Professional Documents
Culture Documents
510 (K) Premarket Notification2
Uploaded by
kuttyjOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
510 (K) Premarket Notification2
Uploaded by
kuttyjCopyright:
Available Formats
510(k) Premarket Notification
A to Z Index Follow FDA En Español
Home Food Drugs Medical Devices Radiation-Emitting Products Vaccines, Blood & Biologics Animal & Veterinary Cosmetics Tobacco Products
510(k) Premarket Notification
FDA Home Medical Devices Databases
-
510(k) | DeNovo | Registration & Listing | Adverse Events | Recalls | PMA | HDE | Classification | Standards
CFR Title 21 | Radiation-Emitting Products | X-Ray Assembler | Medsun Reports | CLIA | TPLC
New Search Back To Search Results
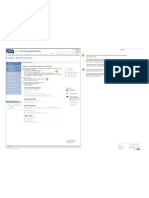
Device Classification Name Prosthesis, Knee, Patellofemorotibial, Semi-Constrained, Cemented, Polymer + Additive/Metal/Polymer + Additive
510(K) Number K111433
Device Name DEPUY STTUNE (TM) PS KNEE SYSTEM
Applicant DEPUY ORTHOPAEDICS, INC.
700 ORTHOPAEDIC DRIVE
Warsaw, IN 46581 -0988
Applicant Contact Nancy Friddle
Correspondent DEPUY ORTHOPAEDICS, INC.
700 ORTHOPAEDIC DRIVE
Warsaw, IN 46581 -0988
Correspondent Contact Nancy Friddle
Regulation Number 888.3560
Classification Product Code OIY
Subsequent Product Code JWH
Date Received 05/23/2011
Decision Date 08/30/2011
Decision Substantially Equivalent (SESE)
Regulation Medical Specialty Orthopedic
510k Review Panel Orthopedic
Summary Summary
Type Traditional
Reviewed By Third Party No
Combination Product No
-
Page Last Updated: 02/18/2019
Note: If you need help accessing information in different file formats, see Instructions for Downloading Viewers and Players.
Language Assistance Available: Español | 繁體中文 | Tiếng Việt | 한국어 | Tagalog | Русский | | Kreyòl Ayisyen | Français | Polski | Português | Italiano | Deutsch | 日本語 | |
English
Accessibility Contact FDA Careers FDA Basics FOIA No FEAR Act Site Map Nondiscrimination Website Policies
U.S. Food and Drug Administration Combination Products
10903 New Hampshire Avenue Advisory Committees
Silver Spring, MD 20993
Science & Research
Ph. 1-888-INFO-FDA (1-888-463-6332)
Contact FDA Regulatory Information
Safety
Emergency Preparedness
International Programs
For Government For Press
News & Events
Training and Continuing Education
Inspections/Compliance
You might also like
- FDA SofwaveDocument9 pagesFDA SofwavePhúc LâmNo ratings yet
- MODULE 1 Overview of The InstitutionDocument45 pagesMODULE 1 Overview of The InstitutionShannen CostoNo ratings yet
- Medical Device Regulation-USFDADocument38 pagesMedical Device Regulation-USFDAMADDINENI AVANEESHWARNo ratings yet
- York, PA 17402 USA: 510 (K) SummaryDocument6 pagesYork, PA 17402 USA: 510 (K) SummaryASHOKNo ratings yet
- Literature Search ProtocolDocument11 pagesLiterature Search ProtocolEon RegulatoryNo ratings yet
- SS 620Document20 pagesSS 620jennalyn miraflor100% (1)
- Official ITEP Preparation GuideDocument92 pagesOfficial ITEP Preparation GuideLeon Delgado85% (13)
- Placement Test: Michael Mccarthy Jeanne Mccarten Helen SandifordDocument103 pagesPlacement Test: Michael Mccarthy Jeanne Mccarten Helen SandifordDavid CarvalhoNo ratings yet
- Mass and Count NounDocument10 pagesMass and Count NounAiza CuregNo ratings yet
- 9G U3 Animal Farm Unit Guide March 2022Document21 pages9G U3 Animal Farm Unit Guide March 2022Fernando Folkis100% (1)
- 510 (K) Summary Caresorb CPT Sutures Co., Ltd.Document11 pages510 (K) Summary Caresorb CPT Sutures Co., Ltd.amit545No ratings yet
- Documents - Pub 236324905 Linguistics Across Cultures Robert Lado Chapters 1 and 3pdfDocument22 pagesDocuments - Pub 236324905 Linguistics Across Cultures Robert Lado Chapters 1 and 3pdfHeba HanafyNo ratings yet
- 510 (K) Premarket NotificationDocument1 page510 (K) Premarket NotificationkuttyjNo ratings yet
- 510 (K) Premarket Notification: FDA Home Medical Devices DatabasesDocument8 pages510 (K) Premarket Notification: FDA Home Medical Devices DatabaseskuttyjNo ratings yet
- 510 (K) Premarket Notification: FDA Home Medical Devices DatabasesDocument10 pages510 (K) Premarket Notification: FDA Home Medical Devices DatabaseskuttyjNo ratings yet
- Ge CT Hispeed AdvantageDocument3 pagesGe CT Hispeed AdvantageAdrian CastroNo ratings yet
- 510 (K) Summary: Exhibit ZDocument6 pages510 (K) Summary: Exhibit ZLuduvina PerloraNo ratings yet
