Professional Documents
Culture Documents
Zhan Et Al 2014 Synthesis of Montmorillonite Acrylic Acid Acrylamide Tricopolymer and Its Super Absorbent Properties
Uploaded by
n2hadianaOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Zhan Et Al 2014 Synthesis of Montmorillonite Acrylic Acid Acrylamide Tricopolymer and Its Super Absorbent Properties
Uploaded by
n2hadianaCopyright:
Available Formats
Synthesis of Montmorillonite/Acrylic Acid/Acrylamide Tricopolymer and Its Super Absorbent Properties
Synthesis of Montmorillonite/Acrylic Acid/Acrylamide Tricopolymer
and Its Super Absorbent Properties
Xiao-yuan Zhan1,2, Fang Wang, Xing-wei Li, and Min Wang
1
College of Material Science and Engineering, SDUST, Qingdao, Shandong 266510, China
2
State Key Laboratory of Mining Disaster Prevention and Control, SDUST, Qingdao, Shandong 266510, China
Summary
Montmorillonite/acrylic acid/acrylamide tricopolymer was synthesized by using aqueous solution polymerization.
Some factors affecting the water absorbency of super absorbent composite material, such as the neutralization
of acrylic acid, the amount of added montmorillonite, initiator and crosslinking agent were investigated. The
best formula for absorbing the running water was proposed, it included 40% montmorillonite, 80% monomer
neutralization degree, 0.08% crosslinker amount, and 0.15% initiator, and the water absorbency of the obtained
composite materials was 150 times. The FTIR research indicated that montmorillonite was effectively compounded
with polymer, it is found that the composite with a good water retention and heat resistance is significant for
reducing the product cost and improving the comprehensive property of the composite.
Keywords: Super absorbent, Montmorillonite, Sodium poly-acrylate, Composite
1. Introduction Super absorbent mineral is mainly inhibitor, inert gas and foam had been
various natural inexpensive clay used with certain unideal effects2. Take
Super absorbent polymer(SAP) is mineral with certain reactive functional advantage of the good water retention
one kind of polymer contains a groups, for instances, montmorillonite, of super absorbent polymer for fighting
large amount of strong hydrophilic kaolinite, attapulgite (surface contains fire, when sprayed into the mouth, it
groups, such as -OH, -COOH, a lot of “ OH”, “Si-O” and other can realize flame extinguishing due
which has special structure with reactive groups), which act as the graft to water evaporation. In the case of
copolymerization crosslinking. This section of base resign material. Super non-spontaneous combustion, the
polymer can absorb abundant water absorbent mineral can not only reduce SAP can absorb water vapor in the
compared with general water absorbing the cost of traditional SAP raw material, roadway, cover the coal well, cut
materials in which the absorbed water is but also can improve their defects. off the interaction between coal and
hardly removable even under pressure. Therefore, it is currently becoming oxygen, then achieve the purpose of
The traditional SAP is formed by an important research direction to fire prevention and control.
hydrophilic group of alkene complex, prepare excellent composite SAP
which is widely used in modern material. Super absorbent mineral/ Domestic researchers Zhou Meng3,
daily life. However, there are some polymer composite can be applied as Lin Jianming4, Wei Yuelin5, Wan Tao6
drawbacks, for example, high material water-retentive material in fields of had used montmorillonite (bentonite)
cost, low gel strength, poor water dry farming, gardening, ecological and acrylic acid (acrylamide/starch)
retention and poor biodegradability. In environment management, also used in as raw materials to successfully
view of aforementioned disadvantages, coal mines to do as anti-extinguishing synthesize super absorbent composite
people have committed themselves to materials by virtue of its low cost, materials with special features in raw
the development of the SAP functional high strength and good salt resistance. materials ratio, synthesis process
research for a long time1, specially super In the practice of fighting mine fires, and properties characterization. This
absorbent mineral/polymer composite. pumping water, mud, inorganic salts article described the preparation and
water absorbency of montmorillonite/
acrylic acid/acrylamide tri-copolymer
in detail. Some factors affecting the
performance of this tricopolymer, such
©
Smithers Information Ltd., 2014 as the neutralization of acrylic acid,
Polymers & Polymer Composites, Vol. 22, No. 5, 2014 489
Xiao-yuan Zhan, Fang Wang, Xing-wei Li, and Min Wang
the amount of added montmorillonite, 2.3 Measurement of the Water will be greatly improved, also the cost
initiator and crosslinking agent were Absorbency7 of product be multiply increased. To
investigated. Then it is found that the seek the best balance between cost and
A common method of measuring water
composite with a good water retention water absorbency, fixed acrylic acid and
absorbency is filtration. The typical
and heat resistance is significant acrylamide in a mass ratio of 2:1, 80%
behavior begins by dissolving 0.50 g
for reducing the product cost and monomer neutralization degree, 0.08%
of super absorbent composite material
improving the comprehensive property crosslinker amount, and 0.15% initiator
in water within a 1000 ml beaker in
of the composite. were added to synthesize the super
order to saturate. Prior to use, this gel
absorbent polymer. Then we studied
must be filtrated for adequate time with
the influence of the amount of added
2. Experiment 0.15 mm (100 mesh) sieve and placed
montmorillonite on water absorbency.
