Professional Documents
Culture Documents
Acid Base Balance Flashcards Quizlet
Uploaded by
apolloOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Acid Base Balance Flashcards Quizlet
Uploaded by
apolloCopyright:
Available Formats
Acid Base Balance Upgrade
Study
Study sets, textbooks, questions
Science Chemistry
Acid Base Balance
9 studiers today 4.3 (9 reviews)
In-class activity
Classic Live
Checkpoint
Self-study activity
Flashcards
Learn
Test
Match
NurseBeauty20
Top creator on Quizlet
Share
Terms in this set (55) Original
pH
~The negative log (base power) of the H+
concentration or the FREE H+ in a solution
~inversely proportional (↓ H+ → ↑ pH)
~Normal body function is dependent on
NARROW range
Hydrogen Ion and pH
~H+ is imperative for CELL MEMBRANE
integrity
~Concentration very small in the body
~0.0000001 mEq/L or pH 7.0
Acids
"DONATE" or release H+ in aqueous
solution; "Acidic"
i.e. Hydrochloric acid
Bases
"ACCEPT" H+ in aqueous solution; "Alkaline"
i.e. Bicarbonate
Acidosis
Blood pH of <7.35
Alkalosis
Blood pH of > 7.45
Buffer
Substance which MINIMIZES/PREVENTS
large changes in pH when either an acid or
base is added to a solution containing the
________; Helps us to get body back to midline
range
Compensation
What one system does to make up for
another system that is not functioning; The
primary disorder is not corrected!!!
~Physiologic RESPONSE of the body
~Body NEVER over-________
Correction
When the "sick system" moves toward
regaining normal function
~Made by interventions to TREAT THE
CAUSES
~Healthcare Professional CAN over-________
Strong Acids
GREATER potential to change the pH of a
solution; DISSOCIATE easily
Weak Acids
LESS ability to change the pH of a solution;
DO NOT dissociate easily
Volatile Acids
Can move from LIQUID → GAS and then
be exhaled via the LUNGS
i.e. carbonic acid
Non-Volatile Acids
CANNOT change into gas form, therefore
are excreted via the KIDNEY
i.e. lactic, ketoacids, sulfuric, phosphoric
acids
pH Balance
Depends on ratio of HCO³ to H₂CO₃ (20:1)
- Very Important!!!
Normal Acid-Base Balance Importance
~Nearly all chemical and enzyme reactions
function OPTIMALLY in a narrow pH range
~H+ and HCO₃ and other ions affect the
EXCITABILITY/RESPONSIVITY of neural,
muscle, and other cells
i.e. acidosis causes arrhythmias
pH in Body
Blood - 7.35 - 7.45
Urine - 6.0
Gastric Acid - 4
Relationship btw pH and H+
pH is INVERSELY related to H+ ion
concentration
↑H+ = pH↓
↓H+ = pH↑
Relationship btw pH and Acid-Base
Balance
Acids are formed as END PRODUCTS of
protein, carbohydrates, and fat metabolism
~H+ must be neutralized or excreted
~BONES, LUNGS, KIDNEYS are the major
organs involved in the regulation of acid
and base balance
Three Part System of Compensation
Buffer System
Excretion
Cellular Ion Exchange
Buffer Systems
Can ABSORB or DONATE H+ ions ->
minimize/prevent LARGE pH changes
Excretion Sites
LUNGS (CO₂)
KIDNEYS (HCO₃ and H+)
GI TRACT (HCO₃ and H+)
Cellular Ion Exchange
Exchange of H+ or HCO₃ ion for another
LIKE-CHARGE ion across the cell
membrane
Buffers
Consists of a WEAK acid and its
CONJUGATE base
~The MOST important plasma buffering
system are the carbonic acid - bicarbonate
and hemoglobin systems
Carbonic acid-bicarbonate System
WEAK acid (H₂CO₃) + Conjugate base
(HCO₃)
Location - plasma (primarily) and interstitial
fluid; KIDNEY TUBULES
Protein
Have NEGATIVE charges, so they can serve
as buffers for H+ (Mainly in the cells)
How Buffer Systems Work
GAIN acid or LOSE base = excess H+ ions
bind with conjugate base becoming a weak
acid → little H+ remains free and pH
change is minimized
(Weak acid does not readily give up the H+)
How Buffer Systems Work
