Professional Documents
Culture Documents
Thsinh
Uploaded by
tvkhang93182018Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Thsinh
Uploaded by
tvkhang93182018Copyright:
Available Formats
Amylase
-1st tube (4o C): The solution turned purplish blue.
Explanation: Amylase is the enzyme that catalyzes the convertion of starch into glucose. At 4oC,
amylase was deactivated, so more starch was left than sugar.
-2nd tube (40oC): The solution turned cloud white.
Explanation: Amylase is the enzyme that catalyzes the convertion of starch into glucose. At 40oC,
amylase is at its ideal condition so there was little starch left in the solution.
-3rd tube (100oC): The solution turned dark blue.
Explanation: Amylase is the enzyme that catalyzes the convertion of starch into glucose. At 100oC,
amylase is denatured, so most of the starch remained.
Conclusion: Amylase is most effective at temperature of approximately 40oC and therefore convert
more starch into glucose.
Bromelain
-1st tube (room temperature): The gelatin pieces was dissolved in the solution.
Explanation: Bromelain is the enzyme that catalyzes the breakdown of protein. At room
temperature, bromelain is at its ideal condition.
-2nd tube (100oC): The gelatin pieces did not dissolve in the solution.
Explanation: Bromelain is the enzyme that catalyzes the breakdown of protein. At 100oC, bromelain
was denatured.
Conclusion: Bromelain is more effective at room temperature than at 100oC and therefore consume
less time for protein digestion.
Protein
-1st tube (water): The solution turned light blue
Explanation: The light blue color appeared due to the formation of Cu(OH)2.
Conclusion: There is no protein in water.
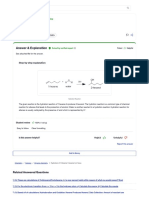
-2nd tube (gelatin): The solution turned light purple.
Explanation: The peptide bonds in gelatin reacted with Cu2+ in the Biuret reagent.
Conclusion: There is protein in gelatin.
-3rd tube (Peanut extract): The solution turned cloud pink.
Explanation: The peptide bonds in peanut reacted with Cu2+ in the Biuret reagent.
Conclusion: There is protein in peanut.
-4th tube (milk): The solution turned pinkish purple.
Explanation: The peptide bonds in milk reacted with Cu2+ in the Biuret reagent.
Conclusion: There is protein in milk.
Carbohydrate
-Monosaccharide: There is orange precipitation
Explanation: The Barfoed reagent is made up of copper sulphate and acetic acid. Since Monosaccharide
is a strong reducing agent, within 2 mins of heating, Copper(II) sulphate turned into Cu2(I)O.
Conclusion: The sample given is monosaccharide.
-Disaccharide: There is no precipitation.
Explanation: The Barfoed reagent is made up of copper sulphate and acetic acid. 2 mins of heating is
not enough for disaccharides to react so no Copper(II) sulphate turned into Cu2(I)O.
Conclusion: The sample given is disaccharide.