the gel on the mesh for 15 min. At last,
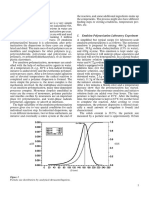
2.1 Experimental Materials As we can see from Figure 1, the
we calculate its water absorbency by
water absorbency decreases along with
• Montmorillonite, purity of 90%- the following formula:
increasing amount of montmorillonite,
98%, industrial grade, Qingdao but when the added amount exceeds
Yu z h o u C h e m i c a l l i m i t e d Water absorbency = the quality of 40%, the decreased tendency becomes
Company; the gel after absorbing water/ the somewhat gentle. Due to the high
quality of the gel amount of added montmorillonite, the
• Acrylic acid (C2H4O2), AA, purity
≥ 99.5%, AR, Tianjin Damao cost of super absorbent composite can
3. Results and be greatly reduced.
Chemical Reagent Factory;
discussion
• Acrylamide (C2H5NO), AM, purity
3.1.2 Initiator
of 98.0%, AR, Tianjin Guangfu 3.1 Effect Factors on Water
Fine Chemical Research Institute; Absorbency Initiator can promote the activity
of monomers that involved in the
• Sodium hydroxide (NaOH), CP, 3.1.1 The Amount of Added poly-reaction. The higher activity of
Tianjin Damao Chemical Reagent Montmorillonite monomers that more difficult to control
Factory; Montmorillonite is one kind of the poly-reaction process is easier
• Crosslinking agent: N, hydrophilic inorganic mineral to form highly crosslinked polymer,
N-methylene-bis-acrylamide materials which can inflate to 10 to 30 which lead to lower water absorbency.
(C 7 H 10 N 2 O 2 ), MBA, purity ≥ times when it absorbs water8. But with If the activity of monomers is low,
98.5%, Chengdu Kelong Chemical respect to super absorbent polymer, then the response time prolongs and
Reagent Factory; its water absorbency is extremely the degree of crosslinking lessens, so
low. However when combines it with that the water soluble property will
• Initiators: potassium persulfate organic polymer, its water absorbency increase, but the water absorbency
(K2S2O8), AR, Tianjin Chemical
Reagent Sixth Factory. Figure 1. The effect of the amount of montmorillonite on water absorbency
2.2 Preparation of the Super
Absorbent Polymer
The process starts by dispersing
appropriate amount of montmorillonite
into a beaker containing some deionized
water, then placing the beaker in an
ultrasonic cleaner to sufficiently disperse
and adding an appropriate amount of
acrylic acid and acrylamide after cooling.
Separately, NaOH is dissolved in the
mixture in order to neutralize C2H4O2
and mixed with K2S2O8 and C7H10N2O2.
It is then placed into a thermostatic water
bath to the sufficiently reaction. Finally,
the production obtained is cut into small
pieces and placed into a drying oven
at 110 °C to get the super absorbent
material.
490 Polymers & Polymer Composites, Vol. 22, No. 5, 2014
Synthesis of Montmorillonite/Acrylic Acid/Acrylamide Tricopolymer and Its Super Absorbent Properties
will decrease. To study the effect of and water retention is poor. On the absorbency is fine when the amount
added initiator, fixed acrylic acid and contrary, the water absorbency also is is 0.06% and it attains the maximum
acrylamide in a mass ratio of 2:1, 80% low when the amount of crosslinking while the amount reaches to 0.08%10.