GAIN base or LOSE acid = excess base
combines with the weak acids to form H₂O
and a SALT → ↓s the effect of the stronger
base on pH
NaOH→Na + OH-
OH- + H₂CO₃→ H₂O + HCO₃
Respiratory Effects on pH
EXCRETE acids
Lungs exhale CO₂ as a waste product→ ↓
blood levels of H₂CO₃ (carbonic acid)
Hyperventilation → CO₂ LOSS
Hypoventilation → CO₂ EXCESS
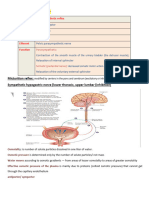
Renal Effects on pH
EXCRETE non-volatile acid and regulate
HCO₃
~Reabsorbs HCO₃ from renal tubules; but
can compensate for alkalosis too
~ "Titratable acids" - NH3 and PO₄ combine
with H+ ions to be excreted in the urine
Potassium
Maintains acid-base equilibrium, and it has
a significant and inverse relationship to pH:
↓ in pH of 0.1 = ↑ K+ level by 0.6
~It moves INSIDE the RBCs into the
extracellular fluid, while H+ moves into the
RBC (in Acidotic states)
Calcium
Alkalosis: ↑ blood pH causing intracellular
uptake of Ca+ ↔ for intracellular H+
(become hypocalcemic)
Acidosis: Long-standing acidosis can result
in osteoporosis and release of Ca+ into
circulation as H+ moves into the cells
(smokers at risk for osteoporosis)
Chloride Shift
Shift of Cl- ions from the plasma → the
RBCs upon the + of HCO₃ from the tissues,
and the reverse movement when CO₂ is
released in the lungs
Acid-Base Disorders
Respiratory Alkalosis
Respiratory Acidosis
Metabolic Alkalosis
Metabolic Acidosis
Respiratory Alkalosis
HIGH pH
Low PaCO₂
Respiratory Acidosis
LOW pH
HIGH PaCO₂
Metabolic Alkalosis
HIGH pH
HIGH HCO₃
HIGH Base Excess
Metabolic Acidosis
LOW pH
LOW HCO³
LOW Base Excess
Arterial Blood Gases (ABGs)
ARTERIAL blood, not venous; Able to
measure O₂, CO₂, HCO₃, pH and O₂
Saturation
Respiratory Alkalosis
↓PaCO₂ < 38 mmHg
↑ in pH
Cause: HYPERVENTILATION "blowing off
CO₂"
Compensation: KIDNEYS
~Active secretion of HCO₃
~↓ secretion/excretion of H+
~Requires 2-4 days
Clinical Manifestations: Respiratory
Alkalosis
Hypocalcemia: intracellular uptake of Ca+
↔ intracellular H+
~Rapid respirations (Hyperventilation)
~CNS: dizziness, muscle contractions
~Changes in LOC
Treatment: Respiratory Alkalosis
Rebreathing CO₂ (paper bag)
Treat underlying cause
Respiratory Acidosis
↑ PaCO₂ > 45 mmHg
↓ pH
Causes:
Obstructive lung dz
Restrictive lung dz
Nueral damage or disruption
Hypoventilation
Compensation: Kidneys
~Little or no secretion of HCO₃ (hold on to
it)
~Secrete H+ into renal tubule to be
excreted in the urine
~Requires 2 - 4 days
Clinical Manifestations: Respiratory Acidosis
Hypoventilation (Respiratory depression)
Headache
Behavior changes
Changes in LOC
Treatment: Respiratory Acidosis
~Treat underlying cause
~Mechanical ventilation
~Bronchodilators, bronchial hygiene
~Judicious use of narcotics, sedatives,
tranquilizers (can depress the treatment)
Metabolic Alkalosis
↑ HCO3 or loss of acids
↑pH
Causes:
~Loss of acid: vomiting
~↑ HCO₃ levels (digestion of antacids)
~↓ Fluid Volume (hypovolemia - DIURESIS)
~Electrolyte levels
(Hypokalemia/Hypochloremia)
Compensation: Metabolic Alkalosis
(Respiratory) Lungs: Hypoventilating to ↑
PaCO₂; Occurs immediately
Clinical Manifestations: Metabolic Alkalosis
~HypoVentilation (compensatory
mechanism)
~HypoVolemia s/s
~HypoKalemia s/s
~Possible neurological irritability
Treatment: Metabolic Alkalosis
~Fluid, K+, Cl- replacements as needed
~Carbonic anhydrase inhibitor
Metabolic Acidosis
Gain of NON-VOLATILE acids (↓pH)
and/or loss of base (HCO₃)
High Anion Gap
~Starvation/protein malnutrition
~Diabetic ketoacidosis
~Lactic acid
~Alcoholic ketoacidosis
~Uremic acidosis
~Toxic ingestion
Normal Anion Gap
~Acid gain
~HCO³ loss
Anion Gap
~Gap represents the anions NOT
ROUTINELY measures: PO₄, proteins, SO₄,
etc.
