Professional Documents
Culture Documents
Set1 Ka, KB, Pka, PKB, PH
Uploaded by
EdcademiaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Set1 Ka, KB, Pka, PKB, PH
Uploaded by
EdcademiaCopyright:
Available Formats
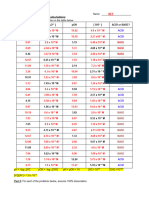
Set 1 Questions
1 .Calculate the K a for a weak acid if the concentration of hydronium ions ¿is 1.5 ×
10 M and the concentration of the undissociated acid [ HA ] is 0.02 M .
−3
2. Determine the K b for a weak base if the concentration of hydroxide ions [ OH ] is
2.0 × 10−4 M and the concentration of the undissociated base [ B ] is 0.01 M .
3. Find the p K a of a weak acid if its K a is 2.5 ×10−5.
4. Calculate the p K b for a weak base if its K b is 8.0 ×10−7.
5. Calculate the pH of a solution with a hydronium ion concentration of
−9
3.0 ×10 M .
6. If the pOH of a solution is 4.5 , find the corresponding pH .
7. Determine the K a for a weak acid if its p K a is 3.8 .
8. Find the K b for a weak base if its p K b is 7.2 .
9. Calculate the concentration of hydroxide ions ¿in a solution with a pOH of 10.2
.
10. If the pH of a solution is 2.5 , determine the concentration of hydronium ions
¿.
Set 1 Answers:
1
( 1.5 × 10−3 ) ( 1.5 ×10−3 ) −4
Ka= =1.125× 10
0.02
2
( 2.0 × 10−4 )( 2.0 × 10−4 ) −6
Kb= =4.0 ×10
0.01
3
p K a=−log 10 ( 2.5 × 10−5 ) ≈ 4.6
4
p K b=−log 10 ( 8.0 × 10−7 ) ≈ 6.1
5
pH ≈ 8.52
6
pH ≈ 9.5
7
−4
K a ≈ 1.58 ×10
8
−8
K b ≈ 1.58 ×10
[ OH- ] =10−10.2
10
[ H_3O+ ]=10−2.5
You might also like
- Spring 2022 CHEM 123 Recitation Activity #8 - KEYDocument5 pagesSpring 2022 CHEM 123 Recitation Activity #8 - KEYdkNo ratings yet
- Solve Ka and KB Problems Using Ice MethodsDocument4 pagesSolve Ka and KB Problems Using Ice Methodsapi-258903855100% (2)
- ISM Chapter 04Document19 pagesISM Chapter 04宇涵鄒No ratings yet
- Ionic HW PDFDocument18 pagesIonic HW PDFKaustubh ThakerNo ratings yet
- Solutions For The Problems About Calculation of PH in The Case of Monoprotic Acids and Bases"Document9 pagesSolutions For The Problems About Calculation of PH in The Case of Monoprotic Acids and Bases"krizelNo ratings yet
- 650d4cf65709d80018c3c617 - ## - Ionic Equilibrium DPP 04 (Extra)Document3 pages650d4cf65709d80018c3c617 - ## - Ionic Equilibrium DPP 04 (Extra)Brijesh MishraNo ratings yet
- KSP & Reaction QuotientDocument2 pagesKSP & Reaction QuotientAndy TanNo ratings yet
- 01 - Ionic Equilibrium (Solved Example) DDocument17 pages01 - Ionic Equilibrium (Solved Example) DNishant JanuNo ratings yet
- Stuff: Please Read Ahead and Don't Fall Behind, One Big Push at The End Will Help ManyDocument12 pagesStuff: Please Read Ahead and Don't Fall Behind, One Big Push at The End Will Help ManyCybrille Fleur Siobhan QúeensNo ratings yet
- U08 CW03 Acid and Base Properties of Salts Worksheet v2Document2 pagesU08 CW03 Acid and Base Properties of Salts Worksheet v2Muyao ChenNo ratings yet
- CH Cooh CH CoohDocument5 pagesCH Cooh CH CoohPiyah RahmanNo ratings yet
- PH Worksheet SolutionsDocument3 pagesPH Worksheet Solutionsxdiep10No ratings yet
- Acid Dissociation Constants of OrganicsDocument6 pagesAcid Dissociation Constants of OrganicsAhmedNo ratings yet
- 6.ionic Equilibrium Exercise PDFDocument35 pages6.ionic Equilibrium Exercise PDFGaurav SinghNo ratings yet
- Polyprotic and Solubility PDT Equilibria 2021Document1 pagePolyprotic and Solubility PDT Equilibria 2021John Mar OrnaNo ratings yet
- Chem12 SM 08 5Document9 pagesChem12 SM 08 5Tithi ChoksiNo ratings yet
- 4.2 Problems - Acid Base SolutionsDocument3 pages4.2 Problems - Acid Base SolutionsTanisha DamleNo ratings yet