monomer neutralization degree, 0.08% agent is extra high. Therefore
crosslinker amount, and 0.15% initiator controlling the appropriate amount
3.2 Performance of the Super
were added to synthesize the super of crosslinking agent is one pivotal
factor to successfully synthetise super
Absorbent Polymer
absorbent polymer. Figure 2 displays
that the variation of water absorbency absorbent polymer. To study the effect On the basis of those factors affecting
from increased to decreased along with of crosslinking agent, fixed acrylic acid water absorbency of super absorbent
added initiator amount. And the water and acrylamide in a mass ratio of 2:1, composite material, such as the
absorbency reaches to the best when 80% monomer neutralization degree, neutralization of acrylic acid, the
the amount is 0.15%. 0.08% crosslinker amount, and 0.15% amount of added montmorillonite,
initiator were added to synthesize initiator and crosslinking agent were
3.1.3 The Neutralization of Acrylic the super absorbent polymer. It is investigated. The best formula for
illustrated from Figure 4 that the water absorbing water was proposed, it
Acid
We can learn that electric charge density
of material is an important factor which Figure 2. The effect of initiator on water absorbency
affects water absorbency by Flory’s
swelling theory. The reason why super
absorbent polymer has high water
absorbency is because of the presence
of different hydrophilic groups. As
to the montmorillonite/acrylic acid/
acrylamide tricopolymer, -COO- is
superior to -COOH, therefore the
neutralization degree has becoming a
great factor effects on water absorbency.
The super absorbent polymer shows
good water absorbency due to the
interactions between functional groups
when the degree of neutralization and
the proportion of -COOH, -COONa,
and -CONH2 in the polymer chain
are appropriate. To study the effect
of neutralization, fixed acrylic acid
and acrylamide in a mass ratio of 2:1,
80% monomer neutralization degree, Figure 3. The effect of neutralization degree on water absorbency
0.08% crosslinker amount, and 0.15%
initiator were added to synthesize the
super absorbent polymer. As we can see
from Figure 3, the water absorbency
keeps increasing as the degree of
neutralization from 60% to 80%, but
has no significant increase when the
degree is over 80%.
3.1.4 Crosslinking Agent
In the synthetic process of super
absorbent polymer, the less amount
of crosslinking agent is, the less dense
it is. Because of poor crosslinking
among monomers, the formative
network structure is relatively simple,
then its water absorbency is low
Polymers & Polymer Composites, Vol. 22, No. 5, 2014 491
Xiao-yuan Zhan, Fang Wang, Xing-wei Li, and Min Wang
included 40% montmorillonite, 80% of montmorillonite still exists after bonds took place between acrylate and
monomer neutralization degree, polymerization. However, the hydroxy montmorillonite, and then effected
0.08% crosslinker amount, and bending, stretching and vibrating the water bending, stretching and
0.15% initiator, then measured its became wide and strong in the high vibrating. Simultaneously there were
performance. frequency area of 3427 cm-1.That characteristic functional groups of
was explained probably generating acrylamide what meant the super
3.2.1 Fourier Transform Infrared hydrogen bonds under the effect absorbent polymer was synthetised
of acrylic hydroxy vibrating which following the effective polymerization
Spectroscopy (FTIS)
inserted into silicate layers. Meanwhile reaction of montmorillonite, acrylic
From Figure 4 and Table 1, it is quite wide and strong absorption peaks of acid and acrylamide.
clear that the sample reserves some 1675 cm-1 (carbonyl) and 1573 cm-1
absorption peaks of montmorillonite: (carboxylate) took the place of 3.2.2 Thermal Gravimetric Analysis
the water between silicate layers 1636 cm-1.In the same time, there were (TGA)
bending, stretching and vibrating at characteristic peaks of 1459 cm-1 and
3427 cm-1, the O-H swing nearby Figure 6 displays that there is a
1413 cm-1 which caused by the -COOH-
917 cm-1 and 624 cm-1, the Si-O big but narrow endothermic valley
symmetrical bending, stretching
nearby 110° for the reason that the
crooking and vibrating. Above and vibrating at low frequency area
super absorbent polymer releases
characteristics shows that the structure nearby. It showed that some chemical
absorbed water when heated. And
the TGA curve tends to smooth from
Figure 4. The effect of crosslinker on water absorbency 200 to 350°. A wide exothermic peak
appears at 650° caused by organic
decomposition and combustion that
lead to structural damage. It is shown
from the TGA curve that continuous
weight loss (water release and organic
decomposition). This particular sample
ultimately happens to contain a
invariable amount at 40 wt.%, hence,
adding montmorillonite at the mount
of 40% in the process of synthetising
the super absorbent polymer.