~Normal 10 - 14
~Used to distinguish btw different types of
METABOLIC ACIDOSIS
Abnormal Anion Gap
Occurs as a result of an ↑ level of an
abnormal unmeasured anion
As abnormal anions accumulate, the
measured anions have to ↓ to maintain
electroneutrality
Causes: Anion Gap
Metabolic acidosis w/ HIGH Anion Gap
↑ organic aids:
Lactate
Alcohol
Uremia
Toxins/Poisons
Ketones
Metabolic acidosis w/ NORMAL Anion Gap
↑ Cl-
↓ HCO₃
Compensation: Metabolic Acidosis
(Respiratory) Lungs: Hyperventilation;
Expiring more CO₂
~Requires minutes to hours
~Protein and cellular buffering systems in
action
Clinical Manifestations: Metabolic Acidosis
~Hyperventilation (compensatory
mechanisms)
~Nueromuscular depression (poor muscle
function and mental status changes)
~N/V (↓ GI motility)
~Cardiac dysrhythmias
~Hypotension
~↓ Cardiac contractility, and
responsiveness to drugs
Treatment: Metabolic Acidosis
Treat the cause, HCO₃ replacement,
dialysis
About us For students
About Quizlet Flashcards
How Quizlet works Test
Careers Learn
Advertise with us Solutions
Get the app Q-Chat
For teachers Resources
You might also like
- Jill Getchell, BS, CCP, LCP, LPNDocument115 pagesJill Getchell, BS, CCP, LCP, LPNapi-301270014No ratings yet
- ACID-BASE BALANCEDocument78 pagesACID-BASE BALANCEAndy SelvianNo ratings yet
- LECTURE ON Acid-Base BalanceDocument229 pagesLECTURE ON Acid-Base BalanceNayyer Khan100% (1)
- Acid Base TutorialDocument74 pagesAcid Base TutorialuedmarineNo ratings yet
- Maintaining Acid-Base Balance: pH, Bicarbonate, Lungs & KidneysDocument3 pagesMaintaining Acid-Base Balance: pH, Bicarbonate, Lungs & KidneysJenny VargheseNo ratings yet
- Arterial Blood GasDocument55 pagesArterial Blood GasLal NandaniNo ratings yet
- Acid Base Disorders DR Kwaifa - PPTX 1Document99 pagesAcid Base Disorders DR Kwaifa - PPTX 1DICKSONNo ratings yet
- Understanding Acid-Base BalanceDocument43 pagesUnderstanding Acid-Base Balanceanju KvNo ratings yet
- "If One Advances Confidently in The Direction of His Dreams, He Will Meet With A Success Unexpected in Common HoursDocument44 pages"If One Advances Confidently in The Direction of His Dreams, He Will Meet With A Success Unexpected in Common Hourssantana2007No ratings yet
- Acid Base Balance..slideDocument12 pagesAcid Base Balance..slideezebelluciNo ratings yet
- Acid Base BalanceDocument59 pagesAcid Base BalanceFratila IuliaNo ratings yet
- Arterial Blood Gas Analysis: Sample Collection and InterpretationDocument140 pagesArterial Blood Gas Analysis: Sample Collection and InterpretationJeeb Mc TayongNo ratings yet
- Kertas Litmus Merupakan Alat Untuk Mengukur Bahan Yang Mempunyai Asid Atau Alkaline.Document56 pagesKertas Litmus Merupakan Alat Untuk Mengukur Bahan Yang Mempunyai Asid Atau Alkaline.Rizal FarieNo ratings yet
- (Final) ACID BASE BALANCEDocument68 pages(Final) ACID BASE BALANCEPauline SalvadorNo ratings yet
- Acid-Base Balance Lecture - Part 1Document43 pagesAcid-Base Balance Lecture - Part 1yigermalamanuel32No ratings yet
- Acid Base Fall 2008Document34 pagesAcid Base Fall 2008anon-252165No ratings yet
- Acid base disorders simplified/TITLEDocument48 pagesAcid base disorders simplified/TITLEAGUNG SETIADI NUGROHONo ratings yet