- K211346 - Zeiss FL400 510k Letter For Kinevo PenteroDocument8 pagesK211346 - Zeiss FL400 510k Letter For Kinevo PenteroJoeSchmoeScribdNo ratings yet
- K203209-FDA - AirphysioDocument10 pagesK203209-FDA - AirphysioNandhini SivakumarNo ratings yet
- Camera System & Light SourceDocument9 pagesCamera System & Light Sourcevenkat_bhagavatiNo ratings yet
- FDA Easy on-PCDocument5 pagesFDA Easy on-PCVictor CuellarNo ratings yet
- Register Blood Bank Scenario v4 Blood Topic PageDocument5 pagesRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731No ratings yet
- Giving Blood Scenario v2Document3 pagesGiving Blood Scenario v2api-894731No ratings yet
- Register Blood Bank Scenario v2Document4 pagesRegister Blood Bank Scenario v2api-894731No ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document10 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993kamanNo ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- Biologics Topic Page OnlyDocument1 pageBiologics Topic Page Onlyapi-894731No ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document11 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993AlaaNo ratings yet
- Allura Xper FD Series or Allura Xper or Table SeriesDocument7 pagesAllura Xper FD Series or Allura Xper or Table Series476143969No ratings yet
- Biologics Topic Page OnlyDocument5 pagesBiologics Topic Page Onlyapi-894731No ratings yet
- MHC Medical Products: 11930 Kemper Springs DriveDocument5 pagesMHC Medical Products: 11930 Kemper Springs DriveMark ManzanasNo ratings yet
- Department of Health & Human ServicesDocument8 pagesDepartment of Health & Human ServicesRenato OllosNo ratings yet
- FDA K160341 ColposcopeDocument8 pagesFDA K160341 ColposcopeRoxana Berrocal SaccaNo ratings yet
- Manufacturer Page Rewritten - HTMDocument1 pageManufacturer Page Rewritten - HTMapi-894731No ratings yet
- K160743 FDA CT PhilipsDocument13 pagesK160743 FDA CT PhilipsThiết bị Điện Tử Y SinhNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document8 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Muhammad AwaisNo ratings yet
- Department of Health & Human ServicesDocument10 pagesDepartment of Health & Human ServiceshadjinaumovaNo ratings yet
- U.S. Food & Drug Administration 10903 New Hampshire Avenue: Silver Spring, MD 20993Document11 pagesU.S. Food & Drug Administration 10903 New Hampshire Avenue: Silver Spring, MD 20993664214458No ratings yet
- U .S. Food & Drug Administration: 10903 New Hampshire Avenue Silv Er Spring, MD 20993Document5 pagesU .S. Food & Drug Administration: 10903 New Hampshire Avenue Silv Er Spring, MD 20993Larissa GolucciNo ratings yet
- Describe 510 (K)Document7 pagesDescribe 510 (K)buyersstrikewpNo ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document6 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993pofoya3158No ratings yet
- Department of Health & Human ServicesDocument55 pagesDepartment of Health & Human ServicesinfoabhaypNo ratings yet
- Establishment Registration & Device ListingDocument2 pagesEstablishment Registration & Device ListingCarlos Gonzalez0% (1)
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document6 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993JebakumarNo ratings yet
- Prelim PrpiDocument118 pagesPrelim PrpiShannen CostoNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document8 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Jorge AntunesNo ratings yet
- Department of Health & Human Services: September 5, 2014Document5 pagesDepartment of Health & Human Services: September 5, 2014مصعب بابكرNo ratings yet
- Department of Health & Human ServicesDocument7 pagesDepartment of Health & Human ServicesVruddhi BhatiaNo ratings yet
- 510 (K) Summary: Jua/N 22zDocument6 pages510 (K) Summary: Jua/N 22zJuasadf IesafNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document14 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Mohammed SairawanNo ratings yet
- 1.4: 510 (K) SUMMARY: A. Submitter InformationDocument5 pages1.4: 510 (K) SUMMARY: A. Submitter InformationjsdanielinNo ratings yet
- Allura Xper FD Series and Allura Xper ORDocument7 pagesAllura Xper FD Series and Allura Xper OR476143969No ratings yet
- U.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993Document8 pagesU.S. Food & Drug: Administration 10903 New Hampshire Avenue Silver Spring, MD 20993QFCarlosCQNo ratings yet