3.2.3 Measuring Water Absorbency
The method begins by pulverizing the
synthetic super absorbent polymer
in a sieve (100 mesh) and drying to
constant weight at 110°. Afterwards,
Table 1. The FTIR spectra of the characteristic functional groups of the super absorbent resin
Wavenumbers Mode of vibration Functional group
3330~2500cm -1
O—H bending, stretching and vibrating O—H in carboxylic acid
2940 cm-1 C—H bending, stretching and vibrating C—H in carboxylic acid
1725~1695 cm-1 C=O bending, stretching and vibrating C==O absorption spectrum in carboxylic acid
1430 cm -1
O—H outward bending vibration O—H in carboxylic acid
1250 cm-1 C—O bending, stretching and vibrating C—O in carboxylic acid
1616~1540 cm -1
—COO-anti-symmetrical bending, stretching and vibrating —COO- in carboxylate
1450~1400 cm-1 —COO- symmetrical bending, stretching and vibrating —COO- in carboxylate
3450~3225 cm -1
NH2 bending, stretching and vibrating NH2 in primary amides
1690~1610 cm-1 C=O bending, stretching and vibrating C=O absorption spectrum in amides
1620 cm-1 NH2 transformative vibrating NH2 in primary amides
700~625 cm -1
NH2 outward bending vibration NH2 in primary amides
492 Polymers & Polymer Composites, Vol. 22, No. 5, 2014
Synthesis of Montmorillonite/Acrylic Acid/Acrylamide Tricopolymer and Its Super Absorbent Properties
it is placed into 1000 ml water and Figure 5. The FTIR spectra of the super absorbent resin
weigh its absorbed weight every half
hour. As we can see from Figure 7,
the super absorbent polymer reaches
to constant weight after 4 hours and
its water absorption ratio is 150 times.
4. Conclusions
Montmorillonite/acrylic acid/
acrylamide tricopolymer was
synthesized by using aqueous solution
polymerization which was simple
and easy to operate. The best formula
for absorbing the running water
was proposed, it included 40%
montmorillonite, 80% monomer
neutralization degree, 0.08%
crosslinker amount, and 0.15%
initiator, and the water absorbency of Figure 6. The thermal gravimetric analysis and differential thermal analysis of
the obtained composite materials was the super absorbent polymer
150 times after 4 hours. Associating
organic monomers with hydroxy in
montmorillonite can improve the
performance of the super absorbent
composite and reduce its cost.
Acknowledgements
This work was supported by
National Program on Key Basic
Research Project(973 Program)(N0.
2012CB723104) and Postgraduate
Fund of Science and Technology
Innovation of Shandong University
of Science and Technology(No.
YC130341).
Figure 7. The water absorption ratio of the super absorbent resin
References
1. Fan Liren, Xu Zhiliang, and Shen
Shangyue, Research progress in
mineral/polymer super absorbent
composites. Journal of Functional
Materials, 36(12) (2005) 1827-
1830,1836.
2. Xu Mangui, Xu Jingcai, Wen Hu,
et al., Current research status of
mechanism and extinguishing
methods of spontaneous combustion
of coal mine, Journal of Xi’an
University of Science & Technology,
21(1) (2001) 4-7.
3. Zhou Meng, Lin Jianming, and Wu
Jihuai, Synthesis and property of
clay-organic resin super absorbent
Polymers & Polymer Composites, Vol. 22, No. 5, 2014 493
Xiao-yuan Zhan, Fang Wang, Xing-wei Li, and Min Wang
composite, China Mining Magazine, 6. Wan Tao, He Wenqiong, Xi 8. Wang Hongxi, Bentonite, Beijing:
9(2) (2000) 72-74. Quanshou, et al., Study on bentonite Geological Publishing House, 1980.
4. Lin Jianming, Yang Zhenfang, Pu composite polysodium acrylate- 9. Flory P.J. Principles of Polymer
Minli, et al., Research on bentonite/ acrylamide high water-absorbing Chemistry, New York: Cornell
polyacrylic acid sodium salt super copolymer resin by inverse University Press, 1953.
absorbent composite, Mineralogica suspension polymerization, China
10. Wu Jihuai, Wei Yueling, and Lin
Sinica, 21(3) (2001) 427-430. Elastomerics, 13(2) (2003) 8-12.