- Acid Base Balance: Carol Johns, MSN, RNDocument36 pagesAcid Base Balance: Carol Johns, MSN, RNkatrinasdNo ratings yet
- Acid-Base Regulation and Disorders: Key ConceptsDocument54 pagesAcid-Base Regulation and Disorders: Key ConceptsPaolo Uccello100% (1)
- Acid-Base DisorderDocument68 pagesAcid-Base DisorderPrafulla Paudel100% (3)
- Acid Base Balance: Dr. Rochana PereraDocument37 pagesAcid Base Balance: Dr. Rochana Pererasanjeewa_sNo ratings yet
- Normal Values & DefinitionsDocument30 pagesNormal Values & DefinitionsAmy GonzalesNo ratings yet
- Blood Buffer SystemDocument10 pagesBlood Buffer Systemmd hasib munsiNo ratings yet
- Ivma Ce Acid BaseDocument11 pagesIvma Ce Acid BaseGhozy IANo ratings yet
- 9 - (D) Acid Base Balance Dec 4.17Document61 pages9 - (D) Acid Base Balance Dec 4.17khaledNo ratings yet
- Maintaining Acid-Base BalanceDocument49 pagesMaintaining Acid-Base BalanceDarshini Nagarajan100% (1)
- Acid Base Balance Pathophysiology NursingDocument7 pagesAcid Base Balance Pathophysiology Nursinggrad_nurse_2015100% (2)
- Acid-Base Balance: Key Concepts and Clinical PresentationsDocument59 pagesAcid-Base Balance: Key Concepts and Clinical PresentationsSarvess MuniandyNo ratings yet
- AbgDocument52 pagesAbgm07wwpNo ratings yet
- Acid-Base Imbalances ExplainedDocument13 pagesAcid-Base Imbalances ExplainedFayeh Harah PadrillanNo ratings yet
- CHEMISTRYDocument36 pagesCHEMISTRYlOUIE TICARNo ratings yet
- Acid Base BalanceDocument41 pagesAcid Base BalanceMohammed FarahNo ratings yet
- Fluid and Electrolytes - Acid Base BalanceDocument38 pagesFluid and Electrolytes - Acid Base BalanceAmy Glend BaseNo ratings yet
- SEMINAR ON Acid-base balance & imbalanceDocument72 pagesSEMINAR ON Acid-base balance & imbalanceAnusha Verghese100% (2)
- Physiology Lec 3Document8 pagesPhysiology Lec 3allid2872No ratings yet
- Unit 1: Renal System: Acid-Base BalanceDocument48 pagesUnit 1: Renal System: Acid-Base BalancePPP MOHD SUKRINo ratings yet
- BLOOD-GASESDocument51 pagesBLOOD-GASESrbm121415chyNo ratings yet
- Fluid-Electrolytes and Acid-Base Disturbance in Surgery: Haidi Hu, MD, PHDDocument68 pagesFluid-Electrolytes and Acid-Base Disturbance in Surgery: Haidi Hu, MD, PHDHUNEL KimNo ratings yet
- Abg PPT NewDocument69 pagesAbg PPT NewMalaka Atapattu100% (2)
- High Yield ItemsDocument790 pagesHigh Yield ItemsErin HillNo ratings yet
- ACID Base Balance EUADocument7 pagesACID Base Balance EUATykee OkonkwoNo ratings yet
- Fe 4Document36 pagesFe 4api-3697326No ratings yet
- Detect and correct acid-base imbalancesDocument6 pagesDetect and correct acid-base imbalancesjohnkuysNo ratings yet
- Acid-Base Balance ExplainedDocument8 pagesAcid-Base Balance ExplainedNicole TangcoNo ratings yet
- Week 4 Buffers, Carbohydrates Metabolic PathwaysDocument16 pagesWeek 4 Buffers, Carbohydrates Metabolic PathwaysangeliaNo ratings yet
- Clinical Chemistry 2: Acid-Base BalanceDocument37 pagesClinical Chemistry 2: Acid-Base BalanceTweetie SiaNo ratings yet
- Referat Acid-Base SamdiDocument47 pagesReferat Acid-Base SamdiSamdiSutantoNo ratings yet
- Acid Base BalanceDocument104 pagesAcid Base BalanceKevin VillaranteNo ratings yet
- Fluids and Electrolytes Reabsorption and FiltrationDocument76 pagesFluids and Electrolytes Reabsorption and FiltrationIola JaneNo ratings yet
