Professional Documents
Culture Documents
Chemistry Poster 2019
Uploaded by
tcolakoglu2020Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chemistry Poster 2019
Uploaded by
tcolakoglu2020Copyright:
Available Formats
Nobel Prize® and the Nobel Prize® medal
design mark are registered trademarks of
The Royal Swedish Academy of Sciences has
The Nobel Prize 2019 in Chemistry
decided to award the Nobel Prize in
Chemistry 2019 to John B. Goodenough,
M. Stanley Whittingham and Akira Yoshino
the Nobel Foundation.
“for the development of lithium-ion batteries”.
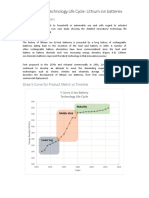
The battery that
C 1985
Petroleum coke
changed the world
John B. Goodenough, M. Stanley Whittingham and Akira Yoshino are
rewarded with the Nobel Prize in Chemistry 2019 for the development
of the lithium-ion battery, the world’s best rechargeable battery. It has B 1980
provided the basis for wireless electronics and makes a fossil fuel-free
world possible. The battery is now used to power mobile phones, laptops
and electric vehicles, and to store energy from solar and wind power.
Cobalt oxide
It has proven difficult to develop powerful Lithium is also the lightest metal and takes
rechargeable batteries. The foundation for very little space, which is why lithium-
lead batteries and alkaline batteries was laid ion batteries are lightweight despite
back in the 19th century, but the research having a high capacity and lasting many
field then stood still for a long time, so it was a hours before needing to be recharged.
technological revolution when the first lithium- Lithium-ion batteries now power every-
Metallic lithium
ion batteries entered the market in 1991. thing from mobile phones and laptops to
One major advantage with the lithium-ion hearing aids and pacemakers. They have
battery is that it is not based on chemical enabled the development of long range A 1976
reactions that break down the electrodes electric vehicles and the storage of energy
Photo: portrait of John B. Goodenough: Cockrell School of Engineering, The University of Texas at Austin; portrait of M. Stanley Whittingham: Jonathan Cohen © Binghamton University; portrait of Akira Yoshino: © 2019 Asahi Kasei Corporation.
and destroy the battery, but rather on lithium from renewable sources, such as solar and
ions that flow backwards and forwards wind power. They contribute to a wireless
between the anode and cathode. These and fossil fuel-free society, and are of
batteries can be charged hundreds of times the greatest benefit to humankind.
before their performance declines.
Lithium ion
Cobalt oxide Electron
Metallic lithium
Lithium Lithium ions
John B. Goodenough M. Stanley Whittingham Akira Yoshino
Born 1922 in Jena, Born 1941 in Born 1948 in Suita,
Germany. Virginia H. Nottingham, UK. Distin- Japan. Honorary
Electrolyte Cockrell Centennial guished Professor at Fellow at Asahi Kasei
Barrier Chair in Engineering Binghamton University, Corporation, Tokyo,
at The University of State University of Japan, and Professor
Texas at Austin, USA. New York, USA. at Meijo University,
Nagoya, Japan.
Titanium disulphide
A 1976 B 1980 C 1985
An old element Lithium is reactive Whittingham laid the foundation Goodenough makes the battery more powerful Yoshino creates a sustainable battery
The first lithium atoms were created shortly Lithium has only one electron in its outer shell, In the 1970s, during the oil crisis, Stanley Thanks to his specialist knowledge about Akira Yoshino created the first commercially
after the Big Bang. The element was discovered which it happily releases. When this happens, Whittingham worked on developing fossil the interior of matter, John Goodenough viable lithium-ion battery in 1985, using
by Swedish chemists in 1817. Its name comes a positively charged lithium ion is formed. fuel-free energy technology. He researched predicted that the cathode material would Goodenough’s cathode as a basis. Instead
from the Greek word for stone, lithos, but it is superconductors and discovered an extremely have an even higher potential if it was built of reactive lithium in the anode, he used
the lightest metal despite its weighty name. energy-rich material. From this, he created using a metal oxide instead of titanium petroleum coke, a carbon material which,
an innovative cathode in a lithium battery. disulphide. After a systematic search, in 1980 like the cobalt oxide in the cathode, can
It consisted of titanium disulphide which has he showed that cobalt oxide with intercalated intercalate lithium ions. The result was a
spaces at the molecular level that lithium ions could provide a voltage as high light, sustainable battery. The first lithium
can house – intercalate – lithium ions. as four volts. This breakthrough led towards -ion battery entered the market in 1991,
The battery literally had great potential, lighter, more powerful batteries that can that which led to a technological revolution.
just over two volts, but the anode was partly can be used in mobiles and screwdrivers.
metallic lithium, which is reactive and difficult
to handle. The battery could even explode.