Jianming, Preparation of a starch-
5. Wei Yuelin, Wu Jihuai, Huang 7. Feng Qiming, Wang Weiqing, Li graft-acrylamide/kaolinite super
Yunfang, et al., Synthesis and surface Jinli, Synthesis and properties absorbent composite and the
modification of kaolin/poly(acrylic of bentonite/acrylic resin water- influence of the hydrophilic group
acid) super absorbent composite, absorbent & retaining agent, Non- on its water absorbency, Polymer
China Mining Magazine, 25(4) Metallic Mines, 32(6) (2009) 6-9, 60. International, 52 (2003) 1909-1912.
(2005) 379-384.
494 Polymers & Polymer Composites, Vol. 22, No. 5, 2014
You might also like
- Polymer Flooding IntroductionDocument50 pagesPolymer Flooding IntroductionAlexandra Cuellar GuasdeNo ratings yet
- Organic Acrylate Binder Synthesis ThrougDocument7 pagesOrganic Acrylate Binder Synthesis ThrougAmr Abdelmegid abdelsalam husseinNo ratings yet
- Binders: 1. Compaction Behaviour of Organic Binders in Alumina Ceramics (PVA & PEG) General FactsDocument13 pagesBinders: 1. Compaction Behaviour of Organic Binders in Alumina Ceramics (PVA & PEG) General FactsPranav KumarNo ratings yet
- Ion Exchange Resins and Adsorbents in Chemical Processing: Second EditionFrom EverandIon Exchange Resins and Adsorbents in Chemical Processing: Second EditionRating: 5 out of 5 stars5/5 (1)
- Bulk and Suspenshion Polynerization of MMA Into PMMADocument5 pagesBulk and Suspenshion Polynerization of MMA Into PMMADavid Meza CarbajalNo ratings yet
- POLARIMETRYDocument17 pagesPOLARIMETRYShaise JacobNo ratings yet
- Rajiv Gandhi University of Health Sciences, Karnataka II.B.D.S Degree Examination - JUNE 2013 Dental Materials (RS-3) QP CODE: 1185Document12 pagesRajiv Gandhi University of Health Sciences, Karnataka II.B.D.S Degree Examination - JUNE 2013 Dental Materials (RS-3) QP CODE: 1185drpnnreddyNo ratings yet
- Synthesis of Superabsorbent Starch-graft-Poly (Potassium Acrylate-Co-Acrylamide) and Its PropertiesDocument7 pagesSynthesis of Superabsorbent Starch-graft-Poly (Potassium Acrylate-Co-Acrylamide) and Its Propertiesdarg111No ratings yet
- Fang - 2018 - IOP - Conf. - Ser. - Earth - Environ. - Sci. - 178 - 012018Document8 pagesFang - 2018 - IOP - Conf. - Ser. - Earth - Environ. - Sci. - 178 - 012018Karlina DewiNo ratings yet
- Synthesis and Characterization of Maleic Acid and Sodium Methallyl Disulfonate New Copolymer: Application As A Barium Sulfate Scale InhibitorDocument10 pagesSynthesis and Characterization of Maleic Acid and Sodium Methallyl Disulfonate New Copolymer: Application As A Barium Sulfate Scale InhibitorAri WijayaNo ratings yet
- Graft Copolymerization, Characterization, and Degradation of Cassava Starch-G-Acrylamide/itaconic Acid SuperabsorbentsDocument17 pagesGraft Copolymerization, Characterization, and Degradation of Cassava Starch-G-Acrylamide/itaconic Acid SuperabsorbentsCaty CerasNo ratings yet
- Lab 3Document4 pagesLab 3Eng mohammadNo ratings yet
- Experiment 1: Emulsion Polymerization of StyreneDocument11 pagesExperiment 1: Emulsion Polymerization of StyreneNurshahanimNo ratings yet
- Synthesis and Properties of Starch-Graft-Acrylic Acid/Na-Montmorillonite Superabsorbent Nanocomposite HydrogelsDocument7 pagesSynthesis and Properties of Starch-Graft-Acrylic Acid/Na-Montmorillonite Superabsorbent Nanocomposite HydrogelsKaeksi Sekar ArumNo ratings yet
- Ammonia Removalfromaqueoussolutionsusinghollow FiberDocument8 pagesAmmonia Removalfromaqueoussolutionsusinghollow FiberSilvia Rahmi EkasariNo ratings yet