- Buffer System: Danica Alyssa C. Cruz, RMTDocument27 pagesBuffer System: Danica Alyssa C. Cruz, RMTDanica Alyssa CruzNo ratings yet
- Acid-Base Balance: George A. Tanner, PH.DDocument22 pagesAcid-Base Balance: George A. Tanner, PH.DDawlat SlamaNo ratings yet
- Fluid, Electrolytes, Acid Base BalanceDocument18 pagesFluid, Electrolytes, Acid Base Balanceashdmb217100% (5)
- Abg Analysis - 2014Document28 pagesAbg Analysis - 2014Dylan ThomasNo ratings yet
- Acid - Base DisturbancesDocument41 pagesAcid - Base DisturbancesIbrahim AkinbolaNo ratings yet
- Acid-Base Imbalance and Electrolyte ImpactDocument40 pagesAcid-Base Imbalance and Electrolyte ImpactahmadNo ratings yet
- Acid Base Disorders Peter SehaDocument29 pagesAcid Base Disorders Peter SehaPeter SehaNo ratings yet
- Arterial Blood Gas Analysis Learning Objectives:: Introduction/ OverviewDocument5 pagesArterial Blood Gas Analysis Learning Objectives:: Introduction/ OverviewjanorberteNo ratings yet
- Physiology of Acid Base Balance by Dr. ROOMIDocument70 pagesPhysiology of Acid Base Balance by Dr. ROOMIMudassar Roomi100% (1)
- The Alkaline Diet Made Easy: Reclaim Your Health, Lose Weight & Heal NaturallyFrom EverandThe Alkaline Diet Made Easy: Reclaim Your Health, Lose Weight & Heal NaturallyNo ratings yet
- Metabolic Alkalosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsFrom EverandMetabolic Alkalosis, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNo ratings yet
- Impact of The COVID-19 Pandemic On Mental Health and Quality of Life Among Local Residents in Liaoning Province, China: A Cross-Sectional StudyDocument12 pagesImpact of The COVID-19 Pandemic On Mental Health and Quality of Life Among Local Residents in Liaoning Province, China: A Cross-Sectional StudyieykatalibNo ratings yet
- H EarlyDocument1 pageH EarlyapolloNo ratings yet
- The GNEP Coupled End-to-End (CETE) Research, Development, and Demonstration Project: Overview of The CETE Testbed Capabilities and OperationsDocument5 pagesThe GNEP Coupled End-to-End (CETE) Research, Development, and Demonstration Project: Overview of The CETE Testbed Capabilities and OperationsapolloNo ratings yet
- Birthday Message - MotherDocument1 pageBirthday Message - MotherMaryam100% (4)
- Henry Moseley, The Atomic Number, and Synthesis of Elements: By: Sirthon AzuelaDocument39 pagesHenry Moseley, The Atomic Number, and Synthesis of Elements: By: Sirthon Azuelaapollo100% (1)
- Project completion notice for Elijane QuemanDocument1 pageProject completion notice for Elijane QuemanapolloNo ratings yet
- Moseley's Law Revolutionized Atomic Number ConceptDocument2 pagesMoseley's Law Revolutionized Atomic Number Conceptklmnt0% (1)
- Birthday Message - MotherDocument1 pageBirthday Message - MotherMaryam100% (4)
- Handout For Inquiries Investigations and ImmersionDocument5 pagesHandout For Inquiries Investigations and Immersionapollo100% (1)
- Physical Science11 - Q1 - 1 5Document107 pagesPhysical Science11 - Q1 - 1 5apollo100% (2)
- 3is Midterm ReviewerDocument2 pages3is Midterm Reviewerapollo100% (3)
- Children 1. Gerardo 2. Jay-Tina-Wife 3. Gina 4. Jenny-Edwin-Husband 5. Joseph 6. Jake 7. JaniceDocument2 pagesChildren 1. Gerardo 2. Jay-Tina-Wife 3. Gina 4. Jenny-Edwin-Husband 5. Joseph 6. Jake 7. JaniceapolloNo ratings yet