LEARN MORE ABOUT THE NOBEL PRIZES AT WWW.KVA.SE More information about the Nobel Prize in Chemistry 2019 is available at www.kva.se/nobelchemistry2019 and www.nobelprize.org, with detailed information about the Prize and the Laureates, suggested reading, videos and more. Editors: Claes Gustafsson, Gunnar von Heijne, and Olof Ramström, Printing and © The Royal Swedish Academy of Sciences
the Nobel Committee for Chemistry, the Royal Swedish Academy of distribution Box 50005, SE-104 05 Stockholm, Sweden
Sciences; Ann Fernholm, Science Writer; Clare Barnes, Translator; made possible by +46 8 673 95 00, kva@kva.se, www.kva.se
Sara Gustafsson, Editor, och Emma Stohne, Nobel Assistant, the Royal Posters may be ordered free of charge
Swedish Academy of Sciences. Graphic design: IVY Agency AB at www.kva.se/nobelposters
Illustrations: Johan Jarnestad/Infographics.se Print: Åtta45
kemi2019.indd 2 2019-11-18 15:53
You might also like
- 11 Marine Fuel Properties.Document7 pages11 Marine Fuel Properties.Kevin LeysonNo ratings yet
- Casting Material Austenitic Ductile Iron in ASTM A439Document3 pagesCasting Material Austenitic Ductile Iron in ASTM A439Alex007No ratings yet
- A Study of Electro Materials For Lithium-Ion BatteriesDocument204 pagesA Study of Electro Materials For Lithium-Ion Batteriesmarchelo_cheloNo ratings yet
- S Curve Analysis of Battery TechnologyDocument5 pagesS Curve Analysis of Battery TechnologyAkhil Jose100% (1)
- Comparison of Batteries Used in Electrical Vehicles: (A Review)Document5 pagesComparison of Batteries Used in Electrical Vehicles: (A Review)pierre abreu mackleine100% (1)
- The Birth of The Lithium-Ion BatteryDocument4 pagesThe Birth of The Lithium-Ion Batterytamilmuthu100% (1)
- Energy and The Environment GCSE IGCSE Environmental Management Geography 0680Document54 pagesEnergy and The Environment GCSE IGCSE Environmental Management Geography 0680IGCSE Physics & ChemistryNo ratings yet
- Advanced Technical CeramicsFrom EverandAdvanced Technical CeramicsShigeyuki SomiyaNo ratings yet
- Molten Salt BatteryDocument9 pagesMolten Salt BatteryUpendraJavaNo ratings yet
- Battery Atlas 2022 PDFDocument11 pagesBattery Atlas 2022 PDFRaúlNo ratings yet
- Chemical & Engineering News - Lithium Nobel PrizeDocument1 pageChemical & Engineering News - Lithium Nobel PrizeGavin D. J. HarperNo ratings yet
- Nobel Prize Presentation ChemistryDocument20 pagesNobel Prize Presentation Chemistryjatin gandhiNo ratings yet
- Lithium Ion BatteryDocument14 pagesLithium Ion BatteryKyiubi el mundoNo ratings yet
- Nobelposter2017 ChemistryDocument1 pageNobelposter2017 Chemistrytcolakoglu2020No ratings yet
- Current Li-Ion Battery Technologies in ElectricDocument21 pagesCurrent Li-Ion Battery Technologies in Electricrosca raulNo ratings yet
- 2018-The - Chemical - Record-KOMABA-towards K Ion and Na Ion As Beyond Li-IonDocument21 pages2018-The - Chemical - Record-KOMABA-towards K Ion and Na Ion As Beyond Li-IonMarty LGNo ratings yet
- Application Note - MattDocument7 pagesApplication Note - Mattمجتبى مهند سمير عبد عليNo ratings yet
- (Paper) Geological Sources Ofmetals in Coal Andcoal ProductsDocument33 pages(Paper) Geological Sources Ofmetals in Coal Andcoal Productsriko dwi kurniawanNo ratings yet
- Zinc As Versatile Battery MaterialDocument5 pagesZinc As Versatile Battery MaterialPeter GuoNo ratings yet
- Technology Strategy Assessment - Zinc BatteriesDocument22 pagesTechnology Strategy Assessment - Zinc BatteriesSridhar KasichainulaNo ratings yet
- Generation of Electricity Using Nitinol: S K Haaris G Madhu Murali Siran2Document3 pagesGeneration of Electricity Using Nitinol: S K Haaris G Madhu Murali Siran2Deepak BhopeNo ratings yet
- Lib-Introduction - WikipediaDocument29 pagesLib-Introduction - WikipediaNguyễn Văn HùngNo ratings yet