- Lemper ABSDocument12 pagesLemper ABSmiftahudinNo ratings yet
- Questions 1 - 20 PDFDocument2 pagesQuestions 1 - 20 PDFUjjawal kumarNo ratings yet
- K Co/CaDocument2 pagesK Co/CaWine FebriantiNo ratings yet
- Unit 8 1630427312380Document41 pagesUnit 8 1630427312380l082haripriyakotariNo ratings yet
- Kimia Kapita SelektaDocument2 pagesKimia Kapita SelektaIta AsfuriyahNo ratings yet
- AcidBase PEQ1Document2 pagesAcidBase PEQ1Aaron LeQuangNo ratings yet
- ch18 PDFDocument45 pagesch18 PDFHafidz RafiqiNo ratings yet
- Post-Lab 10 Ksp-SolutionsDocument3 pagesPost-Lab 10 Ksp-SolutionsUzo Paul NwabuisiNo ratings yet
- Acid Base ActivityDocument2 pagesAcid Base ActivityNICOLE CA�ARESNo ratings yet
- NCM 103 Final ExamDocument5 pagesNCM 103 Final ExamRichmond LacadenNo ratings yet
- H3PO4 Reactions X PHDocument3 pagesH3PO4 Reactions X PHAntonioNo ratings yet
- Solutions To Review Sample Exercises 2014Document6 pagesSolutions To Review Sample Exercises 2014Pedro Ian QuintanillaNo ratings yet
- 4-Ionic Equilibrium - SolutionDocument4 pages4-Ionic Equilibrium - SolutionMohammad OsamaNo ratings yet
- 11.9 Ionic Equilibrium Solution - PremiumDocument24 pages11.9 Ionic Equilibrium Solution - PremiumJonathan ParkerNo ratings yet
- Soal Kimia 1700Document6 pagesSoal Kimia 1700daniel hendrik molleNo ratings yet
- Resolução Cap 10 AtkinsDocument40 pagesResolução Cap 10 Atkinsrcrm17No ratings yet
- Cro Ag Cro S) 2 Ag (Aq) +CR O Aq) K K K KDocument4 pagesCro Ag Cro S) 2 Ag (Aq) +CR O Aq) K K K KCamiloNo ratings yet
- Ib - HL Acid and Base Paper 1Document14 pagesIb - HL Acid and Base Paper 1Arda RahmainiNo ratings yet
- 2022 H2 CAS Tutorial 8.1 Section A AnsDocument8 pages2022 H2 CAS Tutorial 8.1 Section A AnsGareth WongNo ratings yet
- 3811 Acids Bases WanswersDocument2 pages3811 Acids Bases WanswersClark Ivan TorresNo ratings yet
- UntitledDocument3 pagesUntitledkartik bankarNo ratings yet
- Solved Example: °C The Degree of Ionization of Water Was Found × 10Document4 pagesSolved Example: °C The Degree of Ionization of Water Was Found × 10PrashantNo ratings yet
- Chemistry: PH and pOH Calculations: Part 1: Fill in The Missing Information in The Table BelowDocument6 pagesChemistry: PH and pOH Calculations: Part 1: Fill in The Missing Information in The Table BelowCaryl Ann C. SernadillaNo ratings yet
- Calculating PHDocument3 pagesCalculating PHRosella Bethany CorreaNo ratings yet
- PH, Ka, Pka and KW Exam Questions MarkschemeDocument3 pagesPH, Ka, Pka and KW Exam Questions Markscheme장채윤No ratings yet
- 9,3 Dan 9,4Document1 page9,3 Dan 9,4Gielf CiaNo ratings yet
- Analitik Kimya İzahlı TestlərDocument6 pagesAnalitik Kimya İzahlı TestlərValiNo ratings yet
- 01 - Primera Ley de La TermodinámicaDocument6 pages01 - Primera Ley de La TermodinámicaVane HuanNo ratings yet
- PH Problems: FormulasDocument4 pagesPH Problems: FormulasJoshua NaemonNo ratings yet
- Forum Diskusi Modul 5 KB2 ProfesionalDocument5 pagesForum Diskusi Modul 5 KB2 ProfesionalputraNo ratings yet
- Chapter 3 Answers 2019-2020Document11 pagesChapter 3 Answers 2019-2020Nuraina NabihahNo ratings yet
- Topic92 AnswersDocument10 pagesTopic92 AnswersNguyen Quang KhaiNo ratings yet
- Module 7: Determination of The KSP of Various SolidsDocument5 pagesModule 7: Determination of The KSP of Various SolidsPatrick Niel Gicanal GelvoleaNo ratings yet
- Acid Base Equilibrium Practice TestDocument3 pagesAcid Base Equilibrium Practice Testapuszis100% (2)