- Investigation of Effect of Pour Point Depressant On Wax DepositionDocument3 pagesInvestigation of Effect of Pour Point Depressant On Wax DepositionAfzal AktharNo ratings yet
- Ma 2020Document12 pagesMa 2020Bui Ngo Que NghiNo ratings yet
- 3 s2.0 B0080431526004939 MainDocument6 pages3 s2.0 B0080431526004939 MainMohammed JamaliNo ratings yet
- v33s 039 043 PDFDocument5 pagesv33s 039 043 PDFMariam AsgharNo ratings yet
- Preparation and Application of Environmental Friendly AmPAM Flocculant in The Treatment of Tannery WastewaterDocument7 pagesPreparation and Application of Environmental Friendly AmPAM Flocculant in The Treatment of Tannery WastewaterLaili AnieyNo ratings yet
- Company Profile Publication Water Treat DC3Document13 pagesCompany Profile Publication Water Treat DC3tito rahmanNo ratings yet
- Ref. 1Document11 pagesRef. 1alinealmuzainiNo ratings yet
- Riaz 2018 IOP Conf. Ser.: Mater. Sci. Eng. 414 012020Document8 pagesRiaz 2018 IOP Conf. Ser.: Mater. Sci. Eng. 414 012020Abdul ZahirNo ratings yet
- 12 Composite ResinsDocument20 pages12 Composite ResinssimranNo ratings yet
- Journal of Molecular Structure: T. Ngulube, J.R. Gumbo, V. Masindi, A. MaityDocument11 pagesJournal of Molecular Structure: T. Ngulube, J.R. Gumbo, V. Masindi, A. Maityall green associatesNo ratings yet
- Amine-Based Solutions Using A Solvent ResistantDocument8 pagesAmine-Based Solutions Using A Solvent ResistantCharlesNo ratings yet
- Polymer, 10Document9 pagesPolymer, 10Mohammed MNo ratings yet
- Development of Key Additives For Organoclay-Free oDocument6 pagesDevelopment of Key Additives For Organoclay-Free oAgustin CantilloNo ratings yet
- Synthesis and Characterization of A Novel Super-Absorbent Based On Wheat StrawDocument6 pagesSynthesis and Characterization of A Novel Super-Absorbent Based On Wheat StrawAlejandro Flores SanchezNo ratings yet
- Warburton, Parkhill Et Al, 1973 PDFDocument6 pagesWarburton, Parkhill Et Al, 1973 PDFNikhil NaniNo ratings yet
- Reactive & Functional Polymers: Jinzhang Gao, Aixiang Wang, Yan Li, Yan Fu, Jianlin Wu, Youdi Wang, Yujing WangDocument7 pagesReactive & Functional Polymers: Jinzhang Gao, Aixiang Wang, Yan Li, Yan Fu, Jianlin Wu, Youdi Wang, Yujing WangEdward GustafNo ratings yet
- Ammonium Hydroxide Function in Free EmulsifierDocument6 pagesAmmonium Hydroxide Function in Free EmulsifierHermawanRandiNo ratings yet
- Crisis Covered Bio Degradable &water Soluble Copolymer (VA-AA)Document1 pageCrisis Covered Bio Degradable &water Soluble Copolymer (VA-AA)Gopal AeroNo ratings yet
- CVL100 EfDocument5 pagesCVL100 EfHarsh AgarwalNo ratings yet
- Polymers ChemistryDocument26 pagesPolymers ChemistryMessaoud AmrouneNo ratings yet
- 1034-Article Text-3281-1-10-20230708Document6 pages1034-Article Text-3281-1-10-20230708risfiNo ratings yet
- Comparative Experiments On Polymer Degradation OilfieldDocument7 pagesComparative Experiments On Polymer Degradation Oilfieldxxtryxx48No ratings yet
- Brando 2010Document7 pagesBrando 2010LILIANANo ratings yet
- Spe 174541 PaDocument11 pagesSpe 174541 Pare_alvaroNo ratings yet
- Acrylic Allergies InformationDocument7 pagesAcrylic Allergies Informationdrgayen6042No ratings yet
- Carbohydrate Polymers: Fang Yang, Gang Li, Yan-Gang He, Feng-Xia Ren, Gui-Xiang WangDocument5 pagesCarbohydrate Polymers: Fang Yang, Gang Li, Yan-Gang He, Feng-Xia Ren, Gui-Xiang WangAzril M IrfanNo ratings yet
- Costa 2012Document7 pagesCosta 2012Cristofer Newton ChigualaNo ratings yet