- Message From Dr. Akira YoshinoDocument5 pagesMessage From Dr. Akira Yoshino123 456No ratings yet
- The Lithium Ion Polymer Rechargeable Battery: A "Magic Box" That Has Evolved So FarDocument2 pagesThe Lithium Ion Polymer Rechargeable Battery: A "Magic Box" That Has Evolved So FarRheza Janitra KurniawanNo ratings yet
- L2 Structure of The Atom ActivityDocument4 pagesL2 Structure of The Atom ActivityFe Anne Thea De Guzman100% (1)
- Bakelite 80 Years Since The First Synthetic ResinDocument1 pageBakelite 80 Years Since The First Synthetic ResinnicolasNo ratings yet
- Hatfield Group - CLT Whitepaper2Document11 pagesHatfield Group - CLT Whitepaper2erleenhat8056No ratings yet
- Antonio S and Julia L (W4.S3) Poster-For-DownloadDocument1 pageAntonio S and Julia L (W4.S3) Poster-For-DownloadluisakdelimaNo ratings yet
- Popular Chemistryprize2019Document7 pagesPopular Chemistryprize2019jbmccavalcantiNo ratings yet
- Simplification of Aluminum Air Battery by GelationDocument8 pagesSimplification of Aluminum Air Battery by GelationChemistryNo ratings yet
- Microstructural Investigations in Cordierite-Mullite RefractoriesDocument16 pagesMicrostructural Investigations in Cordierite-Mullite RefractoriesuvsarathiNo ratings yet
- Medusa Analog Horror Episode OneDocument6 pagesMedusa Analog Horror Episode OneDylan James VibarNo ratings yet
- PT Inter World Steel MillsDocument20 pagesPT Inter World Steel Millssugiarto budiNo ratings yet
- New Solid Battery CanditatesDocument11 pagesNew Solid Battery CanditatesEero IiskolaNo ratings yet
- Chem 362 PaperDocument9 pagesChem 362 Paperapi-506270148No ratings yet
- History of PiezopolymersDocument14 pagesHistory of PiezopolymersrachmajuwitaNo ratings yet
- Nobelposterphysics2014 HighDocument1 pageNobelposterphysics2014 Hightcolakoglu2020No ratings yet
- Morinobu EndoDocument14 pagesMorinobu EndoAnonymous gUjimJKNo ratings yet
- The Haus Am Horn in Weimar Climate AdaptDocument107 pagesThe Haus Am Horn in Weimar Climate AdaptBatool Al-kharabshehNo ratings yet
- Paper Bettery by Vipib BansalDocument16 pagesPaper Bettery by Vipib BansalVipin BansalNo ratings yet
- Lead Acid Battery Recycling For The Twenty-First Century: Royal Society Open Science May 2018Document13 pagesLead Acid Battery Recycling For The Twenty-First Century: Royal Society Open Science May 2018ABHISHEK SINGHNo ratings yet
- Dry CellDocument1 pageDry CellAli Mubin FitranandaNo ratings yet
- Burial Thermal and Maturation History in PDFDocument3 pagesBurial Thermal and Maturation History in PDFangeysaccisNo ratings yet
- Did You Know ... : 1988 World Snooker ChampionshipDocument3 pagesDid You Know ... : 1988 World Snooker ChampionshipRachel DollisonNo ratings yet
- Did You Know ... : 1988 World Snooker ChampionshipDocument3 pagesDid You Know ... : 1988 World Snooker ChampionshipLGU-Castilla Raquel J. Dollison, MPDCNo ratings yet
- Kubota - WikipediaDocument28 pagesKubota - WikipediaNUTHI SIVA SANTHANNo ratings yet
- Ordonez 2016Document12 pagesOrdonez 2016Sansung AceNo ratings yet
- Lithium - Ion BatteryDocument19 pagesLithium - Ion BatteryAditya ManeNo ratings yet
- Heterogeneous Photocatalyst Materials For Water Splitting2009chemical Society ReviewsDocument27 pagesHeterogeneous Photocatalyst Materials For Water Splitting2009chemical Society ReviewsDaniel Camilo CanoNo ratings yet
- Conductive PolymersDocument7 pagesConductive PolymersDHANUSH RacerNo ratings yet
- BCG 34 HTDDocument2 pagesBCG 34 HTDMansoor AbbasNo ratings yet
- Academic Reading SampleDocument3 pagesAcademic Reading Samplereddy100% (1)
- Dimming of The Light BulbDocument3 pagesDimming of The Light BulbJayati AgrawalNo ratings yet
- Formation of Titanium Oxide NanotubeDocument4 pagesFormation of Titanium Oxide NanotubeSuraj Singh SainiNo ratings yet