- Ionic Equilibrium - DPP 06 (Of Lec-08) - Yakeen 2.0 2024 (Legend) (Physical Chemistry Legend)Document2 pagesIonic Equilibrium - DPP 06 (Of Lec-08) - Yakeen 2.0 2024 (Legend) (Physical Chemistry Legend)UTKARSH BHATTNo ratings yet
- Equilibrium CalculationsDocument8 pagesEquilibrium Calculationseduardo3000No ratings yet
- 17.1 - The Solubility of Slightly Soluble SaltsDocument14 pages17.1 - The Solubility of Slightly Soluble SaltsveronicaNo ratings yet
- Ionic Equilibrium: DPP 04 (Of Lec 08) - Arjuna JEE 2024Document2 pagesIonic Equilibrium: DPP 04 (Of Lec 08) - Arjuna JEE 2024Manas DubeyNo ratings yet
- Ap ChemDocument4 pagesAp ChemEthan NguyenNo ratings yet
- Assignment 5 Ionization (LEC)Document8 pagesAssignment 5 Ionization (LEC)Poison PinkNo ratings yet
- Answers To Saqs: Cambridge International A Level ChemistryDocument4 pagesAnswers To Saqs: Cambridge International A Level ChemistryGeorgeNo ratings yet
- Show My Learning 1Document14 pagesShow My Learning 1ح UAE2No ratings yet
- Misconceptions On SN1 SN2 ReactionsDocument3 pagesMisconceptions On SN1 SN2 ReactionsEdcademiaNo ratings yet
- 4.3 Rates of ReactionsDocument101 pages4.3 Rates of ReactionsSaadNo ratings yet
- 4.3 Rates of Reactions MSDocument38 pages4.3 Rates of Reactions MSLis ViegasNo ratings yet
- Misconceptions in Thermal PhysicsDocument2 pagesMisconceptions in Thermal PhysicsEdcademiaNo ratings yet
- Misconceptions in BasicityDocument3 pagesMisconceptions in BasicityEdcademiaNo ratings yet
- Misconception On OrbitalsDocument2 pagesMisconception On OrbitalsEdcademiaNo ratings yet
- Misconceptions in The Periodic TableDocument3 pagesMisconceptions in The Periodic TableEdcademiaNo ratings yet
- MathWorksheetsGrade4 2 13Document16 pagesMathWorksheetsGrade4 2 13EdcademiaNo ratings yet
- Refraction CalculationDocument2 pagesRefraction CalculationEdcademiaNo ratings yet
- Misconception On BaseDocument2 pagesMisconception On BaseEdcademiaNo ratings yet
- MathWorksheetsGrade5 2 13Document20 pagesMathWorksheetsGrade5 2 13EdcademiaNo ratings yet
- MathWorksheetsGrade6 2 13Document17 pagesMathWorksheetsGrade6 2 13EdcademiaNo ratings yet
- MathWorksheetsGrade1 2 13Document19 pagesMathWorksheetsGrade1 2 13EdcademiaNo ratings yet
- MathWorksheetsGrade2 2 13Document16 pagesMathWorksheetsGrade2 2 13EdcademiaNo ratings yet
- MathWorksheetsGrade3 2 13Document16 pagesMathWorksheetsGrade3 2 13EdcademiaNo ratings yet
- MathWorksheetsGrade5 2 6Document22 pagesMathWorksheetsGrade5 2 6EdcademiaNo ratings yet
- MathWorksheetsGrade2 2 6Document20 pagesMathWorksheetsGrade2 2 6EdcademiaNo ratings yet
- MathWorksheetsGrade6 2 6Document15 pagesMathWorksheetsGrade6 2 6EdcademiaNo ratings yet
- MathWorksheetsGrade4 2 6Document15 pagesMathWorksheetsGrade4 2 6EdcademiaNo ratings yet
- Unit Conversion TwoDocument1 pageUnit Conversion TwoEdcademiaNo ratings yet
- MathWorksheetsGrade1 2 6Document18 pagesMathWorksheetsGrade1 2 6EdcademiaNo ratings yet
- MathWorksheetsGrade3 2 6Document15 pagesMathWorksheetsGrade3 2 6EdcademiaNo ratings yet
- Electricity CalculationDocument2 pagesElectricity CalculationEdcademiaNo ratings yet
- Administration Oath and Supporting AffidavitDocument2 pagesAdministration Oath and Supporting AffidavitEdcademiaNo ratings yet
- O Level Pure Chem SummaryDocument75 pagesO Level Pure Chem SummaryEdcademiaNo ratings yet
- Length Conversion OneDocument3 pagesLength Conversion OneEdcademiaNo ratings yet
- 1.1 Cell TheoryDocument1 page1.1 Cell TheoryLucca PiaggioNo ratings yet
- Chemistry Notes PT 1Document55 pagesChemistry Notes PT 1EdcademiaNo ratings yet
- H2 BIO Cell Structure NotesDocument15 pagesH2 BIO Cell Structure NotesEdcademiaNo ratings yet