiyatmiNo ratings yet
- Skin LesionDocument23 pagesSkin Lesion5alifa55No ratings yet
- Rosacea 2022Document10 pagesRosacea 2022ucarecufinochiettoNo ratings yet
- Acne Vulgaris By: Dr. Majid Suhail Prof DermatologyDocument78 pagesAcne Vulgaris By: Dr. Majid Suhail Prof DermatologyMajid SuhailNo ratings yet
- Gangguan Kulit Pada LansiaDocument28 pagesGangguan Kulit Pada LansianoviNo ratings yet
- Skin Cancer TBL 2 2f3Document19 pagesSkin Cancer TBL 2 2f3api-356476029No ratings yet
- Meth Elab Topic N2Document19 pagesMeth Elab Topic N2Group M1869No ratings yet
- Melasma: Evangeline B. Handog and Maria Juliet E. MacarayoDocument17 pagesMelasma: Evangeline B. Handog and Maria Juliet E. Macarayochash75No ratings yet
- Skin CancerDocument5 pagesSkin CancerEl FaroukNo ratings yet
- B. Disorders of Hyperpigmentation: 1. EphelidesDocument4 pagesB. Disorders of Hyperpigmentation: 1. EphelidesIsmail LubisNo ratings yet
- Skin TumDocument9 pagesSkin TumElsa OctaviaNo ratings yet
- Benign Moles and LesionsDocument2 pagesBenign Moles and LesionsMari MariNo ratings yet
- Amyloidosis SkinDocument6 pagesAmyloidosis SkinGoran MaliNo ratings yet
- Melasma: An Up-to-Date Comprehensive Review: Oluwatobi A. Ogbechie-Godec Nada ElbulukDocument14 pagesMelasma: An Up-to-Date Comprehensive Review: Oluwatobi A. Ogbechie-Godec Nada ElbulukDra Natalia Maria ZuluagaNo ratings yet
- Dermatological DiseasesDocument27 pagesDermatological Diseasesdidaryadgar099No ratings yet
- Research Articlal MelasmaDocument9 pagesResearch Articlal MelasmaNikhil ShrivastavaNo ratings yet
- Chapter 26:: Seborrheic Dermatitis:: Dae Hun Suh: Clinical FeaturesDocument10 pagesChapter 26:: Seborrheic Dermatitis:: Dae Hun Suh: Clinical FeaturesmyztNo ratings yet
- What Is Cosmelan and Dermamelan?: The Solution?Document1 pageWhat Is Cosmelan and Dermamelan?: The Solution?Laurynne GouwsNo ratings yet
- Natural Options For Management of MelasmaDocument17 pagesNatural Options For Management of Melasmapuji lestari100% (1)
- Use of CO2 Laser For The Treatment of Acne and Burn Scars in Indian Skin...Document8 pagesUse of CO2 Laser For The Treatment of Acne and Burn Scars in Indian Skin...Phúc LâmNo ratings yet
- Pediatric DermatosesDocument58 pagesPediatric DermatosesSajin AlexanderNo ratings yet
- Acne Vulgaris Clinical FeaturesDocument22 pagesAcne Vulgaris Clinical FeaturesHanako AranillaNo ratings yet
- AJGP 12 2021 Focus Doolan Melasma WEBDocument6 pagesAJGP 12 2021 Focus Doolan Melasma WEBضحى الغزيNo ratings yet
- Melasma Insights and PerspectivesDocument6 pagesMelasma Insights and PerspectivesTatianaNo ratings yet
- Acne and Dermatosurgical TreatmentDocument15 pagesAcne and Dermatosurgical TreatmentBRENNETH CANIBELNo ratings yet
- 2021 The Pathogenesis of Melasma and Implications For TreatmentDocument14 pages2021 The Pathogenesis of Melasma and Implications For Treatmentyezafig4001No ratings yet
- Hpe Final 110529Document98 pagesHpe Final 110529deeps.u.97No ratings yet
- Skin Cancer Лекция - 2 Дополненная Перевод - КопияDocument72 pagesSkin Cancer Лекция - 2 Дополненная Перевод - Копияdrmedico82No ratings yet
- New Microsoft Office Word DocumentDocument6 pagesNew Microsoft Office Word Documentabas_maytham1021No ratings yet
- Dermatita SeboreicaDocument4 pagesDermatita SeboreicaFlorian MaghearNo ratings yet
- Skin CancerDocument9 pagesSkin Cancermuhd izzulNo ratings yet
- Q.1 Components of Skin: The Skin: Basic Structure and FunctionDocument7 pagesQ.1 Components of Skin: The Skin: Basic Structure and Functionnani100No ratings yet
- Benign Skin LesionsDocument109 pagesBenign Skin Lesionsrinaldy IX9No ratings yet
- Benignskinlesions 141227191914 Conversion Gate01 PDFDocument109 pagesBenignskinlesions 141227191914 Conversion Gate01 PDFrinaldy IX9No ratings yet
- Lésions Pigmentées VulvairesDocument9 pagesLésions Pigmentées VulvairesMarande LisaNo ratings yet
- Pics MCQSDocument22 pagesPics MCQStania100% (1)
- Age Spots (Lentigines), A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandAge Spots (Lentigines), A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Dermatology Notes for Medical StudentsFrom EverandDermatology Notes for Medical StudentsRating: 4 out of 5 stars4/5 (5)