- T Up Thy SwordDocument1 pageT Up Thy SwordapolloNo ratings yet
- The GNEP Coupled End-to-End (CETE) Research, Development, and Demonstration Project: Overview of The CETE Testbed Capabilities and OperationsDocument5 pagesThe GNEP Coupled End-to-End (CETE) Research, Development, and Demonstration Project: Overview of The CETE Testbed Capabilities and OperationsapolloNo ratings yet
- VeronaDocument3 pagesVeronaapolloNo ratings yet
- Children 1. Gerardo 2. Jay-Tina-Wife Gina: Steve LanganDocument3 pagesChildren 1. Gerardo 2. Jay-Tina-Wife Gina: Steve LanganapolloNo ratings yet
- Y Are Brought To Life Through A Stage PerformanceDocument1 pageY Are Brought To Life Through A Stage PerformanceapolloNo ratings yet
- Yukio Mishima'S Swaddling Clothes: Steve LanganDocument1 pageYukio Mishima'S Swaddling Clothes: Steve LanganapolloNo ratings yet
- 356-Article Text-1099-1-10-20160622Document16 pages356-Article Text-1099-1-10-20160622apolloNo ratings yet
- Yukio Mishima'S Swaddling Clothes: Steve LanganDocument1 pageYukio Mishima'S Swaddling Clothes: Steve LanganapolloNo ratings yet
- Vehicle Trip Ticket: Name of Driver Type of VehicleDocument2 pagesVehicle Trip Ticket: Name of Driver Type of VehicleapolloNo ratings yet
- 356-Article Text-1099-1-10-20160622Document16 pages356-Article Text-1099-1-10-20160622apolloNo ratings yet
- Yukio Mishima'S Swaddling Clothes: Steve LanganDocument1 pageYukio Mishima'S Swaddling Clothes: Steve LanganapolloNo ratings yet
- Busy Tube Train.: SophieDocument4 pagesBusy Tube Train.: SophieapolloNo ratings yet
- Democratic PracticesDocument1 pageDemocratic PracticesapolloNo ratings yet
- Department of Education: Republic of The PhilippinesDocument10 pagesDepartment of Education: Republic of The PhilippinesapolloNo ratings yet
- Bayog Elementary School Project Proposals for 2016-2017Document6 pagesBayog Elementary School Project Proposals for 2016-2017apolloNo ratings yet
- Project Proposal: Bayog Elementary SchoolDocument6 pagesProject Proposal: Bayog Elementary SchoolapolloNo ratings yet
- Yukio MishimaDocument1 pageYukio MishimaapolloNo ratings yet
- Vehicle Trip Ticket: Name of Driver Type of VehicleDocument2 pagesVehicle Trip Ticket: Name of Driver Type of VehicleapolloNo ratings yet
- 3 - Cell Structures and Their FunctionsDocument12 pages3 - Cell Structures and Their FunctionsPrince TeodocioNo ratings yet
- Teacher Guide: Photosynthesis Lab: Learning ObjectivesDocument4 pagesTeacher Guide: Photosynthesis Lab: Learning ObjectivesRodrigo Simões MarquesNo ratings yet
- Forensic Chemistry and Toxicology QuizDocument7 pagesForensic Chemistry and Toxicology QuizJerick OrtegaNo ratings yet
- Added Photos-Final PROJECT REPOR 8sem (1) PDFDocument50 pagesAdded Photos-Final PROJECT REPOR 8sem (1) PDFravi singhNo ratings yet
- Namibia Current Medicines RegisterDocument382 pagesNamibia Current Medicines RegisterportosinNo ratings yet
- Maxillofacial Prosthesis MaterialsDocument23 pagesMaxillofacial Prosthesis MaterialsAbdinajiib MahamedNo ratings yet
- J Chem Ed 2013 90 345 24781 PDFDocument7 pagesJ Chem Ed 2013 90 345 24781 PDFUmar JuttNo ratings yet
