Professional Documents
Culture Documents
Separation of Mixture and Determination of Density Results
Uploaded by
Parker ReedyCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Separation of Mixture and Determination of Density Results
Uploaded by
Parker ReedyCopyright:
Available Formats
Separation of Mixture and Determination of Density by Parker Reedy
Results:
Part One: Physical separation of heterogeneous mixture including Fe, Sand, and NaCl.
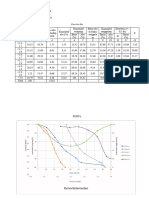
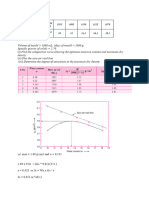
Table 1
Vail # 148 D Mixture Fe Sand NaCl
Mass (g) 3.8401 g 0.3763 g 2.7661 g 0.6977 g
Experimental WT N/A 9.8% 72% 18.2%
%
Actual WT % N/A 7.1% 57.1% 35.7%
(given by
instructor)
Error % N/A 38% 26.1% 49%
Calculations (show one calculation per type with correct units and significant figures for each numerical
value):
Formula mass of component: Mixture mass (g) – (Fe (g) + Sand (g))
Calculation of NaCl: 3.8401g- (0.3763g + 2.7661g )= 0.6977 g
mass component ( g)
Formula percent mass: ×100
total mass of mixture
0.3763 g
Calculation of Fe: ×100=9.8 %
3.8401 g
|experimental percent mass−actual percent mass|
Formula percent error × 100
actual percent mass
|9.8 %−7.1 %|
Calculation of Fe: × 100=38 %
7.1 %
Part 2
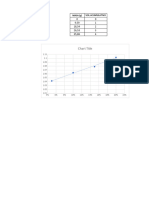
Table 2
Sand Transfer Total mass of Sand (g) Total volume of Sand (mL)
1 1.0466 10.1
2 2.0593 10.12
3 3.0706 10.14
4 4.1015 10.16
Dertimination of Density
1.02E+01
10.16
10.14
10.12
10.1R²
f(x)==0.999979747583194
0.0196536900075316 x + 10.0794998435256
Volume mL
1.01E+01
1.00E+01
0.5 1 1.5 2 2.5 3 3.5 4 4.5
Mass (g)
You might also like
- 1 TestDocument12 pages1 Testabhishekthapa311No ratings yet
- 1.2 Standard Deviation 1.3 MarginDocument4 pages1.2 Standard Deviation 1.3 MarginHalissa MsmyNo ratings yet
- And Thickness (D) Calculation Table: Concentration (G/ML)Document1 pageAnd Thickness (D) Calculation Table: Concentration (G/ML)SayanAttaNo ratings yet
- Ejercicios Procesos Taller 2Document10 pagesEjercicios Procesos Taller 2Juan Jose CalvoNo ratings yet
- Field Density (Undisturbed Sample) : AppendixDocument44 pagesField Density (Undisturbed Sample) : AppendixAbhishek SharmaNo ratings yet
- Pengolahan GrafikDocument2 pagesPengolahan GrafikAhmad FadlanNo ratings yet
- LAB3 KiethDocument13 pagesLAB3 KiethKi KiethNo ratings yet
- Tugas PBGDocument2 pagesTugas PBGmmas21dNo ratings yet
- Principles of Foundation Engineering Ch03Document12 pagesPrinciples of Foundation Engineering Ch03Lee Jia HoeNo ratings yet
- Soil and foundation details for bearing capacity analysisDocument5 pagesSoil and foundation details for bearing capacity analysisDiana CristinaNo ratings yet
- Lampiran PerhitunganDocument7 pagesLampiran PerhitunganRani KurniatiNo ratings yet
- Nama: Raihan Valentino Jaya Saputra NIM: 5111418055Document8 pagesNama: Raihan Valentino Jaya Saputra NIM: 5111418055JamesNo ratings yet
- Specific Gravity TestDocument13 pagesSpecific Gravity Testsengthai100% (1)