You might also like
- Biology Full Report ReferenceDocument3 pagesBiology Full Report ReferenceallenNo ratings yet
- Lab Report (Amylase)Document3 pagesLab Report (Amylase)Chew Kian ShengNo ratings yet
- Exp Bio Lab 3Document3 pagesExp Bio Lab 3Akmal SyahmieNo ratings yet
- The Characterization of Biological Macromolecules PDFDocument5 pagesThe Characterization of Biological Macromolecules PDFAngelo Phillip BautistaNo ratings yet
- Metabolic Biochemistry Experiment 8Document7 pagesMetabolic Biochemistry Experiment 8门门No ratings yet
- Analysis of HoneyDocument3 pagesAnalysis of HoneyGIRI PRESENTSNo ratings yet
- General Color Tests For Carbohydrates-2 PDFDocument48 pagesGeneral Color Tests For Carbohydrates-2 PDFTimothy John BautistaNo ratings yet
- Experiment No. 3 - ProteinsDocument7 pagesExperiment No. 3 - Proteinskat films “Kat”No ratings yet
- Organic Chem ExpDocument9 pagesOrganic Chem ExpFat Asian BoyNo ratings yet
- Chem Lab (Ii) 3Document11 pagesChem Lab (Ii) 3Nurul Hasanah88% (75)
- CHEMISTRY Project AMIT KUMARDocument15 pagesCHEMISTRY Project AMIT KUMARRishi AnandNo ratings yet
- Experiment 12: Digestion in The MouthDocument16 pagesExperiment 12: Digestion in The Mouthkirstie guill100% (2)
- Cloruro de Hexamino Cobalto IIIDocument5 pagesCloruro de Hexamino Cobalto IIIElizabeth Ayala BlancoNo ratings yet
- Analysis of HoneyDocument4 pagesAnalysis of HoneyAyush RaghuvanshiNo ratings yet
- Analysis of Honey PDFDocument4 pagesAnalysis of Honey PDFGangaram ParitNo ratings yet
- Chem Lab Report 2 Deol ADocument5 pagesChem Lab Report 2 Deol AMagnolia Kaye Deola100% (1)
- Name NIM Class: Nevta Fatikha Ariyani 4411421027 Biology 1ADocument59 pagesName NIM Class: Nevta Fatikha Ariyani 4411421027 Biology 1ANevta FatikhaNo ratings yet
- Qualitative and Quantitative Tests Study GuideDocument16 pagesQualitative and Quantitative Tests Study GuideJanNo ratings yet
- BM Chemistry ProjectDocument9 pagesBM Chemistry ProjectSabir S XI-ANo ratings yet
- Analysis of HoneyDocument13 pagesAnalysis of HoneyBharat Bajpai100% (1)
- Procedure Act 2Document8 pagesProcedure Act 2Rhealyn Legaspi100% (2)
- Title: Chemical Properties of AlkenesDocument8 pagesTitle: Chemical Properties of AlkenesLeeshaaLenee Paramanantha KumarNo ratings yet
- StarchDocument6 pagesStarchPatrice CampbellNo ratings yet
- Title: Chemical Properties of AlkanesDocument9 pagesTitle: Chemical Properties of AlkanesLeeshaaLenee Paramanantha KumarNo ratings yet
- Exp 3-Reduction of Cyclohexanone With Sodium BorohydrideDocument11 pagesExp 3-Reduction of Cyclohexanone With Sodium Borohydrideakuserai100% (3)
- Quantitative Analysis of Carbohydrates I - Lab 4Document27 pagesQuantitative Analysis of Carbohydrates I - Lab 4Noriko Medoruma0% (3)
- CHM1024 Report 3: Identification of HydrocarbonsDocument15 pagesCHM1024 Report 3: Identification of HydrocarbonsAkmal Adib Fadzil90% (41)
- Apparatus:: 1. Test For PotassiumDocument3 pagesApparatus:: 1. Test For PotassiumRItesh KumarNo ratings yet
- Qualitative Tests For CarbohydratesDocument22 pagesQualitative Tests For CarbohydratesHelal HamadNo ratings yet
- Lab Bio 3Document5 pagesLab Bio 3Nada Nabila63% (8)
- Analysis of HoneyDocument14 pagesAnalysis of HoneyMomo PlayerNo ratings yet
- CHEM35.1 E2 Aromatic Side Chain OxidationDocument3 pagesCHEM35.1 E2 Aromatic Side Chain OxidationGlenn Vincent TumimbangNo ratings yet