- 510 (K) Summary of Safety and Effectiveness Syneron Medical Ltd. VelashapeDocument4 pages510 (K) Summary of Safety and Effectiveness Syneron Medical Ltd. VelashapeksztaltosferaNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document7 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993venkat_bhagavatiNo ratings yet
- U.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993Document7 pagesU.S. Food & Drug Administration: 10903 New Hampshire Avenue Silver Spring, MD 20993liuyonglogNo ratings yet
- 'Ikca: Manufactfuring CorpDocument5 pages'Ikca: Manufactfuring CorpF CPNo ratings yet
- E8Document36 pagesE8Li Chiang SiaNo ratings yet
- Matig Gloves - Certifications - OpulentDocument17 pagesMatig Gloves - Certifications - Opulentsaisridhar99No ratings yet
- K203505 DORO QR3 Headrest System Aluminum 29.03.2021Document2 pagesK203505 DORO QR3 Headrest System Aluminum 29.03.2021anish kcNo ratings yet
- Guidance Documents Traditional and Abbreviated 510 (K) S PDFDocument11 pagesGuidance Documents Traditional and Abbreviated 510 (K) S PDFSebastián MardonesNo ratings yet
- Argos 510KDocument10 pagesArgos 510KNorman GuntsonNo ratings yet
- Medical Device Alert - Hoya Vivinex Toric IOLsDocument12 pagesMedical Device Alert - Hoya Vivinex Toric IOLsBio AllianceNo ratings yet
- Contract Research and Development Organizations-Their History, Selection, and UtilizationFrom EverandContract Research and Development Organizations-Their History, Selection, and UtilizationNo ratings yet
- #1 - Strong and Weak - NovakDocument2 pages#1 - Strong and Weak - NovakRomero FlaviaNo ratings yet
- Upper Intermediate Glossary: Unit 1 - Business or Pleasure?Document17 pagesUpper Intermediate Glossary: Unit 1 - Business or Pleasure?Kavi kNo ratings yet
- Grade 2 Spelling Word List UpdatedDocument4 pagesGrade 2 Spelling Word List UpdatedDhara Mirani ChawdaNo ratings yet
- National Workshop BrochureDocument2 pagesNational Workshop BrochureLonely Lark0% (1)
- Negotiating With A Client CompanyDocument4 pagesNegotiating With A Client CompanyAngela AlqueroNo ratings yet
- January NightDocument7 pagesJanuary NightShazia ParveenNo ratings yet
- Direct Speech and Indirect SpeechDocument4 pagesDirect Speech and Indirect SpeechAndreea PatrauteanuNo ratings yet
- What Is The Common European Framework (CEFR) ?: Second Edition Pre-Intermediate and CEFR Level B1Document6 pagesWhat Is The Common European Framework (CEFR) ?: Second Edition Pre-Intermediate and CEFR Level B1Antonela MasottaNo ratings yet
- Rising Sindh Grammar Lecture 1Document11 pagesRising Sindh Grammar Lecture 1Nadeem RindNo ratings yet
- 1-Billy La Bufanda Project 30 Points True Newest FinalDocument1 page1-Billy La Bufanda Project 30 Points True Newest Finalapi-293617676No ratings yet
- Taller Ingles Verbo To BeDocument4 pagesTaller Ingles Verbo To BeSunny Tour Sunny TourNo ratings yet
- A Study For The Effect of The Emphaticness and LanDocument18 pagesA Study For The Effect of The Emphaticness and LanMontaser OwdaNo ratings yet
- Context Free GrammarsDocument27 pagesContext Free Grammarsh0u3wvfg9aNo ratings yet
- Aung Ko U English Language Proficiency Class: Else and OtherDocument2 pagesAung Ko U English Language Proficiency Class: Else and OtherkhinthetNo ratings yet
- Presentation About Noun ClausesDocument13 pagesPresentation About Noun Clausesclaudia clavioNo ratings yet
- 1606228406259Document20 pages1606228406259YASH BHAI GAMING KANNADA100% (2)
- Materi PembelajaranDocument19 pagesMateri PembelajaranAdam Herman100% (1)
- Grammar Summary Unit 3Document2 pagesGrammar Summary Unit 3Rony RamosNo ratings yet
- Blaine's Beast - An MM Beauty An - Joel AbernathyDocument186 pagesBlaine's Beast - An MM Beauty An - Joel Abernathyairamaguilera566No ratings yet
- Clase de Español 1Document9 pagesClase de Español 1yveysikhNo ratings yet
- Properties of A Well Written TextDocument10 pagesProperties of A Well Written TextKrztyl AbayonNo ratings yet
- Language Proficiency: by Ana Marie PamintuanDocument3 pagesLanguage Proficiency: by Ana Marie PamintuanJudah Ben Ng DucusinNo ratings yet
- In Search of A Source of Pentatonic Hemitonic and Anhemitonic Scales in Southeast AsiaDocument33 pagesIn Search of A Source of Pentatonic Hemitonic and Anhemitonic Scales in Southeast AsiaJavier CastañedaNo ratings yet
- App2brain Cheat Sheet Finnish Basic VerbsDocument3 pagesApp2brain Cheat Sheet Finnish Basic VerbsJuLie Ann DeGuzman GeslaniNo ratings yet
- PASIVDocument4 pagesPASIVSandra VitkovićNo ratings yet