- Materials Science For Energy TechnologiesDocument6 pagesMaterials Science For Energy TechnologiesAngel Jair BarbosaNo ratings yet
- Nickelvol33no2summer2018 FB EngDocument16 pagesNickelvol33no2summer2018 FB Engeugenio.gutenbertNo ratings yet
- Bakelite WikipediaDocument4 pagesBakelite WikipediaFAizan AbbasNo ratings yet
- 2007 2008TechnologyDivisionAwardsDocument8 pages2007 2008TechnologyDivisionAwardsGirish NaravaneNo ratings yet
- Lead 68: Edited Proceedings, Third International Conference on Lead, VeniceFrom EverandLead 68: Edited Proceedings, Third International Conference on Lead, VeniceNo ratings yet
- Solef PVDF Design and Processing Guide - EN PDFDocument64 pagesSolef PVDF Design and Processing Guide - EN PDFJosé Luis Pinto VergaraNo ratings yet
- 2014 Course CatalogDocument376 pages2014 Course CatalogТимур ИстемировNo ratings yet
- Fluid and ElectrolytesDocument13 pagesFluid and ElectrolytesMarcus, RN100% (7)
- CHENG Frank 2012-08-20 PipelineDocument15 pagesCHENG Frank 2012-08-20 PipelineSandeepSinghNo ratings yet
- Nano-Emulsions: New Applications and Optimization of Their PreparationDocument7 pagesNano-Emulsions: New Applications and Optimization of Their PreparationYuli CartrinaNo ratings yet
- XI STUDYING - BOTANY and ZOOLOGY - NEET + COMPREHENSIVE TEST PACKAGE (CTP)Document1 pageXI STUDYING - BOTANY and ZOOLOGY - NEET + COMPREHENSIVE TEST PACKAGE (CTP)Dharmendra JajalNo ratings yet
- Emcephob LE enDocument2 pagesEmcephob LE enpetronela.12No ratings yet
- Compresed GasDocument3 pagesCompresed GasAsan IbrahimNo ratings yet
- 7.5.9.5.8 Methods of SterilizationDocument3 pages7.5.9.5.8 Methods of SterilizationDedi LihawaNo ratings yet
- Gpe Cat FlexonDocument14 pagesGpe Cat FlexonCristian PopescuNo ratings yet
- Quantum Wells Wires Dots - Lecture 8-2005Document17 pagesQuantum Wells Wires Dots - Lecture 8-2005samara gul100% (1)
- KORAFIT Adhesive Is The Really Reliable ConnectionDocument2 pagesKORAFIT Adhesive Is The Really Reliable ConnectionhudileksonoNo ratings yet
- Cooling Towers Information PackageDocument25 pagesCooling Towers Information Packagemdawg467No ratings yet
- Perfluoroelastomer and Fluoroelastomer Seals For Photovoltaic Cell Manufacturing ProcessesDocument12 pagesPerfluoroelastomer and Fluoroelastomer Seals For Photovoltaic Cell Manufacturing ProcessesJagdish PatelNo ratings yet
- Petroleum Testing Laboratory Manual With CalculationDocument17 pagesPetroleum Testing Laboratory Manual With CalculationKarthikeshwaran RamasamyNo ratings yet
- Water Safety ForumDocument31 pagesWater Safety ForumShirlee Alicor L MacheteNo ratings yet
- STPM Trials 2009 Chemistry Paper 1 (Malacca)Document14 pagesSTPM Trials 2009 Chemistry Paper 1 (Malacca)sherry_christyNo ratings yet
- Analytical TechniquesDocument42 pagesAnalytical Techniquessidqy radinalNo ratings yet
- Amaravathi Capital Master Plan ImagesDocument88 pagesAmaravathi Capital Master Plan ImagesveerNo ratings yet
- Basic Concepts of ChemistryDocument82 pagesBasic Concepts of ChemistryGowri ShankarNo ratings yet
- Toyota Genuine ATF WSDocument14 pagesToyota Genuine ATF WSKirillNo ratings yet
- Cell, Cellular Components and Their FunctionDocument3 pagesCell, Cellular Components and Their FunctionNur SyahirahNo ratings yet
- Bentocryl 86 Product Data SheetDocument1 pageBentocryl 86 Product Data SheetAnonymous S7Cq7ZDgP50% (2)
- Pds Hempatex Hi-Build 46410 En-GbDocument2 pagesPds Hempatex Hi-Build 46410 En-GbMohamed ChelfatNo ratings yet
- Skum Silv-Ex G F201132 - FDS14121 0214 LR PDFDocument2 pagesSkum Silv-Ex G F201132 - FDS14121 0214 LR PDFAhmed El Sayed SalamaNo ratings yet
- Form 4 Physics RevisionDocument5 pagesForm 4 Physics RevisionannmarieNo ratings yet
- MSDS Na K TartratDocument6 pagesMSDS Na K TartratRaihana RizkaNo ratings yet
- Cetamine f3100 enDocument1 pageCetamine f3100 enatlagh ayoubNo ratings yet