- Bio JadiDocument8 pagesBio JadiIka DamayantiNo ratings yet
- Jawahar Navodaya Vidyalaya MYSURU 2021-22: Chemistry Investigatory ProjectDocument14 pagesJawahar Navodaya Vidyalaya MYSURU 2021-22: Chemistry Investigatory ProjectMr Suhas gowdaNo ratings yet
- Investigatory ProjectDocument18 pagesInvestigatory ProjectArwin NanduNo ratings yet
- Analysis of Honey Xii ADocument10 pagesAnalysis of Honey Xii ACHENNAI HOLIC100% (1)
- Cellular RespirationDocument33 pagesCellular Respirationapi-202349222100% (1)
- Carbohydrates Lab ReportDocument7 pagesCarbohydrates Lab ReportRameesh IshakNo ratings yet
- Chemistry Investigatory ProjectDocument15 pagesChemistry Investigatory Projectshankaranand200517No ratings yet
- Gabrielle Morgan - Lab 2Document4 pagesGabrielle Morgan - Lab 2Gabrielle MorganNo ratings yet
- PercobaanfermentasiDocument2 pagesPercobaanfermentasinovembermanlyNo ratings yet
- Honey AnalysisDocument15 pagesHoney AnalysisAyyappan SudharshanNo ratings yet
- Analysis of Honey: Sri Sathya Sai Vidya Vihar, IndoreDocument20 pagesAnalysis of Honey: Sri Sathya Sai Vidya Vihar, IndoreShiv ShrïvƛstƛvaNo ratings yet
- Protein: Group 1 Abasolo, Banguiran, Boston, Carrillo, Haron, Lamban, Pajigal, Steenkamp Bsmls-2CDocument14 pagesProtein: Group 1 Abasolo, Banguiran, Boston, Carrillo, Haron, Lamban, Pajigal, Steenkamp Bsmls-2CKeith Jason CortesNo ratings yet
- Protein: Group 1 Abasolo, Banguiran, Boston, Carrillo, Haron, Lamban, Pajigal, Steenkamp Bsmls-2CDocument14 pagesProtein: Group 1 Abasolo, Banguiran, Boston, Carrillo, Haron, Lamban, Pajigal, Steenkamp Bsmls-2CKeith Jason CortesNo ratings yet
- Sample + Silver Nitrate (1 Spatula) + Dil NH (0.5 ML) + Dil. Naoh (0.5Ml) (Silver Mirror Deposit)Document14 pagesSample + Silver Nitrate (1 Spatula) + Dil NH (0.5 ML) + Dil. Naoh (0.5Ml) (Silver Mirror Deposit)Zunaira AliNo ratings yet
- Chemistry Project:: Analysis of HoneyDocument15 pagesChemistry Project:: Analysis of HoneyNilamya PrakashNo ratings yet
- CHM 2962 Report 4Document9 pagesCHM 2962 Report 4Yun KiatNo ratings yet
- Biochem Lab ReviewerDocument6 pagesBiochem Lab ReviewerDarlin Maree JamonNo ratings yet
- Lab 3 Aspirin ReportDocument3 pagesLab 3 Aspirin ReportMsShu93100% (1)
- Hydroboration Oxidation or (1R) (+) Alpha PineneDocument4 pagesHydroboration Oxidation or (1R) (+) Alpha Pinenewilso279100% (1)
- Experiment 2: Course: Sko 3033 (Organic Chemistry 2) Group: B Lecturer'S Name: En. Mohamad Syahrizal BinDocument5 pagesExperiment 2: Course: Sko 3033 (Organic Chemistry 2) Group: B Lecturer'S Name: En. Mohamad Syahrizal BinNapsiah NasuchiNo ratings yet
- Lab Denaturing AlbuminDocument3 pagesLab Denaturing AlbuminMinh HoangNo ratings yet
- Sargin, Şevval Qualitative Aminoacid and ProteinsDocument11 pagesSargin, Şevval Qualitative Aminoacid and ProteinsŞEVVAL SARGINNo ratings yet
- Molisch and Iodine TestDocument29 pagesMolisch and Iodine TestTom Anthony Tonguia0% (4)
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastFrom EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastNo ratings yet
- The Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterFrom EverandThe Chemistry of Dairy Products - A Chemical Analysis of Milk, Cream and ButterNo ratings yet
- Kathrein 80010430 PDFDocument1 pageKathrein 80010430 PDFRaúl Fernández SánchezNo ratings yet