- Research Article: Synthesis and Properties of Adhesive Polymer-Methylmethacrylate MaterialsDocument10 pagesResearch Article: Synthesis and Properties of Adhesive Polymer-Methylmethacrylate MaterialsMuradNo ratings yet
- A Laboratory Approach To Measure Carbonate RocksDocument7 pagesA Laboratory Approach To Measure Carbonate RocksAYAUWU LOVEDAYNo ratings yet
- S - 8 Composites Composition, Classification & PolymerisationDocument36 pagesS - 8 Composites Composition, Classification & PolymerisationShaliniNo ratings yet
- Dye1 PDFDocument15 pagesDye1 PDFمروة فؤاد حسن شعبانNo ratings yet
- A Fieldscale Simulation Study of Surfactant and Polymer Flooding Insandstone Heterogeneous Reservoir 2157 7463 1000366Document6 pagesA Fieldscale Simulation Study of Surfactant and Polymer Flooding Insandstone Heterogeneous Reservoir 2157 7463 1000366David LópezNo ratings yet
- 06 07 08 Chemical EORDocument72 pages06 07 08 Chemical EORAndre WibawaNo ratings yet
- Ion-Exchange Capability For Ammonium Removal Using Zeolite Modified by Potassium PermanganateDocument6 pagesIon-Exchange Capability For Ammonium Removal Using Zeolite Modified by Potassium PermanganateArash AbbasiNo ratings yet
- He 2015 - Urea-Assisted Aqueous Exfoliation of GraphiteDocument5 pagesHe 2015 - Urea-Assisted Aqueous Exfoliation of Graphitejohn_k7408No ratings yet
- Stability and Performance Study of Water-in-Oil-in-Water Emulsion: Extraction of Aromatic AminesDocument8 pagesStability and Performance Study of Water-in-Oil-in-Water Emulsion: Extraction of Aromatic AminesJaydeep BaradNo ratings yet
- Plastic and Polymer Industries (2022)Document45 pagesPlastic and Polymer Industries (2022)sandraNo ratings yet
- SPE-177914-MS Successful Chemical Water Shut-Off Treatment in An Omani Field Heavy-Oil WellDocument19 pagesSPE-177914-MS Successful Chemical Water Shut-Off Treatment in An Omani Field Heavy-Oil Wellnia setya budiningtyasNo ratings yet
- Study Using Nacl As SolventDocument7 pagesStudy Using Nacl As SolventBarisNo ratings yet
- GICDocument26 pagesGICJyotsna vaishnavNo ratings yet
- Study of Starch Based Coagulant in The Treatment of Dairy Waste Water in Coimbatore Tamil Nadu IJERTV3IS110917Document4 pagesStudy of Starch Based Coagulant in The Treatment of Dairy Waste Water in Coimbatore Tamil Nadu IJERTV3IS110917Cédric RochatNo ratings yet
- Synthesis and Characterization of Silicone Modified Acrylic Resin and Its Uses in The Emulsion PaintsDocument13 pagesSynthesis and Characterization of Silicone Modified Acrylic Resin and Its Uses in The Emulsion PaintsfredyNo ratings yet
- Ammar Et Al 2023 Ultraviolet Uv Curable Hybrid Material Based On Palm Oil Plasticization Effect and Flame RetardancyDocument12 pagesAmmar Et Al 2023 Ultraviolet Uv Curable Hybrid Material Based On Palm Oil Plasticization Effect and Flame Retardancy발라드님No ratings yet
- Seminar RPDocument20 pagesSeminar RPcgoldcafeNo ratings yet
- Study Using Nacl As Solvent2Document8 pagesStudy Using Nacl As Solvent2BarisNo ratings yet
- TMP D578Document5 pagesTMP D578FrontiersNo ratings yet
- Regulator Broch Flxflo SurgerelieverDocument12 pagesRegulator Broch Flxflo SurgerelieverCarlos SandinoNo ratings yet
- EnzomologyDocument26 pagesEnzomologyToga Brandon100% (1)
- GC1 Q1 Week-5c PDFDocument12 pagesGC1 Q1 Week-5c PDFLovely MavilNo ratings yet
- Starch and GlycogenDocument20 pagesStarch and GlycogenLaksilu Viduraga Peiris100% (1)