- Improving Students' Ability to Name and Write Chemical FormulasDocument8 pagesImproving Students' Ability to Name and Write Chemical FormulasWisdom AbotsiNo ratings yet
- ALTERNATIVE METHODS FOR PRODUCING IODIZED SALTDocument3 pagesALTERNATIVE METHODS FOR PRODUCING IODIZED SALTfghhnnnjmlNo ratings yet
- Distinction Tests 12th Shobhit NirwanDocument1 pageDistinction Tests 12th Shobhit NirwanArush DhawalNo ratings yet
- The Separation of Kyanite From Quartz by Flotation at Acidic PH 2016 Minerals EngineeringDocument8 pagesThe Separation of Kyanite From Quartz by Flotation at Acidic PH 2016 Minerals EngineeringW ZuoNo ratings yet
- Boron Removal: Ion Exchange ResinsDocument2 pagesBoron Removal: Ion Exchange Resinsnermeen ahmedNo ratings yet
- Fundamentals of Petroleum and Petrochemical Engineering: Uttam Ray ChaudhuriDocument70 pagesFundamentals of Petroleum and Petrochemical Engineering: Uttam Ray ChaudhuriEmad AliNo ratings yet
- 12th Chem Full Book MCQs With Answer KeyDocument57 pages12th Chem Full Book MCQs With Answer KeyTHE PROFESSORS'No ratings yet
- Sillitoe 1973 Geología Del Pórfido Cuprifero Los PelambresDocument10 pagesSillitoe 1973 Geología Del Pórfido Cuprifero Los PelambrescatalinaNo ratings yet
- Brochure SunflagDocument12 pagesBrochure SunflagkunalNo ratings yet
- Astm d1356Document14 pagesAstm d1356Jose Luis Villegas EchalarNo ratings yet
- Almeida Et Al 2022Document7 pagesAlmeida Et Al 2022Otacílio de AlmeidaNo ratings yet
- AGR001 Introduction To Agriculture Ceyann2Document8 pagesAGR001 Introduction To Agriculture Ceyann2Lloyd VenturaNo ratings yet
- Berger Paints to Manufacture Japanese CMP Marine Coatings in BangladeshDocument45 pagesBerger Paints to Manufacture Japanese CMP Marine Coatings in Bangladeshnur hasanNo ratings yet
- CH 4af20 1570 ProkaryotesDocument73 pagesCH 4af20 1570 Prokaryotesb9wtknwz24No ratings yet
- Lehninger Principles of Biochemistry Test Bank CH 4Document13 pagesLehninger Principles of Biochemistry Test Bank CH 4Jubeena SadiqueNo ratings yet
- Chemical Calc (1) .Problems (Volumetric Analysis)Document28 pagesChemical Calc (1) .Problems (Volumetric Analysis)Peter Yin100% (4)
- WINSEM2021-22 CHE1014 TH VL2021220501387 Reference Material I 03-03-2022 Module 5 Purification of Petroleum ProductsDocument65 pagesWINSEM2021-22 CHE1014 TH VL2021220501387 Reference Material I 03-03-2022 Module 5 Purification of Petroleum ProductsJod KillerNo ratings yet
- Acetylene Degradation by New Isolates of Aerobic Bacteria and Comparison of Acetylene Hydratase EnzymesDocument6 pagesAcetylene Degradation by New Isolates of Aerobic Bacteria and Comparison of Acetylene Hydratase Enzymesראול אפונטהNo ratings yet
- WJEC GCE Adaptations Consultation Booklet Summer 2022Document60 pagesWJEC GCE Adaptations Consultation Booklet Summer 2022Adrian ColborneNo ratings yet
- Metales CasarettDocument50 pagesMetales CasarettMaximiliano OrtegaNo ratings yet
- GEOPOLYMER CONCRETE RESEARCHDocument1 pageGEOPOLYMER CONCRETE RESEARCHnuraina aqilahNo ratings yet
- Doc-20221212-Wa0006 221213 205551Document11 pagesDoc-20221212-Wa0006 221213 205551Divyanshu AswalNo ratings yet
- Nomenclature Homework 2Document6 pagesNomenclature Homework 2James PerriamNo ratings yet