- Manual de Programacao r6 N 1.3Document160 pagesManual de Programacao r6 N 1.3Ian MechuraNo ratings yet
- BPCL Training ReportDocument34 pagesBPCL Training ReportVishalVaishNo ratings yet
- ch12 칼리스터 재료과학과 공학 답지Document71 pagesch12 칼리스터 재료과학과 공학 답지hayun9999999No ratings yet
- Person Name: NDT Management CoordinatorDocument4 pagesPerson Name: NDT Management CoordinatorDendy PratamaNo ratings yet
- DSP Lab 6Document7 pagesDSP Lab 6Ali MohsinNo ratings yet
- CapacitanceDocument71 pagesCapacitanceTharaj ThajNo ratings yet
- Astm D698Document13 pagesAstm D698Jorge CarrascoNo ratings yet
- Design of Torque ArmDocument16 pagesDesign of Torque ArmRonak PanchalNo ratings yet
- Design of Absorption ColumnDocument33 pagesDesign of Absorption ColumnAli Hassan50% (2)
- Solar and Wind Hybrid Power GenerationDocument35 pagesSolar and Wind Hybrid Power Generationlatest advance guruji 2018No ratings yet
- Rational and Irrational NumbersDocument16 pagesRational and Irrational NumbersSumit JoshiNo ratings yet
- RoboticsDocument41 pagesRoboticsMark Jason100% (3)
- Spring State Machine TransitionsDocument6 pagesSpring State Machine TransitionsGroza CristiNo ratings yet
- Quality and Reliability in Analytical ChemistryDocument108 pagesQuality and Reliability in Analytical ChemistryLuffy NamiNo ratings yet
- Math Ed 04: TrigonometryDocument5 pagesMath Ed 04: TrigonometryCharles SilerioNo ratings yet
- Kisi Kisi Soal LKS 2021Document23 pagesKisi Kisi Soal LKS 2021Gusti RahmadiNo ratings yet
- Modem StandardsDocument28 pagesModem StandardsShammer ShaNo ratings yet
- Siddartha Institute of Science and Technoloy, Puttur: Seminar On Audio SpotlightingDocument19 pagesSiddartha Institute of Science and Technoloy, Puttur: Seminar On Audio SpotlightingRasiNo ratings yet
- SIMULATION of EMERGENCY ROOMS USING FLEXSIMDocument10 pagesSIMULATION of EMERGENCY ROOMS USING FLEXSIMBrandon VarnadoreNo ratings yet
- Product and Service DesignDocument17 pagesProduct and Service DesignRahul KhannaNo ratings yet
- (Solved) Hydration of 1-Hexene 1-Hexene To 2 Hexanol Equation Write The Equation of The Reaction - Course HeroDocument2 pages(Solved) Hydration of 1-Hexene 1-Hexene To 2 Hexanol Equation Write The Equation of The Reaction - Course HeroJdkrkejNo ratings yet
- 74LVC14APWDHDocument11 pages74LVC14APWDHIlie GrecuNo ratings yet
- Solution Stoichiometry 1Document54 pagesSolution Stoichiometry 1Johncy MoradaNo ratings yet
- Statistics Fall2013 - Final Sample Test 01Document7 pagesStatistics Fall2013 - Final Sample Test 01Thanh VyNo ratings yet
- Generalized CoordinatesDocument8 pagesGeneralized CoordinatesJoshua WoodNo ratings yet
- Sci Problem SolutionsDocument10 pagesSci Problem SolutionsVS PUBLIC SCHOOL BangaloreNo ratings yet
- GD33-12 12V33AH: GD SERIES-Deep Cycle BatteryDocument2 pagesGD33-12 12V33AH: GD SERIES-Deep Cycle BatteryMarcel BaqueNo ratings yet
- Engineeringinterviewquestions Com RCC Structures Design Multiple Choice QuestionDocument87 pagesEngineeringinterviewquestions Com RCC Structures Design Multiple Choice QuestionRajeev BansalNo ratings yet
- Spirometric Evaluation of Pulmonary Function Tests in Bronchial Asthma PatientsDocument6 pagesSpirometric Evaluation of Pulmonary Function Tests in Bronchial Asthma PatientsdelphineNo ratings yet