- Physics Lab Report - PendulumDocument8 pagesPhysics Lab Report - PendulumRyan SongNo ratings yet
- Full ReportDocument10 pagesFull ReportazmieNo ratings yet
- 解答20220929Document2 pages解答20220929Denny LuNo ratings yet
- AppendixDocument11 pagesAppendixVishal PatadiaNo ratings yet
- PR TIKUMWEEK5Document7 pagesPR TIKUMWEEK5Edyson CitraNo ratings yet
- Gauge and Higgs Bosons Gauge and Higgs Bosons Gauge and Higgs Bosons Gauge and Higgs BosonsDocument6 pagesGauge and Higgs Bosons Gauge and Higgs Bosons Gauge and Higgs Bosons Gauge and Higgs Bosons420No ratings yet
- Lab Report Iodine Clock ReactionDocument6 pagesLab Report Iodine Clock ReactionYoonseo (Elin) ChaNo ratings yet
- Forward Divided Difference MethodDocument14 pagesForward Divided Difference MethodObed Reinhard SiregarNo ratings yet
- Activity 7 - Circular Motion and Centripetal Force (Data Sheet) - Alban, Ronel D.Document2 pagesActivity 7 - Circular Motion and Centripetal Force (Data Sheet) - Alban, Ronel D.Ronel AlbanNo ratings yet
- Bhargavi23 01 2017Document7 pagesBhargavi23 01 2017Bhargavi ChowdaryNo ratings yet
- Perla Espinosa Professor Dr. Ricci Physics 101 25 of October 2021 Lab#6Document4 pagesPerla Espinosa Professor Dr. Ricci Physics 101 25 of October 2021 Lab#6perla espinosaNo ratings yet
- Excel LikuifaksiDocument6 pagesExcel Likuifaksicressytambunan100% (1)
- Lampiran A Perhitungan Standarisasi SimplisiaDocument10 pagesLampiran A Perhitungan Standarisasi SimplisiaRahayu Maulida RNo ratings yet
- 0-Problems Solved PDFDocument9 pages0-Problems Solved PDFFlovgrNo ratings yet
- Contoh Perhitungan Data Uji SondirDocument8 pagesContoh Perhitungan Data Uji SondirYoga PriyantNo ratings yet
- Excel Geo 1 Kelompok 2Document6 pagesExcel Geo 1 Kelompok 2h2svpj9prhNo ratings yet
- Concrete mix design trail batch proportionsDocument5 pagesConcrete mix design trail batch proportionsMohamed Amine ZemouriNo ratings yet
- Density and Concentration of Salt Solutions Lab Report PDFDocument4 pagesDensity and Concentration of Salt Solutions Lab Report PDFapi-346879601No ratings yet
- Newtons Law of Motion - Phys213Document8 pagesNewtons Law of Motion - Phys213Shanelle EstradaNo ratings yet
- EXP8 - Jet Impinging On Blade Report Part 1Document5 pagesEXP8 - Jet Impinging On Blade Report Part 1Poochi DougNo ratings yet
- Tugas 1: Berat Basah (W) (g/cm3)Document4 pagesTugas 1: Berat Basah (W) (g/cm3)Yemima NggebuNo ratings yet
- Assigment: Conductivity (MS/CM) Vs Concentration G/M 3Document5 pagesAssigment: Conductivity (MS/CM) Vs Concentration G/M 3Thiv TriNo ratings yet
- GRAFIK 1Document2 pagesGRAFIK 1S. Alfisyah Nur AzizaNo ratings yet
- Archimedes' Principle and Moments ExperimentDocument13 pagesArchimedes' Principle and Moments ExperimentLance ShahNo ratings yet
- compactionDocument5 pagescompactionbrkmre00No ratings yet
- AnalysisDocument3 pagesAnalysisAnnamarie SanDiegoNo ratings yet
- CBR.2 TemplateDocument4 pagesCBR.2 TemplateBenjamin DagbeyNo ratings yet
- CHM 1103 Lab - 2 - Student Report - Pdf.docxDocument4 pagesCHM 1103 Lab - 2 - Student Report - Pdf.docxjesseNo ratings yet