- The Thermal Degradation of Poly (Vinyl Acetate) Measured by Thermal Analysis Fourier Transform Infrared SpectrosDocument5 pagesThe Thermal Degradation of Poly (Vinyl Acetate) Measured by Thermal Analysis Fourier Transform Infrared SpectrosalmasNo ratings yet
- Brief Byk-066 N enDocument7 pagesBrief Byk-066 N enGİZEM DEMİRNo ratings yet
- Contribution of Starch To The Flavor of Rice-Based Instant FoodDocument13 pagesContribution of Starch To The Flavor of Rice-Based Instant FoodKeynaya MahayuNo ratings yet
- Mokennene 1Document30 pagesMokennene 1Estifanos EndalewNo ratings yet
- Electrochemical Energy Systems: IIT KanpurDocument12 pagesElectrochemical Energy Systems: IIT KanpurAjeet kumarNo ratings yet
- Tushal KyadaDocument11 pagesTushal KyadaDevashish JoshiNo ratings yet
- Dheyab 2020Document21 pagesDheyab 2020Felipe AgudeloNo ratings yet
- 2 - Thermodynamics ProblemsDocument3 pages2 - Thermodynamics Problemskhalid.jm70No ratings yet
- Journal of Cleaner Production: Mohammad Shahid, Shahid-ul-Islam, Faqeer MohammadDocument22 pagesJournal of Cleaner Production: Mohammad Shahid, Shahid-ul-Islam, Faqeer MohammadZhulietaNo ratings yet
- Pangmalakasang Mesl Problem Part 5Document331 pagesPangmalakasang Mesl Problem Part 5aljay balingitNo ratings yet
- Grinding Valve JournalDocument12 pagesGrinding Valve JournalmuhammadshultonskNo ratings yet
- Review Problems From Diff BooksDocument9 pagesReview Problems From Diff BooksHannah AzucenaNo ratings yet
- BOOKS-2019-0034 Proof HiDocument76 pagesBOOKS-2019-0034 Proof HiLudi D. LunarNo ratings yet
- Student Notes: HPCT: Davao Doctors College Medical Laboratory Science DepartmentDocument4 pagesStudent Notes: HPCT: Davao Doctors College Medical Laboratory Science DepartmentJym TampusNo ratings yet
- Fispq Graxa Staburags Nbu 12Document7 pagesFispq Graxa Staburags Nbu 12andremirandadantasNo ratings yet
- Metals and Non Metals Notes Class 10Document19 pagesMetals and Non Metals Notes Class 10Deepayan PaikNo ratings yet
- Carbonated Hydroxyapatite - Materials, Synthesis, and Applications-Pan Stanford Publishing (2015) PDFDocument272 pagesCarbonated Hydroxyapatite - Materials, Synthesis, and Applications-Pan Stanford Publishing (2015) PDFKENAN ABBARANo ratings yet
- June 2018 MS - Paper 1 AQA Biology As-LevelDocument16 pagesJune 2018 MS - Paper 1 AQA Biology As-Levelmarim ahmedNo ratings yet
- Midterm CorrosionDocument4 pagesMidterm CorrosionAngelica FerrerNo ratings yet
- Efficiency and Potential Environmental Impacts of Different Cleaning Agents Used On Contaminated Railway BallastDocument7 pagesEfficiency and Potential Environmental Impacts of Different Cleaning Agents Used On Contaminated Railway BallastWesley MachiniNo ratings yet
- Isothermal Titration Calorimetry: Presented By: Ms. Prajakta S.Pawar. Guided byDocument54 pagesIsothermal Titration Calorimetry: Presented By: Ms. Prajakta S.Pawar. Guided byVrushali Puranik-GokhaleNo ratings yet
- Experiment-3 Metals and Their Properties UpdatedDocument4 pagesExperiment-3 Metals and Their Properties UpdatedSnehaNo ratings yet
- Henry & Guidotti 2002 The Ti Saturation Surafce For Low To Medium PressureDocument13 pagesHenry & Guidotti 2002 The Ti Saturation Surafce For Low To Medium PressureVictor ValdiviaNo ratings yet
- Roquette Cosmetics Brochure Formulation Guide A6Document11 pagesRoquette Cosmetics Brochure Formulation Guide A6Sathyapriya SubramaniNo ratings yet
- Isensee Robert W1943Document17 pagesIsensee Robert W1943DŨNG VŨ NGUYỄN TUẤNNo ratings yet