- Microorganisms: What Is A Disease?Document8 pagesMicroorganisms: What Is A Disease?Rosa -No ratings yet
- Clinical Progress: Report of Expert CommitteeDocument22 pagesClinical Progress: Report of Expert CommitteecacingkucingNo ratings yet
- Pertuss Surv Report 2019 508Document1 pagePertuss Surv Report 2019 508cdsaludNo ratings yet
- ComplaintDocument52 pagesComplaintmarwaNo ratings yet
- 2ENGLISH10 - Q1 - Module 2 - Week2 - Determine The Effect of Textual Aids On Understanding A Text - v4BDocument25 pages2ENGLISH10 - Q1 - Module 2 - Week2 - Determine The Effect of Textual Aids On Understanding A Text - v4Bcodm PlayerNo ratings yet
- VA Complete RegsDocument47 pagesVA Complete RegsTJSNo ratings yet
- Short Learning Topics On Biomedical Equipments by Heer Thoshani 22998Document55 pagesShort Learning Topics On Biomedical Equipments by Heer Thoshani 22998Salim AloneNo ratings yet
- Model Nursing Care PlanDocument35 pagesModel Nursing Care PlanAnuchithra RadhakrishnanNo ratings yet
- Hearing PresentationDocument27 pagesHearing PresentationTaremwa PeterNo ratings yet
- Rehabilitation EngineeringDocument33 pagesRehabilitation EngineeringMuhammad MoazzamNo ratings yet
- The Basic Facts About: TinnitusDocument2 pagesThe Basic Facts About: TinnitusAkhil AnandNo ratings yet
- UTS 5. KOMPLIKASI POST PARTUS-English 2019Document63 pagesUTS 5. KOMPLIKASI POST PARTUS-English 2019Rafiq SamtoNo ratings yet
- REQUEST FOR ASSESSMENT EIM NC II - Dec 19, 2020 NEW PDFDocument39 pagesREQUEST FOR ASSESSMENT EIM NC II - Dec 19, 2020 NEW PDFChe MoralesNo ratings yet
- Last ProposalDocument30 pagesLast ProposalRahmet AbdulfetahNo ratings yet
- Current Affairs: Year 5 - Vol. 1 - Total Vol. 38 - January 2023Document44 pagesCurrent Affairs: Year 5 - Vol. 1 - Total Vol. 38 - January 2023Abhishek KumarNo ratings yet
- Vitamin A DeficiencyDocument16 pagesVitamin A DeficiencyNikhil ShresthaNo ratings yet
- Holistic Dental Approach For Visually Impaired Children: A Narrative ReviewDocument8 pagesHolistic Dental Approach For Visually Impaired Children: A Narrative ReviewIJAR JOURNAL100% (1)
- Comparative Study of Ashwatha Ksheer Sutra and Udumber Ksheer Sutra in The Management of Bhagandara (Fistula in ANO)Document9 pagesComparative Study of Ashwatha Ksheer Sutra and Udumber Ksheer Sutra in The Management of Bhagandara (Fistula in ANO)International Journal of Innovative Science and Research TechnologyNo ratings yet
- Management of Hepatic CystsDocument12 pagesManagement of Hepatic CystsDabessa MosissaNo ratings yet
- Nursing Assessment: Gastrointestinal System (Chapter 39) : Structures and FunctionsDocument4 pagesNursing Assessment: Gastrointestinal System (Chapter 39) : Structures and FunctionsPrince K. TaileyNo ratings yet
- Anesthesia ConsentDocument2 pagesAnesthesia ConsentRAMAPATI SANYALNo ratings yet
- Effectiveness of Epsom Salt Hot Water Application On Knee Joint Pain Among Elderly People at Selected Old Age HomeDocument5 pagesEffectiveness of Epsom Salt Hot Water Application On Knee Joint Pain Among Elderly People at Selected Old Age HomeIJAR JOURNALNo ratings yet
- Full Download Test Bank For The Sociology of Health Illness and Health Care A Critical Approach 7th Edition PDF Full ChapterDocument35 pagesFull Download Test Bank For The Sociology of Health Illness and Health Care A Critical Approach 7th Edition PDF Full Chapterforceepipubica61uxl95% (22)
- Immune Responses To Influenza VirusDocument12 pagesImmune Responses To Influenza Virusdavid onyangoNo ratings yet
- Evidence 2Document3 pagesEvidence 2api-527267271No ratings yet
- Quiz 2Document5 pagesQuiz 2Matelyn OargaNo ratings yet
- CHNN211 Week 2 Health Care Delivery SystemDocument7 pagesCHNN211 Week 2 Health Care Delivery SystemABEGAIL BALLORANNo ratings yet
- Folliate PappilaeDocument9 pagesFolliate PappilaeEga Muhamad YusufNo ratings yet
- Aisha Jangeru Complete Project (1) - 091530Document60 pagesAisha Jangeru Complete Project (1) - 091530Dauda Lawal dareNo ratings yet