- Grafik PMVDocument3 pagesGrafik PMVdendyrandy5No ratings yet
- Extract IvesDocument1 pageExtract IvesVon Adrian RaboyNo ratings yet
- Results Concntration of Na Standard Solution (PPM / MG/L Reading From The Photometer 20 20.00 40 40.00 60 60.00 80 80.00 100 100.00 150 150.00 200Document3 pagesResults Concntration of Na Standard Solution (PPM / MG/L Reading From The Photometer 20 20.00 40 40.00 60 60.00 80 80.00 100 100.00 150 150.00 200piyaNo ratings yet
- CBR LAB TEST RESULTSDocument8 pagesCBR LAB TEST RESULTSAwii Yunus100% (1)
- Hasil Pengamatan dan Pembahasan Adsorpsi NaOH Menggunakan Arang AktifDocument11 pagesHasil Pengamatan dan Pembahasan Adsorpsi NaOH Menggunakan Arang AktifMuhammad AlmadhanyNo ratings yet
- Chart Title: Concentracion DensidadDocument2 pagesChart Title: Concentracion Densidadaspantoja72No ratings yet
- 12 1 18-Soil-MechlectureDocument72 pages12 1 18-Soil-MechlectureCyrus R. Flores100% (1)
- Soil Testing ReportDocument12 pagesSoil Testing ReportEinstein JeboneNo ratings yet
- The Factorial Function The Gamma FunctionDocument2 pagesThe Factorial Function The Gamma FunctionantonioNo ratings yet
- Vmix 1Document8 pagesVmix 1Nohan JoemonNo ratings yet
- 6.0 Result and Calculations Experiment Data Sheet: Experiment 1a G RDocument7 pages6.0 Result and Calculations Experiment Data Sheet: Experiment 1a G RcikaNo ratings yet
- Density of Beverages ExperimentDocument6 pagesDensity of Beverages ExperimentDana VillanNo ratings yet
- Decanter DesignDocument27 pagesDecanter Designnorzaifee nizamudin100% (6)
- Results:: Graph of Percentage Finer Passing Against Sieve SizeDocument6 pagesResults:: Graph of Percentage Finer Passing Against Sieve SizeLim KElvinNo ratings yet
- ECG263 LAB 6 Short Report (Data Only)Document1 pageECG263 LAB 6 Short Report (Data Only)naqibkamarozamanNo ratings yet
- Engr. Besavilla - Lecture 03 - 10 Nov 2023Document15 pagesEngr. Besavilla - Lecture 03 - 10 Nov 2023Rhowelle TibayNo ratings yet
- Determining soil properties from granulometric analysisDocument64 pagesDetermining soil properties from granulometric analysisyeimis rocio hernandez valenciaNo ratings yet
- Managing People and OrganisationsDocument50 pagesManaging People and OrganisationsOmkar DesaiNo ratings yet
- 1920s Irish Crochet Lace Edging OriginalDocument8 pages1920s Irish Crochet Lace Edging OriginalLaura HortopanuNo ratings yet
- Court of Appeals decision on Batara family land disputeDocument19 pagesCourt of Appeals decision on Batara family land disputeKhanini GandamraNo ratings yet
- Vienna Convention 1963Document21 pagesVienna Convention 1963Sonia Celinda aNo ratings yet
- Permalex CatalogDocument24 pagesPermalex CataloggbricksphNo ratings yet
- Error RegconitionDocument15 pagesError RegconitionNguyễn Trần Hoài ÂnNo ratings yet
- Sindh Agriculture University Tandojam: ADMISSION / REGISTRATION FORM (For Subsequent Semester)Document2 pagesSindh Agriculture University Tandojam: ADMISSION / REGISTRATION FORM (For Subsequent Semester)Lochi GmNo ratings yet
- Boston Scientific Corporation v. Mabey, 10th Cir. (2011)Document8 pagesBoston Scientific Corporation v. Mabey, 10th Cir. (2011)Scribd Government DocsNo ratings yet
- Easychair Preprint: Shakti Chaturvedi, Nisha Goyal and Raghava Reddy VaraprasadDocument24 pagesEasychair Preprint: Shakti Chaturvedi, Nisha Goyal and Raghava Reddy VaraprasadSneha Elizabeth VivianNo ratings yet
- Unit 13Document28 pagesUnit 13Tinh NguyenNo ratings yet
- ml350p g8.Document57 pagesml350p g8.Aboubacar N'dji CoulibalyNo ratings yet
- My Little Pony: Friendship Is Magic #14 PreviewDocument10 pagesMy Little Pony: Friendship Is Magic #14 PreviewGraphic Policy100% (3)
- Essay On Causes of Corruption and Its RemediesDocument30 pagesEssay On Causes of Corruption and Its Remediesanoos04100% (2)
- 11 M-Way Search TreesDocument33 pages11 M-Way Search TreesguptharishNo ratings yet
- Sample Midterm Test: Business EconomicsDocument5 pagesSample Midterm Test: Business EconomicsLaura BencsesNo ratings yet
- 5 Schedule Timetable FLIFODocument8 pages5 Schedule Timetable FLIFOAlba FernándezNo ratings yet
- Onu - Escwa (Escwa) Report Workshop On International Migration and Development in The Arab Region: Integrating International Migration Into Development Strategies Beirut, 19-22 July 2010Document21 pagesOnu - Escwa (Escwa) Report Workshop On International Migration and Development in The Arab Region: Integrating International Migration Into Development Strategies Beirut, 19-22 July 2010ddufourtNo ratings yet
- Dangerous Goods Hazmat Material Training Cat-10Document24 pagesDangerous Goods Hazmat Material Training Cat-10Claudio GonzalezNo ratings yet
- Manual ATPDMan56Document288 pagesManual ATPDMan56Alex DavidNo ratings yet
- 2023 Congressional Baseball Sponsorship PackagesDocument2 pages2023 Congressional Baseball Sponsorship PackagesSpencer BrownNo ratings yet
- Bank TellerDocument3 pagesBank Tellerapi-3701112No ratings yet
- Omnidirectional Condenser Lavalier Microphone U.S. Atr Series Lifetime Limited End-User WarrantyDocument2 pagesOmnidirectional Condenser Lavalier Microphone U.S. Atr Series Lifetime Limited End-User WarrantyEduardito De Villa CrespoNo ratings yet
- Logistics Sector DevelopmentDocument182 pagesLogistics Sector DevelopmentAmolSarpeNo ratings yet
- How To Add Message Queuing Feature - Dell IndiaDocument2 pagesHow To Add Message Queuing Feature - Dell Indiayuva razNo ratings yet
- Understanding marketing conceptsDocument19 pagesUnderstanding marketing conceptsMOST SUBSCRIBER WITHOUT A VIDEO50% (2)
- Complex Analysis, Gamelin, II.5 Problems and SolutionsDocument3 pagesComplex Analysis, Gamelin, II.5 Problems and SolutionsC. Ephrem StuyvesantNo ratings yet
- Nesco Utility: Expression of InterestDocument14 pagesNesco Utility: Expression of InterestSanjayaNo ratings yet
- Burj Khalifa Case StudyDocument4 pagesBurj Khalifa Case StudyJATINKUMAR CHAUDHARINo ratings yet
- BatchGenerator UserReferenceDocument13 pagesBatchGenerator UserReferenceLuis OrtizNo ratings yet